Abstract
In human tumors, changes in the surface expression and/or function of major histocompatibility complex (MHC) class I antigens are frequently found and may provide malignant cells with a mechanism to escape control of the immune system. This altered human lymphocyte antigen (HLA) class I phenotype can be caused by either structural alterations or dysregulation of genes encoding subunits of HLA class I antigens and/or components of the MHC class I antigen-processing machinery (APM). Herein we analyze the expression of several proteins involved in the generation of MHC class I epitopes in feline injection site sarcoma, a spontaneously occurring tumor in cats that is an informativemodel for the study of tumor biology in other species, including humans. Eighteen surgically removed primary fibrosarcoma lesions were analyzed, and an enhanced expression of two catalytic subunits of immunoproteasomes, PA28 and leucine aminopeptidase, was found in tumors compared to matched normal tissues. As a functional counterpart of these changes in protein levels, proteasomal activities were increased in tissue extracts from fibrosarcomas. Taken together, these results suggest that alterations in the APM system may account for reduced processing of selected tumor antigens and may potentially provide neoplastic fibroblasts with a mechanism for escape from T-cell recognition and destruction.
Keywords: Proteasome, immunoproteasome, PA28, MHC class I epitopes, antigen processing
Introduction
The immune system continually screens for viral infections and cancers by monitoring whether cells are synthesizing foreign or mutant proteins [1]. The surveillance process depends on the presence of major histocompatibility complex (MHC) class I molecules that bind to and display cytotoxic T lymphocyte (CTL) peptides (known as antigenic peptides or epitopes) that are derived from the entire spectrum of proteins expressed in the cell. Under normal physiological conditions, all MHC class I-presented peptides are derived from normal autologous sequences to which the immune system is nonreactive, owing to self-tolerance. However, if the cell is infected by viruses or expresses mutant gene products (e.g., oncogene products), then nonnative (“foreign”) peptides will be displayed and will stimulate CD8+ lymphocytes (CTL) to kill the anomalous cell. Furthermore, CTL can also be activated by self-peptide-MHC class I complexes expressed at supraphysiological levels [2]. At present, there is a general agreement that the immune system can recognize and destroy nascent transformed cells, thereby preventing tumor formation [3,4].
The majority of antigenic peptides are generated during the degradation of intracellular proteins by the ubiquitin-proteasome pathway [1]. These peptides are then translocated through the TAP transporter into the endoplasmic reticulum, where they bind to MHC class I heterodimers. This binding stabilizes the interaction between MHC class I heavy and light chains and allows the heterodimer to be transported to the cell surface. The active form of the proteasome, which appears to degrade most cellular proteins, is 26S proteasome [5]. This 2.4-MDa complex is formed by the association of a 19S-regulatory particle with one or both ends of the core 20S proteasome. The 26S proteasome degrades ubiquitinated and some nonubiquitinated proteins in an ATP-dependent manner [6]. Proteins are cleaved within the 20S (700 kDa) core proteasome, which is composed of four stacked rings. The two inner β-rings contain six proteolytic sites that differ in substrate specificity: two have chymotrypsin-like activities, two have trypsin-like activities, and two possess caspase-like activities.
The immune system has evolved several mechanisms to modify the activity of the ubiquitin-proteasome pathway and to enhance the efficiency of the generation of MHC class I-presented peptides [1]. Several components of this system are induced by the immune modifier interferon-γ (IFN-γ), including special forms of the proteasome, termed immunoproteasomes, that contain novel active subunits, the α and β subunits of the proteasome activator PA28 [7], and leucine aminopeptidase (LAP) [8], a cytosolic enzyme that can generate antigenic peptides from longer proteasome products but also destroy them [9–12]. Each IFN-γ-inducible proteasomal β subunit is homologous with a constitutive catalytic subunit (LMP2/Y, LMP7/X, and MECL/Z) and can replace its homologue during proteasome assembly.
It has been well established that the presence of these IFN-γ-induced subunits alters catalytic activity against model peptide substrates. They enhance cleavages after hydrophobic, basic, and branched-chain residues, while suppressing cleavages after acidic residues. Therefore, immunoproteasomes generate a different spectrum of oligopeptide products compared to constitutive particles. This new pattern of peptides has been proposed to promote antigen presentation [1] because the transporter associated with antigen presentation (TAP) and MHC class I molecules strongly prefer to bind peptides with carboxyl-terminal hydrophobic and basic residues over acidic ones. In addition, this altered specificity may also enhance the production of N-extended precursors of antigenic peptides [13] that are trimmed to the presented epitopes by aminopeptidases in the cytosol or endoplasmic reticulum [14]. Another IFN-γ-inducible component of the proteasome system that affects antigen presentation is PA28 (also termed REG or 11S), a ring-shaped 180-kDa multimeric complex that can bind to the free ends of 20S or asymmetric (i.d. 19S–20S) 26S proteasomes and dramatically enhance their ability to degrade short peptide substrates [7]. Expression of PA28α or PA28α/β has been reported to enhance the MHC class I antigen presentation of some, but not all, epitopes [1], and so-called “hybrid proteasomes” (i.d. 19S–20S-PA28) have been shown to generate a different set of peptide products from proteins [15]. Therefore, by increasing the variety of peptides generated from a protein substrate, PA28 can presumably enhance the diversity of epitopes presented on the cell surface and thereby increase the possibility that an appropriate CTL response is elicited. Nevertheless, in contrast to the current view that immunoproteasomes are always better suited for the processing of MHC class I-restricted peptides, it has been shown that several tumor-specific antigens and melanoma differentiation antigens, which are efficiently produced by standard proteasomes, are poorly produced by immunoproteasomes [16–18]. Therefore, a switch from normal proteasomes to immunoproteasomes may be one of the mechanisms that allow tumor cells to escape attack from CTLs [19].
So far, however, studies using cultured tumor cell lines, antigen-processing mutants, and surgically removed malignant lesions have demonstrated a reduced expression of IFN-γ-induced proteasome and PA28 subunits [20–26]. These abnormalities correlate with impaired processing of antigenic peptides, low levels or loss of MHC class I surface expression, and resistance to CTL-mediated lysis [20]. Furthermore, deficiencies in the antigen-processing machinery (APM) can be reverted by cytokine treatment, particularly by IFN-γ [21,25]. As only a limited number of studies on alterations of APM components have been performed in surgically removed tumor lesions, we analyzed feline injection site sarcoma (FISS), a spontaneously occurring, highly locally invasive tumor that develops at the site of injection of vaccines or other drugs in cats [27]. Histologically, FISS is a mesenchymal tumor (usually well differentiated) that is characterized by plumps of spindle cells arranged in interwoven bundles with anaplastic tendency, with cells of variable size and shape, pleomorphic nuclei, and an increased number of multinucleated cells [28]. This tumor was chosen because it represents a good informative model for carcinogenesis and genetic susceptibility that is applicable to cancer in all species, including humans [29,30]. In our study, we analyzed 18 surgically removed primary lesions, and we found an enhanced expression of different catalytic β subunits of immunoproteasomes, α and β subunits of PA28, and LAP in tumors compared to matched normal tissues. As a functional counterpart of these changes in protein levels, proteasomal cleavage specificities are modified in tissue extracts from fibrosarcomas. Altogether, the alterations in the APM system that we have identified might account for a reduced processing of some tumor antigens and might potentially provide neoplastic fibroblasts with a mechanism to escape from T-cell recognition and destruction.
Materials and Methods
Total Proteins
Samples of fibrosarcoma and healthy subcutis were collected from the same animal during surgery, placed on ice, and immediately homogenized in ice-cold extraction buffer (50 mM Tris-HCl pH 7.5, 1 mM dithiothreitol, 250 mM sucrose, 5 mM MgCl2, 0.5 mM EDTA, and 2 mM ATP) using an Ultraturax DIAX900 homogenizer (Heidolph Instruments, Kelheim, Germany) and centrifuged at 14,000 rpm for 20 minutes at 4°C. The protein concentration in supernatants was determined using the QUICK START Bradford Dye Reagent 1x (Bio-Rad Laboratories, Hercules, CA) using a standard curve constructed with bovine serum albumin (BSA). Samples were stored at -80°C until use.
Proteasome Activity Assays
Peptidase activities of proteasomes were assayed by monitoring the production of 7-amino-4-methylcoumarin (AMC) from fluorogenic peptides, as previously described [13]. Briefly, Suc-LLVY-AMC (for chymotrypsin-like activity) and Z-YVAD-AMC (for caspase-like activity) (BACHEM, Bubendorf, Switzerland) were used at a final concentration of 100 µM in 20 mM Tris-HCl pH 7.5, 1 mM ATP, 2 mM MgCl2, and 0.2% BSA. Reactions were started by adding an aliquot of tissue extracts, and the fluorescence of released AMC (excitation, 380 nm; emission, 460 nm) was monitored continuously at 37°C with a Carry Eclipse spectrofluorimeter (VARIAN, Palo Alto, CA). Background activity (caused by nonproteasomal degradation) was determined by the addition of the proteasome inhibitor MG132 (BACHEM) at a final concentration of 10 µM.
Immunoblot Analyses
Immunoblot analyses of the proteasomal and immunoproteasomal catalytic β subunits, PA28α/β, and LAP in both fibrosarcomas and normal subcutis tissue extracts were performed as previously described [15]. Briefly, 60 µg of total proteins for X, Y, LMP2, and LMP7, and 40 µg for PA28α, PA28β, and LAP were separated on a 12% SDS-PAGE gel, and proteins were transferred on a Hybond-P membrane (Amersham Pharmacia, Buckinghamshire, UK). Membranes were stained with Ponceau red before incubation with the primary antibody to confirm that similar amounts of proteins had been transferred. The membrane was then incubated in a blocking buffer (5% nonfat milk in 1 x PBS; 0.1% Tween-20), followed by incubation with rabbit antisera against LMP2, LMP7, PA28α, and PA28β (a kind gift of Dr. K. Tanaka, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), X (a kind gift of Prof. A. L. Goldberg, Harvard Medical School, Boston, MA), and LAP (a kind gift of Dr. T. Saric, University of Cologne, Cologne, Germany), and mouse monoclonal anti-Y (a kind gift of Prof. A. L. Goldberg). Bound antibodies were visualized using the ECL technique (ECLPlus Western Blotting Detection Reagents; Amersham Pharmacia), and densitometric analysis of bands was performed with a VersaDoc 1000 Imaging System (Bio-Rad Laboratories) using the Quantity One software (Bio-Rad Laboratories).
Statistical Analysis
Because the Shapiro-Wilk test has proven that densitometric data were not normally distributed (data not shown), nonparametric Wilcoxon test was performed to establish whether protein expression levels significantly differed between fibrosarcomas and related healthy subcutis. For this purpose, values were transformed into log10(densitometric value + 1), and the ratio was expressed as log10(fibrosarcoma/healthy subcutis protein expression). Furthermore, Wilcoxon test was also used to establish whether chymotrypsin-like and caspase-like activities differed significantly between fibrosarcomas and healthy subcutis. P < .05 was considered statistically significant, whereas P < .01 was considered highly significant. Data were graphically visualized using box plots.
Immunohistochemistry
Immunohistochemistry studies were performed with the same antibodies used for Western blot analysis. Tissue sections (3 µm) were dewaxed in xylene, rehydrated in graded ethanol, and washed with PBS (pH 7.0, 1 mM). Endogenous peroxidase activity was quenched by immersion in a solution of 3% hydrogen peroxide in methanol, followed by several rinses in PBS. Nonspecific binding was blocked by incubation with 5% BSA in PBS. Slides were then incubated for 60 minutes at room temperature with primary antibody, rinsed in PBS, and incubated with secondary antibody using the Super Sensitive IHC Detection System (BioGenex, San Ramon, CA). Slides were rinsed in PBS and then stained with the DAKO Cytomation Liquid DAB Substrate Chromogen System (DAKO Corp., Carpinteria, CA). Sections were counterstained with Mayer's hematoxylin solution. Negative controls were run in parallel, replacing the primary antibody with PBS containing 5% BSA. Tissue sections were evaluated by light microscopy to determine anti-X, Y, LMP7, LMP2, PA28α, PA28β, and LAP positivity. Positive cells were counted in 10 high-power fields (x400) for each tissue section, and at least 1000 cells for each antigen were evaluated. The number of cells positive for each antigen was estimated semiquantitatively and was scored as (-) absence of immunoreaction; (+) 1% to 10% positive cells; (++) 11% to 50% positive cells; (+++) > 50% positive cells.
Results
Patients and Tissue Samples
Eighteen fibrosarcoma lesions and matched normal subcutis were obtained from 11 male and 7 female cats with an average age of 10.6 years (range, 5–16 years; median, 10.5 years), which had undergone wide-margin surgery at the Veterinary Teaching Hospital of Turin University. For 17 patients, complete follow-up, in terms of both disease-free survival time and overall survival time, was available. Eleven tumors were located in the subcutis of the interscapular region, three were located on the dorsal neck, one was located on the left scapula, one was located on the right scapula, and two were located on the thorax wall. Fourteen were primary lesions, and four were local recurrences. Histologic examination revealed that all lesions were fibrosarcomas. None of the patients had received any preoperative treatment such as chemotherapy, radiotherapy, or immunotherapy. Tumor staging was based on the more recent World Health Organization Tumor-Node-Metastasis (TNM) classification system [31]. One tumor was classified as T1N0M0, four were classified as T2N0M0, six were classified as T3N0M0, and seven were classified as T4N0M0. The characteristics of each tumor are reported in Table 1.
Table 1.
Characteristics of Fibrosarcoma Tumors Analyzed.
| Number | Age (years) | Gender | Anatomic Site | State at Presentation | Clinical Status* | TNM | Disease-Free Survival* (days) | Overall Survival* (days) |
| 1 | 9 | M | Left scapula | Primary tumor | Dead | T1N0M0 | 537 | 628 |
| 2 | 11 | M | Interscapular | Local recurrence | Dead | T2N0M0 | 988 | 1588 |
| 3 | 9 | F | Interscapular | Primary tumor | Alive | T2N0M0 | 971 | 971 |
| 4 | 13 | F | Neck | Primary tumor | Alive | T2N0M0 | 1023 | 1023 |
| 5 | 13 | F | Thorax | Local recurrence | LFU | T2N0M0 | 248 | 248 |
| 6 | 13 | M | Interscapular | Primary tumor | LFU | T3N0M0 | 158 | 158 |
| 7 | 11 | M | Neck | Primary tumor | LFU | T3N0M0 | 315 | 315 |
| 8 | 10 | F | Interscapular | Primary tumor | Alive | T3N0M0 | 914 | 914 |
| 9 | 10 | F | Interscapular + neck | Primary tumor | LFU | T3N0M0 | 440 | 440 |
| 10 | 5 | F | Interscapular | Primary tumor | Alive | T3N0M0 | 758 | 758 |
| 11 | 10 | M | Interscapular | Primary tumor | Dead | T3N0M0 | 481 | 481 |
| 12 | 10 | M | Interscapular | Primary tumor | Dead | T4N0M0 | 459 | 459 |
| 13 | 12 | M | Interscapular | Primary tumor | Alive | T4N0M0 | 1247 | 1247 |
| 14 | 7 | M | Interscapular | Primary tumor | Alive | T4N0M0 | 1401 | 1401 |
| 15 | 16 | F | Right scapula | Local recurrence | Dead | T4N0M0 | 2121 | 2243 |
| 16 | 13 | M | Neck | Primary tumor | Dead | T4N0M0 | 0 | 0 |
| 17 | 6 | M | Lateral thorax | Local recurrence | LFU | T4N0M0 | 476 | 476 |
| 18 | 12 | M | Interscapular | Primary tumor | Dead | T4N0M0 | 221 | 281 |
F, female; M, male; LFU, lost to follow-up.
On July 25, 2007.
Enhanced LMP2 and LMP7 and Unchanged X and Y Expressions in Fibrosarcoma Lesions
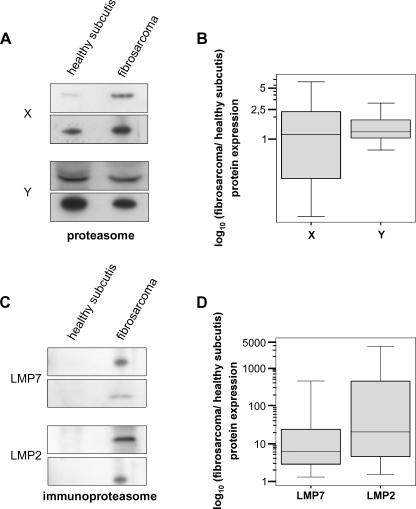
Abnormalities in several components of class I APM are often associated with neoplastic transformation and may confer a nonimmunogenic phenotype to tumor cells [3,4,32]. In particular, a reduced expression of proteasomal subunits is the most frequent alteration described in several cell lines of neoplastic origin or in naturally occurring tumors [20–26]. Therefore, to determine the expression levels of proteasomal and immunoproteasomal catalytic β subunits, tissue extracts from 18 surgically removed fibrosarcoma lesions and matched healthy subcutis were analyzed byWestern blot analysis using antibodies specific for X, Y, LMP2, and LMP7 subunits. This analysis showed (Figure 1A) unchanged expression of X and Y in the neoplastic component compared to corresponding healthy subcutis in all cases evaluated. Densitometric analysis of specific bands revealed an identical expression pattern of these two catalytic proteasomal subunits in both healthy and normal tissues (Figure 1B) (X: Z = -0.457, P = .647; Y: Z = -2.570, P = .10; Wilcoxon test). On the contrary, expression of the IFN-γ-induced catalytic subunits LMP2 and LMP7 was highly enhanced in tumor lesions compared to matched healthy tissues (Figure 1, C and D). Statistical analysis of densitometric data demonstrated that these differences are highly significant (LMP2: Z = -3.724, P = .0002; LMP7: Z = -3.724, P = .0002; Wilcoxon test) but not significantly correlated to tumor stage or clinical outcome (data not shown). Taken together, these results clearly demonstrate that, compared to healthy subcutis, fibrosarcomas have no differences in the expression levels of two catalytic subunits (X and Y) of constitutive proteasomes, whereas two catalytic subunits (LMP2 and LMP7) of immunoproteasomes are strongly induced.
Figure 1.
Levels of proteasomal (X and Y) and immunoproteasomal (LMP2 and LMP7) catalytic β subunits in fibrosarcomas and healthy control subcutis. Eighteen specimens were analyzed. Two representative Western blot analyses for X and Y (A) and two representative Western blot analyses for LMP2 and LMP7 (C) are shown. Expression ratios of X and Y (B) and of LMP2 and LMP7 (D) in fibrosarcomas and healthy subcutis were calculated as reported in Materials and Methods. It is worthy of note that the intensities of bands can only be compared within each single experiment and not between them, as blots were performed with different antibodies.
Enhanced Expression of PA28α/β and LAP in Fibrosarcomas
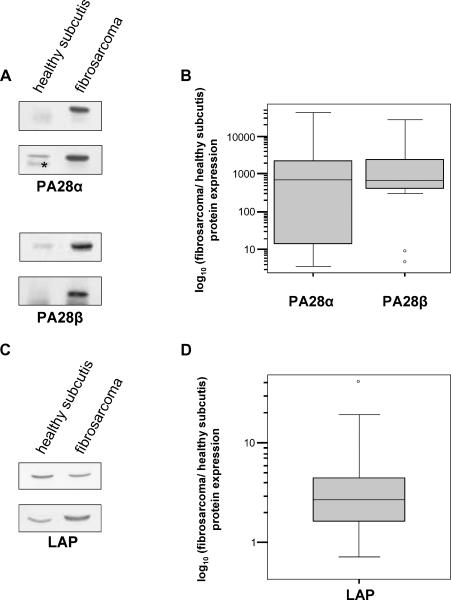
It has been well established that other components of APM collaborate with proteasomes in generating the final versions of epitopes that are presented on the cell surface in association with MHC class I molecules [1]. Specifically, the proteasome activator PA28 has been reported to enhance the generation of several class I epitopes [7], whereas LAP is one of the main enzymes involved in the cytosolic trimming of both epitopes and their N-extended precursors [9–12], which represent major proteasomal products and whose generation is further enhanced by immunoproteasomes [13]. Therefore, we analyzed the expression of the α and β subunits of PA28 and LAP in fibrosarcomas. Interestingly, Western blot analysis showed that expression levels of PA28α/β are much higher in fibrosarcomas compared to healthy control subcutis (Figure 2A) and that these differences are highly significant (Figure 2B) (PA28α: Z = -3.724, P = .0002; PA28β: Z = -3.724, P = .0002; Wilcoxon test). Additionally, LAP expression was enhanced in fibrosarcomas compared to control subcutis, although to a lesser extent (Figure 2, C and D) (LAP: Z = -3.549, P = .0004; Wilcoxon test). Finally, the enhanced expression of these IFN-γ-induced APM components is not significantly correlated with tumor stage or course of the disease (data not shown).
Figure 2.
Expression of PA28α/β and LAP in 18 fibrosarcomas and healthy control subcutis. (A) Two representative Western blot analyses for PA28α and two representative Western blot analyses for PA28β are shown. (B) Box plots of the expression ratios of PA28α/β between fibrosarcomas and healthy subcutis, determined as reported in Materials and Methods. *Nonspecific band. (C) Two representative Western blot analyses for LAP are shown. (D) Box plots of the expression ratios of LAP between fibrosarcomas and healthy subcutis.
Taken together, these results demonstrate that PA28α/β and LAP, three important components of the APM strongly induced by IFN-γ, are expressed at significantly higher levels in fibrosarcomas than in healthy subcutis.
Enhanced Proteasomal Activity in Fibrosarcomas
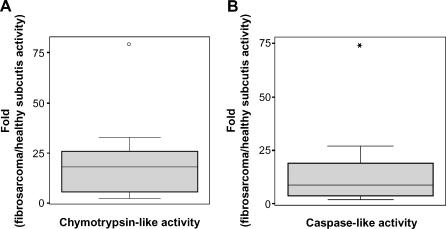
To investigate whether differences in the expression levels of proteasomal and PA28 subunits detected by Western blot analysis correlated with changes in enzymatic activity, we next analyzed the cleavage specificity of proteasomes in homogenates of fibrosarcomas and healthy subcutis. In agreement with our data showing an enhanced expression of two proteasomal catalytic subunits and PA28, chymotrypsin-like activity, the main proteasomal proteolytic activity that is rate-limiting in protein degradation, is nearly 18-fold higher in fibrosarcomas than in healthy subcutis (Table 2 and Figure 3A) (Z = -3.724, P = .0002; Wilcoxon test). Additionally, caspase-like activity was significantly enhanced in fibrosarcomas, albeit to a lesser extent (Table 2 and Figure 3B) (Z = -3.724, P = .0002; Wilcoxon test). Together, these results demonstrate that, in agreement with an enhanced expression of IFN-γ-induced proteasomal catalytic β subunits and PA28, chymotrypsin-like and caspase-like activities of proteasomes are also highly increased in extracts from fibrosarcomas.
Table 2.
Chymotrypsin-Like and Caspase-Like Activities in Fibrosarcoma Lesions and Related Healthy Subcutis.
| Healthy Subcutis | Fibrosarcomas | |
| Chymotrypsin-like activity | 0.29 ± 0.07 | 2.10 ± 0.31 |
| (nmol Suc-LLVY-AMC cleaved/mg min) | (0.12) | (2.13) |
| Caspase-like activity | 0.02 ± 0.004 | 0.10 ± 0.02 |
| (nmol Z-YVAD-AMC cleaved/mg min) | (0.01) | (0.07) |
Data are presented as the mean ± SEM (median) of the specificactivities of all 18 animals analyzed.
SEM, standard error of the mean.
Figure 3.
Increase in proteasomal chymotrypsin-like activity (A) and caspase-like activity (B) in tissue extracts from 18 fibrosarcomas compared to healthy subcutis.
Immunohistochemical Analysis
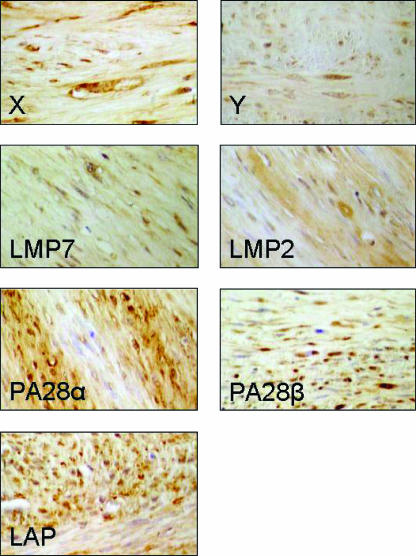
To investigate the expression of APM components at the single-cell level, two formalin-fixed paraffin-embedded surgically removed fibrosarcoma lesions and related healthy subcutis were immunohistochemically analyzed for the expression of X, Y, LMP2, LMP7, PA28α, PA28β, and LAP (Figure 4 and Table 3). This analysis demonstrated that all these proteins are expressed in neoplastic fibroblasts, although to different extents. In particular, the number of cells that are positive for LMP2, LMP7, PA28α, PA28β, and LAP is higher than that for X and Y (Table 3). On the contrary, in normal fibroblasts of the same animals, PA28β is undetectable, whereas the other immunoproteasomal subunits are expressed at lower levels than the subunits of constitutive proteasomes (Table 3). Furthermore, a comparison between neoplastic and healthy tissues revealed an equal expression of constitutive proteasomal catalytic subunits X and Y, but a higher expression of LMP2, LMP7, PA28α, PA28β, and LAP in transformed than in normal fibroblasts (Table 3). Therefore, immunohistochemical analysis clearly confirms Western blot analysis results showing an enhanced expression of IFN-γ-induced APM elements in neoplastic fibroblasts.
Figure 4.
Immunohistochemical detection of different MHC class I antigen-processing components in two different fibrosarcomas. Original magnification, x600.
Table 3.
Immunohistochemical Evaluation of LAP, PA28α/β, LMP2, LMP7, X, and Y in Fibrosarcoma Lesions and Related Healthy Subcutis.
| Antigens | Score | |
| Healthy Subcutis | Fibrosarcomas | |
| X | ++ | +/++ |
| Y | ++ | + |
| LMP7 | + | ++ |
| LMP2 | + | ++ |
| PA28α | - | ++ |
| PA28β | + | ++ |
| LAP | ++ | +++ |
The number of positive cells was estimated semiquantitatively and scored as (-) absence of immunoreaction; (+) 1% to 10% positive cells; (++) 11% to 50% positive cells; (+++) > 50% positive cells. Data refer to the immunohistochemical studies of two different animals.
Discussion
Previous reports have suggested that defects in the production of antigenic peptides for MHC class I assembly and presentation might contribute to the immune escape of tumor cells [3,4,32]. To test this hypothesis, we measured the expression levels of several components of APM in FISS, a locally invasive and histologically aggressive soft-tissue sarcoma that develops at the site of injection administration [27] and that represents a highly informative model for the study of tumor biology in different species, including humans [29,30]. Moreover, to investigate whether differences in protein expression correlate with differences in enzymatic activity, we analyzed the cleavage specificity of proteasomes in homogenates of fibrosarcomas and healthy subcutis.
The results of the present study can be summarized as follows: 1) proteasomal catalytic β subunits X and Y are expressed roughly at the same levels in fibrosarcoma lesions and healthy subcutis; 2) immunoproteasome catalytic β subunits LMP2 and LMP7, α and β subunits of the proteasome activator PA28, and LAP are expressed at much higher levels in fibrosarcomas than in normal subcutis; and 3) tissue extracts from fibrosarcomas present enhanced proteasomal chymotrypsin-like activities and, to a lesser extent, caspaselike activities than healthy subcutis.
In tumors, abnormalities in the expression and/or function of the components of class I APM such as tapasin, TAP, LMP, and PA28 subunits [20,21] may cause a marked downregulation of the cell surface expression of MHC class I antigens, thereby providing malignant cells with a mechanism to escape control of the immune system [33,34]. Such abnormalities are frequently found in malignant cells in both humans and mice, and represent a major obstacle to the successful implementation of T-cell-based immunotherapy. Furthermore, loss of the ability to process immunodominant tumor antigens represents an additional mechanism by which tumor cells can escape immune recognition [4,19,32]. To date, the expression of proteasomal and PA28 subunits has been investigated only in a limited number of surgically removed lesions and tumor cell lines of distinct histology from humans, whereas no data are available on the tumoral expression of LAP, a cytosolic enzyme that can generate antigenic peptides from longer proteasome products but also destroy them [9–12]. Although some discrepancies are present in previous studies, which probably reflect differences in the tumors or cell lines analyzed and/or in the methods used to detect changes in class I peptide-generating machinery, the overall picture that arises is that a general downregulation of IFN-γ-induced proteasomal subunits is associated with malignant transformation of cells. In this context, the impaired expression of LMPs and PA28 might cause reduced generation and presentation of some tumor-associated antigens and therefore provide malignant cells with a mechanism to evade control of the immune system. It was thus unexpected that the expression of several IFN-γ-induced proteasomal components (LMP2, LMP7, PA28α, and PA28β) is highly enhanced compared to normal autologous tissues in FISS. Although we could not directly measure the expression of the third catalytic proteasomal β subunits Z and of its IFN-γ-induced homologue MECL-1 due to lack of cross reactivity for cat proteins of all the antibodies we tested, however, our data are consistent with an enhanced expression of immunoproteasomes in FISS. In fact, incorporation of constitutive (X, Y, and Z) or IFN-γ-induced (LMP2, LMP7, and MECL1) subunits into nascent proteasomal particles is a cooperative process that favors only the assembly of homogeneous proteasomes or immunoproteasomes [35–37].
The molecular mechanisms underlying this upregulation in fibrosarcomas lesions are presently unknown. An enhanced expression of LMP2, LMP7, and other IFN-γ-regulated components may be a direct consequence of malignant transformation, possibly modulated by overexpression of PML and CIITA transcription factors [38,39]; alternatively, it may be caused by high local levels of proinflammatory cytokines such as IFN-α and IFN-γ at the tumor site. In fact, although histologic evaluation has demonstrated a limited presence of lymphocytes, which never exceeds 5% of the total cells in the specimens used in our study (data not shown), it is likely that the inflammatory component present in fibrosarcomas is still responsible for the maintenance of high local levels of cytokines in the neoplastic lesion. Accordingly, chronic inflammation is recognized as one of the possible causes of these tumors [40] developing in a microenvironment characterized by high levels of cytokines that are potent inducers of the immunoproteasomes PA28 and LAP [1]. We are currently exploring these different possibilities. Interestingly, an enhanced expression of immunoproteasomes in FISS is not accompanied by a reduction in constitutive proteasomes. This observation further suggests that neoplastic transformation in this tumor is associated with dysregulation of mechanisms that control proteasome subunits expression. Furthermore, our findings are consistent with previous reports showing that levels of proteasomal constitutive subunits are not negatively regulated by IFN-γ in several cell lines [41–43]. It is, however, clear that incorporation of IFN-γ-induced subunits into proteasomes causes differences in enzymatic activities and cleavage specificities, leading to the production of a different set of antigenic peptides during hydrolysis of endogenous proteins [1]. Degradation experiments performed in the present study using fluorogenic substrates indicate that proteasomal chymotrypsin-like activities and, to a lesser extent, caspaselike activities are highly enhanced in homogenates from fibrosarcomas compared to healthy subcutis. These functional results are in agreement with the observation that fibrosarcomas present a higher content of immunoproteasomes and PA28. In fact, it has been reported that LMP7 substitution of the constitutive subunit X results in stimulation of chymotrypsinlike activity [44]. Moreover, PA28 is known to be a potent activator of all three proteasomal peptidase activities, although to different extents [7]. Finally, in agreement with the observation that LMP2 causes a reduction in caspase-like activity [44], degradation of Z-YVAD-AMC in fibrosarcoma homogenates is less enhanced compared to the degradation of the substrate specific for chymotrypsin-like activity.
Taken together, our results indicate that, in neoplastic fibroblasts, differences in the composition of APM subunits and the subsequent modifications of enzymatic activities may result in the production of a characteristic spectrum of peptides that is different from that produced by other cell types and, specifically, in normal fibroblasts. Importantly, such modifications might not be optimized for the generation and presentation of tumor antigens that can elicit an effective immune response based on the activation of specific anti-tumor-associated antigen CD8+ lymphocytes. In fact, immunoproteasome expression, which causes a change in the type of peptides processed and presented on certain allospecificities, is indicated as a mechanism of tumor escape [19]. Accordingly, the hypothesis that immunoproteasomes are always better suited for the processing of MHC class I-restricted peptides has been challenged in recent years following reports that some epitopes, mainly of self-origin, are not processed by this form of proteasome and that mature dendritic cells constitutively express immunoproteasomes and, therefore, cannot efficiently present such antigenic peptides [16,45]. The first examples of such epitopes that are processed by standard proteasomes, but not by immunoproteasomes, are a peptide derived from a self-protein called RU1 and a peptide derived from a melanoma differentiation protein [16]. Successively, other melanoma antigens belonging to the group of melanocyte differentiation antigens were shown to be poorly generated by immunoproteasomes [17, 18]. Furthermore, it has been reported that several tumorspecific antigens, melanoma differentiation antigens, and antigens derived from ubiquitous self-proteins are recognized less efficiently by specific CTL in cells treated with IFN-γ [16, 18]. Although further studies are required to determine the precise fraction of tumor epitopes that are poorly processed by immunoproteasomes, the observation that proteasomal catalytic subunits induced by IFN-γ are highly upregulated in fibrosarcomas might be invoked as a mechanism to evade immune surveillance in neoplastic fibroblasts. In fact, to the best of our knowledge, this is the first time that a strong upregulation of LMPs and PA28s has been described in a naturally occurring tumor. Interestingly, a proteasome switch caused either by high local levels of cytokines and/or by dysregulation of tumor cells might result in a lack of presentation of tumor antigens and escape from CTL attack. Similarly to immunoproteasomes and PA28, another IFN-γ-induced APM component that is upregulated in FISS is LAP, a cytosolic enzyme that can generate antigenic peptides from longer proteasome products but also destroy them [9–12]. Although a recent report questions its role in the generation or destruction of several antigenic peptides [46], previous studies have clearly identified in LAP the major cytosolic exopeptidase capable of trimming both N-extended precursors of different antigenic peptides and mature epitopes [9,10] and that overexpression of LAP results in a shorter half-life of peptides in living cells [11]. Consequently, enhanced levels of LAP in FISS might contribute to immune escape mechanism by reducing the supply of antigens to MHC class I molecules.
Our findings have potentially important consequences to cancer therapy. In fact, the observation that immunoproteasomes in a naturally occurring cancer are mainly expressed gives rise to several concerns about anticancer treatments based on systemic IFN-γ therapy and could account for observations of increased tumor progression after such treatment [47,48]. In cancers that are similar to FISS and present an enhanced expression of IFN-γ-induced subunits, therapies aimed to reduce immunoproteasome levels (e.g., treatment with neutralizing antibodies specific for IFN-γ) might have better a possibility to achieve positive results. Furthermore, when designing cancer vaccines for these tumors, priority should be given to epitopes that are well generated by immunoproteasomes and that are highly abundant on the surface of neoplastic cells.
Acknowledgements
We thank A. L. Goldberg, K. Tanaka, and T. Saric for providing antibodies.
Abbreviations
- APM
antigen-processing machinery
- CTL
cytotoxic T lymphocyte
- FISS
feline injection site sarcoma
- LAP
leucine aminopeptidase
- MHC
major histocompatibility complex
Footnotes
This research was supported by grants from the MIUR (PRIN) to P. Cascio.
References
- 1.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 2.Anderton SM, Wraith DC. Selection and fine-tuning of the autoimmune T-cell repertoire. Nat Rev Immunol. 2002;2:487–498. doi: 10.1038/nri842. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 4.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 6.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 7.Rechsteiner M, Realini C, Ustrell V. The proteasome activator 11S REG (PA28) and class I antigen presentation. Biochem J. 2000;345:1–15. [PMC free article] [PubMed] [Google Scholar]
- 8.Harris CA, Hunte B, Krauss MR, Taylor A, Epstein LB. Induction of leucine aminopeptidase by interferon-gamma. Identification by protein microsequencing after purification by preparative two-dimensional gel electrophoresis. J Biol Chem. 1992;267:6865–6869. [PubMed] [Google Scholar]
- 9.Beninga J, Rock KL, Goldberg AL. Interferon-gamma can stimulate post-proteasomal trimming of the N terminus of an antigenic peptide by inducing leucine aminopeptidase. J Biol Chem. 1998;273:18734–18742. doi: 10.1074/jbc.273.30.18734. [DOI] [PubMed] [Google Scholar]
- 10.Mo XY, Cascio P, Lemerise K, Goldberg AL, Rock KL. Distinct proteolytic processes generate the C and N termini of MHC class I-binding peptides. J Immunol. 1999;163:5851–5859. [PubMed] [Google Scholar]
- 11.Reits E, Griekspoor A, Neijssen J, Groothuis T, Jalink K, van Veelen P, Janssen H, Calafat J, Drijfhout JW, Neefjes J. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity. 2003;18:97–108. doi: 10.1016/s1074-7613(02)00511-3. [DOI] [PubMed] [Google Scholar]
- 12.Saric T, Graef CI, Goldberg AL. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem. 2004;279:46723–46732. doi: 10.1074/jbc.M406537200. [DOI] [PubMed] [Google Scholar]
- 13.Cascio P, Hilton C, Kisselev AF, Rock KL, Goldberg AL. 26S Proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. EMBO J. 2001;20:2357–2366. doi: 10.1093/emboj/20.10.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5:670–677. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- 15.Cascio P, Call M, Petre BM, Walz T, Goldberg AL. Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J. 2002;21:2636–2645. doi: 10.1093/emboj/21.11.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morel S, Levy F, Burlet-Schiltz O, Brasseur F, Probst-Kepper M, Peitrequin AL, Monsarrat B, Van Velthoven R, Cerottini JC, Boon T, et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000;12:107–117. doi: 10.1016/s1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 17.Van den Eynde BJ, Morel S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Curr Opin Immunol. 2001;13:147–153. doi: 10.1016/s0952-7915(00)00197-7. [DOI] [PubMed] [Google Scholar]
- 18.Chapiro J, Claverol S, Piette F, Ma W, Stroobant V, Guillaume B, Gairin JE, Morel S, Burlet-Schiltz O, Monsarrat B, et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J Immunol. 2006;176:1053–1061. doi: 10.4049/jimmunol.176.2.1053. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 21.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Coordinate downregulation of multiple MHC class I antigen processing genes in chemical-induced murine tumor cell lines of distinct origin. Tissue Antigens. 2000;56:327–336. doi: 10.1034/j.1399-0039.2000.560404.x. [DOI] [PubMed] [Google Scholar]
- 22.Ritz U, Momburg F, Pilch H, Huber C, Maeurer MJ, Seliger B. Deficient expression of components of the MHC class I antigen processing machinery in human cervical carcinoma. Int J Oncol. 2001;19:1211–1220. doi: 10.3892/ijo.19.6.1211. [DOI] [PubMed] [Google Scholar]
- 23.Seliger B, Atkins D, Bock M, Ritz U, Ferrone S, Huber C, Storkel S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin Cancer Res. 2003;9:1721–1727. [PubMed] [Google Scholar]
- 24.Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, Storkel S, Seliger B. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer. 2004;109:265–273. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;64:215–220. doi: 10.1158/0008-5472.can-2522-2. [DOI] [PubMed] [Google Scholar]
- 26.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, Seliger B. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 27.Hendrick MJ, Goldschmidt MH, Shofer FS, Wang YY, Somlyo AP. Postvaccinal sarcomas in the cat: epidemiology and electron probe microanalytical identification of aluminum. Cancer Res. 1992;52:5391–5394. [PubMed] [Google Scholar]
- 28.Goldschmidt MH, Henrick MJ. Tumors of the skin and soft tissues. In: Meuten, DJ, editor. Tumors in Domestic Animals. 4th ed. Ames, IA, USA: Iowa State Press; 2002. pp. 85–86. [Google Scholar]
- 29.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 30.McNiel EA. Vaccine-associated sarcomas in cats: a unique cancer model. Clin Orthop Relat Res. 2001;382:21–27. doi: 10.1097/00003086-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Owen L. TNM Classification of Tumors in Domestic Animals. Geneva: World Health Organization; 1980. [Google Scholar]
- 32.Ferrone S, Finerty JF, Jaffee EM, Nabel GJ. How much longer will tumour cells fool the immune system? Immunol Today. 2000;21:70–72. doi: 10.1016/s0167-5699(99)01569-8. [DOI] [PubMed] [Google Scholar]
- 33.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- 34.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 35.Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci USA. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nandi D, Woodward E, Ginsburg DB, Monaco JJ. Intermediates in the formation of mouse 20S proteasomes: implications for the assembly of precursor beta subunits. EMBO J. 1997;16:5363–5375. doi: 10.1093/emboj/16.17.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, Monaco JJ, Colbert RA. Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 39.Zheng P, Guo Y, Niu Q, Levy DE, Dyck JA, Lu S, Sheiman LA, Liu Y. Proto-oncogene PML controls genes devoted to MHC class I antigen presentation. Nature. 1998;396:373–376. doi: 10.1038/24628. [DOI] [PubMed] [Google Scholar]
- 40.Macy DW, Hendrick MJ. The potential role of inflammation in the development of postvaccinal sarcomas in cats. Vet Clin North Am Small Anim Pract. 1996;26:103–109. doi: 10.1016/s0195-5616(96)50009-4. [DOI] [PubMed] [Google Scholar]
- 41.Akiyama K, Yokota K, Kagawa S, Shimbara N, Tamura T, Akioka H, Nothwang HG, Noda C, Tanaka K, Ichihara A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science. 1994;265:1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- 42.Stohwasser R, Standera S, Peters I, Kloetzel PM, Groettrup M. Molecular cloning of the mouse proteasome subunits MC14 and MECL-1: reciprocally regulated tissue expression of interferon-gamma-modulated proteasome subunits. Eur J Immunol. 1997;27:1182–1187. doi: 10.1002/eji.1830270520. [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Awasthi N, Egwuagu CE, Wagner BJ. Immunoproteasome expression in a nonimmune tissue, the ocular lens. Arch Biochem Biophys. 2002;405:147–153. doi: 10.1016/s0003-9861(02)00341-7. [DOI] [PubMed] [Google Scholar]
- 44.Gaczynska M, Rock KL, Spies T, Goldberg AL. Peptidase activities of proteasomes are differentially regulated by the MHC-encoded genes LMP2 and LMP7. Proc Natl Acad Sci USA. 1994;91:9212–9217. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macagno A, Gilliet M, Sallusto F, Lanzavecchia A, Nestle FO, Groettrup M. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur J Immunol. 1999;29:4037–4042. doi: 10.1002/(SICI)1521-4141(199912)29:12<4037::AID-IMMU4037>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 46.Towne CF, York IA, Neijssen J, Karow ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Neefjes JJ, Rock KL. Leucine aminopeptidase is not essential for trimming peptides in the cytosol or generating epitopes for MHC class I antigen presentation. J Immunol. 2005;175:6605–6614. doi: 10.4049/jimmunol.175.10.6605. [DOI] [PubMed] [Google Scholar]
- 47.Meyskens FL Jr, Kopecky K, Samson M, Hersh E, Macdonald J, Jaffe H, Crowley J, Coltman C. Recombinant human interferon gamma: adverse effects in high-risk stage I and II cutaneous malignant melanoma. J Natl Cancer Inst. 1990;82:1071. doi: 10.1093/jnci/82.12.1071-a. [DOI] [PubMed] [Google Scholar]
- 48.Kowalzick L, Weyer U, Lange P, Breitbart EW. Systemic therapy of advanced metastatic malignant melanoma with a combination of fibroblast interferon-beta and recombinant interferon-gamma. Dermatologica. 1990;181:298–303. doi: 10.1159/000247830. [DOI] [PubMed] [Google Scholar]