Abstract
HIV-1 replication is induced by the excess of iron and iron chelation by desferrioxamine (DFO) inhibits viral replication by reducing proliferation of infected cells. Treatment of the cells with DFO and 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311) inhibit expression of proteins that regulate cell-cycle progression, including cycle-dependent kinase 2 (CDK2). Our recent studies showed that CDK2 participates in HIV-1 transcription and viral replication suggesting that inhibition of CDK2 by iron chelators might also affect HIV-1 transcription. Here we evaluated the effect of a clinically approved orally effective iron chelator, 4-[3,5-bis-(hydroxyphenyl) -1,2,4-triazol-1-yl]-benzoic acid (ICL670) and 311 on HIV-1 transcription. Both ICL670 and 311 inhibited Tat–induced HIV-1 transcription in CEM-T cells, 293T and HeLa cells. Neither ICL670 nor 311 induced cytotoxicity at concentrations that inhibited HIV-1 transcription. The chelators decreased cellular activity of CDK2 and reduced HIV-1 Tat phosphorylation by CDK2. The ICL670 and 311 did not decrease CDK9 protein level but significantly reduced association of CDK9 with cyclin T1 and reduced phosphorylation of Ser-2 residues of RNA polymerase II C-terminal domain. In conclusion, our findings add to the evidence that iron chelators can inhibit HIV-1 transcription by deregulating inhibiting CDK2 and CDK9. Further consideration should be given to the development of iron chelators for future anti-retroviral therapeutics.
Keywords: HIV-1; desferrioxamine (DFO); 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311); 4-[3,5-bis-(hydroxyphenyl) -1,2, 4-triazol-1-yl]-benzoic acid (ICL670); transcription; CDK2; CDK9
INTRODUCTION
Increased iron stores correlated with faster HIV-1 progression in HIV-1 positive thalassemia major patients; in HIV-positive patients who were administered oral iron and in HIV-positive subjects with haptoglobin 2-2 polymorphism associated with higher iron stores (Gordeuk et al., 2001). Moreover, a retrospective study of bone marrow macrophage iron in HIV-positive patients suggested that survival is shorter with higher iron stores (Gordeuk et al., 2001). In cultured T cells, excess of iron stimulated HIV-1 viral replication, whereas iron chelation with desferrioxamine (DFO) lowered viral replication as measured by decreased p24 levels and reverse transcriptase (RT) activity (Traore and Meyer, 2004). Treatment of monocyte-derived macrophages and peripheral blood lymphocytes (PBL) with DFO or deferiprone (CP20) reduced expression of p24 and also cellular proliferation (Georgiou et al., 2000). The orally active bidentate chelators CP502 and CP511 decreased HIV-1 replication and cellular proliferation in a manner similar to DFO and CP20 (Georgiou et al., 2002). Reduction of HIV-1 replication by DFO or by hydroxypyridinone bidentate chelators may be through inhibition of cellular proliferation rather than by a direct antiviral action. Recently, a new oral tridentate iron chelator Deferasirox (ICL670) was introduced by Novartis (Porter, 2006). ICL670 is superior to the previously used DFO and deferiprone and is approved for use in the United States (Porter, 2006). Thus it would be of interest to test the effect of ICL670 on HIV-1. Latent HIV-1 provirus is activated by viral Tat protein that recruits cellular factors to the HIV promoter. Tat interacts with the protein kinases CDK9/cyclin T1 and CDK2/cyclin E, with the acetyltransferases p300/CBP, PCAF and hGCN5, with protein phosphatase-1 and other factors (Nekhai et al., 2006). We previously reported that CDK2 is required for Tat-dependent transcription in vitro (Ammosova et al., 2006; Ammosova et al., 2005a; Deng et al., 2002; Nekhai et al., 2002) and that inhibition of CDK2 by CYC202 (R-roscovitine) (Agbottah et al., 2005) or by siRNA (Ammosova et al., 2005a) efficiently blocks replication of HIV-1. Moreover, Tat is phosphorylated by CDK2 in cultured cells and inhibition of this phosphorylation by mutation of Ser16 and Ser46 residues of Tat blocked HIV-1 transcription and viral replication (Ammosova et al., 2006). Richardson and colleagues showed that the iron chelator 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311) inhibited the expression of CDK2 (Gao and Richardson, 2001). Thus the effect of iron chelators could conceivably affect the activity of CDK2 and thus inhibit HIV-1 transcription and viral replication.
In the present study we analyzed the effect of iron chelators, ICL670 and 311, on HIV-1 transcription, and on the expression and activities of CDK2 and CDK9 in cultured cells.
RESULTS
Iron chelators 311 and ICL670 inhibit Tat-induced HIV-1 transcription in CEM T-cells and 293T cells
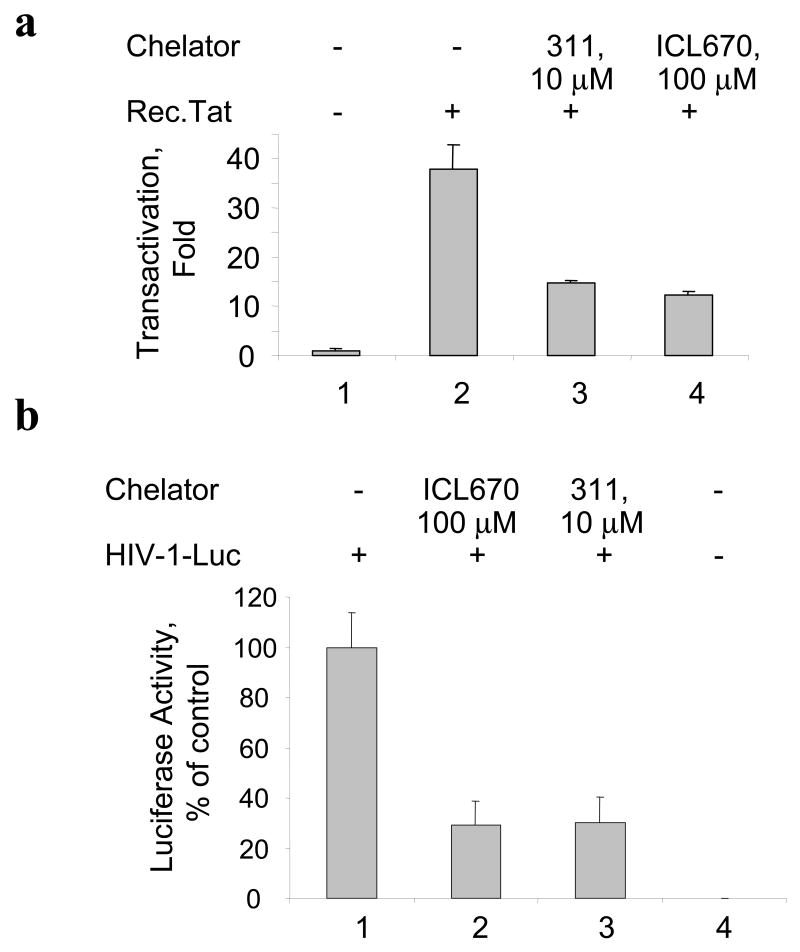
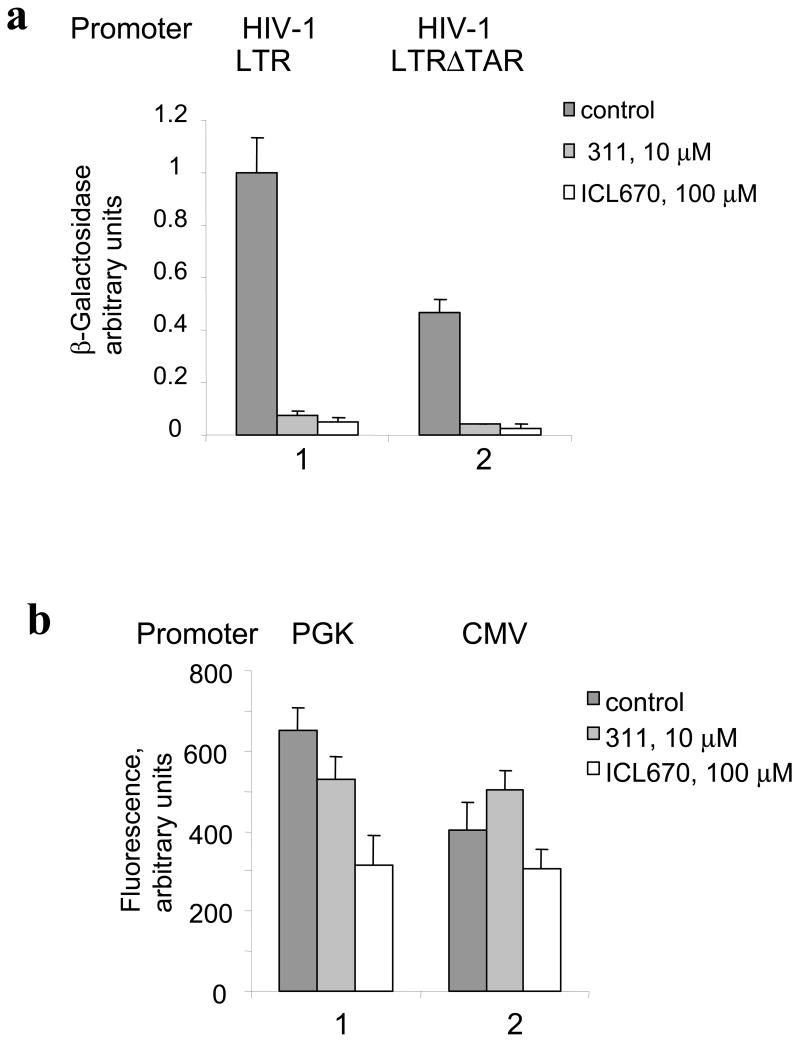
We examined the effect of iron chelators 311 and ICL670 (see their structure in supplemental Fig. 1) on HIV-1 transcription in CEM cells containing an integrated HIV-1 LTR – GFP (CEM-GFP). We infected CEM-GFP cells with adenovirus expressing Tat (Ad-Tat) (Ammosova et al., 2003; Nekhai et al., 2006). In CEM-GFP cells HIV-1 transcription was detectable after infection with Ad-Tat but not with non-relevant Ad-LacZ virus (Fig. 1a). Treatment of the Ad-Tat infected CEM-GFP cells with either ICL670 (100 μM) or 311 (10 μM) resulted in the inhibition of Tat-mediated HIV-1 transactivation as visualized by fluorescence (Fig. 1b). Titration of the iron chelators showed that ICL670 inhibited HIV-1 transcription in CEM-GFP cells with IC50=23 μM (Fig 1c) and that 311 inhibited HIV-1 transcription with IC50=2 μM (Fig 1c). To determine whether the effect of the iron chelators might be due to reduced expression of Tat, we induced Tat-transactivation with purified recombinant Tat protein added to the media in the presence of chloroquine (Frankel and Pabo, 1988). Recombinant Tat potently induced HIV-1 transcription in CEM-GFP cells (Fig. 2a, lane 2). Treatment with 100 μM ICL670 or 10 μM 311 inhibited HIV-1 transcription induced by the extracellular Tat (Fig. 2a, lanes 3 and 4). Thus inhibition of HIV-1 transcription by iron chelators was not the result of decreased expression of Tat. We next analyzed the effect of ICL670 and 311 on HIV-1 transcription from HIV-1 genomic construct pNL4-3 Luc in 293T cells. The 293T cells were transfected with pNL4-3 Luc construct and simultaneously treated with 100 μM ICL670 or 10 μM 311. Treatment with chelators inhibited HIV-1 transcription as evidenced by the decrease of luciferase activity (Fig. 2b, lanes 2 and 3). We next analyzed the effect of chelators on HIV-1 basal transcription by transiently transfecting 293T cells with HIV-1 LTR-LacZ reporter combined with cytomegalovirus (CMV)-EGFP vector to normalize transfection. We also used HIV-1 LTR-LacZ in which the TAR-coding sequence was deleted (HIV-1 LTR ΔTAR (Ammosova et al., 2003)). Treatment with 10 μM 311 or 100 μM ICL670 inhibited basal HIV-1 transcription from WT or TAR-deleted HIV-1 LTR (Fig. 3a). To determine whether the chelators exclusively affect the HIV-1 promoter, we analyzed their effect on viral CMV or cellular phosphoglycerate kinase (PGK) promoters. Neither chelator had a significant effect on transcription from the CMV promoter (Fig. 3b, lane 2). In the case of the PGK promoter, 311 did not inhibit transcription whereas ICL670 had an inhibitory effect although not as prominent as for the HIV-1 (Fig. 3b, lane 1).
Fig. 1. Iron chelators 311 and ICL670 inhibit Tat-induced HIV-1 transcription in CEM T-cells.
(a) CEM-GFP T-cells were grown in 96-well plate and infected with the indicated amounts of Ad-Tat or Ad-LacZ. At 24 h the cells were lysed and GFP fluorescence was measured on a luminescence spectrometer. (b) CEM-GFP cells grown in 96-well plate were infected with 100 Pfu/cell of Ad-Tat and treated with the indicated concentrations of 311 and ICl670. Cell cultures were continued for 24 hours, and photographs were taken. (c) CEM-GFP cells were grown in 96-well plate, infected with 100 Pfu/cell of Ad-Tat and treated with the indicated concentrations of 311 and ICl670. At 24 h the cells were lysed and GFP fluorescence was measured on a luminescence spectrometer.
Fig. 2. (a) Iron chelators inhibit HIV-1 transcription induced by extracellular Tat.
CEM-GFP cells were placed in 96-well plate, supplemented as indicated with recombinant Tat (3 μg/300,000 cells) and 100 μM chloroquine and treated with indicated concentrations of ICL670 or 311. Cell cultures were continued for 24 h. The cells were lysed and GFP fluorescence was measured on Luminescence Spectrometer. (b) Iron chelators inhibit HIV-1 transcription from pNL4-3.Luc. 293T cells were grown in 96-well plate and transfected with pNL4-3.Luc.R-E- construct (lane 1) and treated with indicated concentrations of ICL670 or 311 (lanes 2 and 3). Lane 4, mock transfected cells. At 48 hours posttransfection, the cells were lysed using Luclite luminescence reporter buffer containing luciferase substrate (Perkin Elmer) and luminescence was measured on Luminoscan (Perkin Elmer).
Fig. 3. Iron chelators inhibit basal HIV-1 transcription but not transcription from CMV or PGK promoters.
(a) 293T cells were grown in 96-well plates and transfected with HIV-1 LTR-LacZ and CMV-EGFP (lane 1) or a TAR deleted construct of HIV-1 LTR-LacZ (HIV-1 LTRΔTAR) and CMV-EGFP (lane 2) and treated as indicated with 311 and ICL670. At 24 hours the cells were lysed and analyzed for green fluorescence and for β-galactosidase activity. (b) 293T cells were transfected with vectors expressing EGFP under the control of cytomegalovirus (CMV) (lane 1) or cellular phosphoglycerate kinase (PGK) (lane 2) promoters. At 24 h post transfection, the cells were lysed and GFP fluorescence was measured on Luminescence Spectrometer.
Taken together, our results indicate that iron chelators potently inhibit Tat-induced and basal HIV-1 transcription, but not the transcription from the non-relevant CMV promoter or PGK promoter.
Inhibition of HIV-1 transcription by iron chelators is not due to decreased cellular proliferation or cellular toxicity
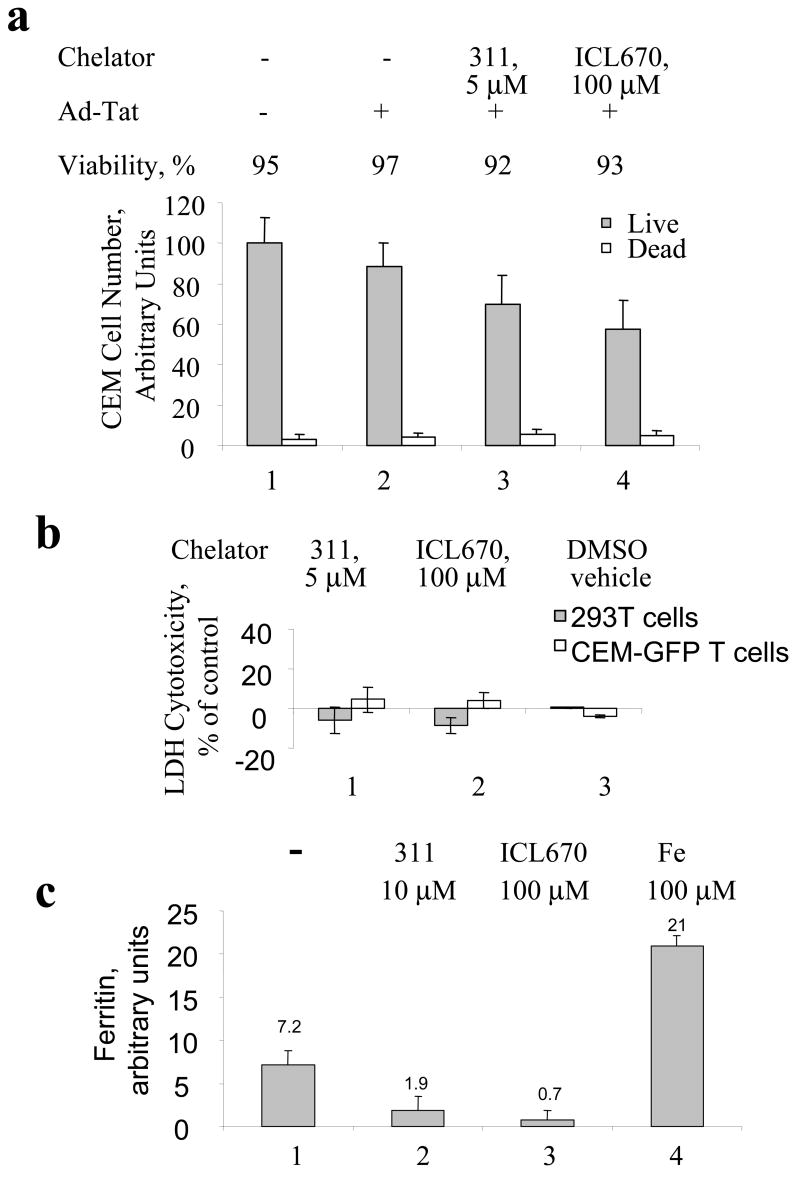
Treatment with ICL670 and 311 did not have a significant effect on proliferation of CEM-GFP cells as determined by cell counting (Fig. 4a). Cellular viability determined from data in Fig. 4a remained above 90% for the cells treated with iron chelators (Fig. 4a). Cytotoxicity of iron chelators was analyzed using lactate dehydrogenase (LDH) release as an indicator of cell membrane damage. Measurement of LDH in the media of cultures treated with iron chelators showed minimal LDH release indicating that iron chelators were not toxic at the concentrations used (Fig. 4b). Analysis of the effect of 311 and ICL670 on HIV-1 transcription and cellular proliferation in HeLa-MAGI cells infected with Ad-Tat (Supplemental Fig. 2a, (Ammosova et al., 2003)) showed that ICL670 and 311 decreased cell proliferation by approximately 20% at concentrations that inhibited HIV-1 transcription by 50% (Supplemental Fig. 2 b and c). In contrast, the effect of DFO on cell proliferation was more pronounced (Supplemental Fig. 2d) thus confirming previous observations that DFO decreases cellular proliferation. To confirm that iron chelators changed intracellular iron levels, we measure intracellular concentrations of ferritin which reflects the iron status of the cells. Treatment with 311 reduced ferritin level 4-fold, and ICL670 – 10-fold (Fig. 4c, compare lane 1 to lanes 2 and 3). Treatment of the cells with 100μM ferric ammonium citrate increased ferritin concentration 3-fold. These results indicate that treatment with iron chelators drastically reduced intracellular iron concentration and that inhibition of HIV-1 transcription was due to iron depletion. When 311 or ICL670 were precomplexed with ferric ammonium citrate, there was no effect on cell proliferation (Supplemental Fig. 2a and b) and the Fe complexes of 311 or ICL670 did not affect Tat-induced transcription (Supplemental Fig. 2c). Together, these results are consistent with the concept that the effects of iron chelators on HIV-1 transcription are due to the depletion of intracellular iron and not the consequence of the increased cellular toxicity.
Fig. 4. Cytotoxicty of iron chelators and their effect on cellular iron.
(a) CEM-GFP cells were grown in 96-well plate, infected with Ad-Tat and treated with the indicated concentration of 311 and ICl670. Cell cultures were continued for 24 hours, and the cells were counted to determine cell viability by Trypan blue exclusion assay. (b) Cytotoxicity of iron chelators was measured by lactate dehydrogenase (LDH) release. CEM-GFP cells or 293T cells were grown in 96-well plate and treated as indicated with 311, ICL670 or DMSO vehicle for 24 h. Cytotoxicity was measured as described in methods. Percentage of cytotoxicity was calculated using the formula [(Absorbance of the treated samples – Absorbance of control untreated cells)/(Absorbance of high control – Absorbance of control)] *100. (c) The effect of iron chelators on cellular ferritin. CEM-GFP cells were grown in 96-well plate and treated as indicated with 311, ICL670 or ferric ammonium citrate for 24 h. Ferritin concentration was measured by ELISA as described in the Methods.
Iron chelators inhibit CDK2 activity and Tat phosphorylation in cultured cells
Previously, CDK2 expression was shown to be reduced upon chelating of intracellular iron by 311 or DFO (Gao and Richardson, 2001). We measured levels and activity CDK2 in 293 cells treated with 311 (10 μM), ICL670 (100 μM) or DFO (100 μM) for 24 hours and 48 hours. Western blot analysis showed that chelators did not have a significant effect on the expression of CDK2 (Fig. 5a). We further analyzed the effect of iron chelators on the enzymatic activity of CDK2 using histone H1, which is a general substrate for a number of serine/threonine kinases including CDK2. Recombinant CDK2/Cyclin E efficiently phosphorylated histone H1 (Fig. 5b, lane 10). CDK2 immunoprecipitated from untreated 293 cells lysates by polyclonal antibody to CDK2 also effectively phosphorylated histone H1 (Fig. 5b, lanes 2 and 6). Treatment of 293 with DFO, 311 or ICL670 dramatically inhibited phosphorylation of histone H1 by CDK2 (Fig. 5b, lanes 5–7 and lane 7–9).
Fig. 5. Iron chelators reduce cellular activity of CDK2.
(a) CDK2 expression determined by Western blotting. 293 cells were grown in 100 mm plates and treated for the indicated time with 100 μM DFO, 10 μM 311 or 100 μM ICL670. The cells were lysed and CDK2 was immunoprecipitated as described in Methods. Lane 1, input control. Lane, preimmune IgG was used for the immunoprecipitation. The precipitated CDK2 was resolved on 10% SDS-PAGE and immunoblotted with antibodies against CDK2. (b) CDK2 was precipitated as in (a) and the immunoprecipitated material was subsequently incubated with histone H1 in the presence of γ-(32P) ATP. Kinase reactions were resolved on 10% SDS-PAGE and analyzed on Phosphor Imager. Lane 1: non-specific preimmune IgG. Lane 10, histone H1 phosphorylation with recombinant CDK2/Cyclin E.
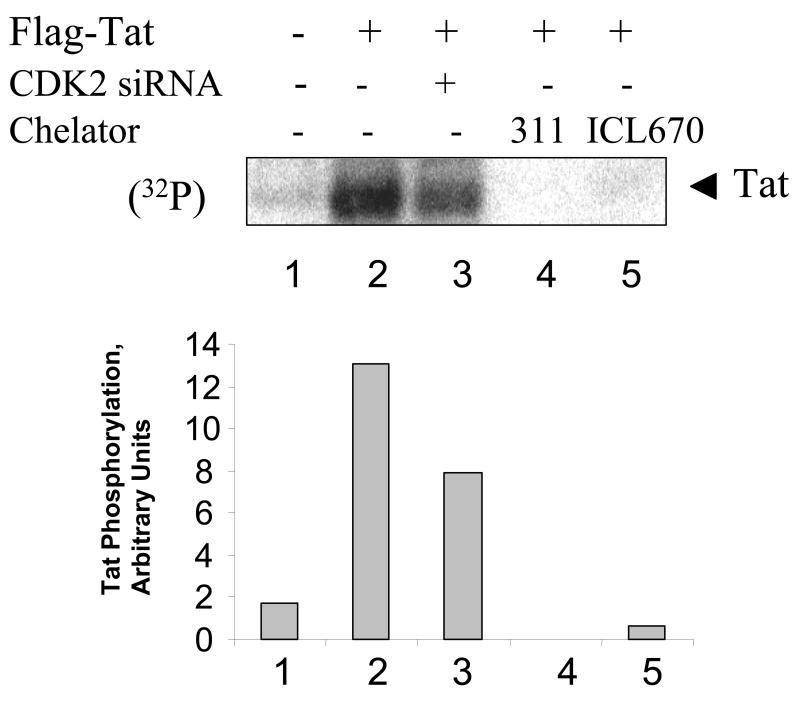
We recently showed that CDK2 phosphorylates HIV-1 Tat in cultured cells (Ammosova et al., 2006). Phosphorylation of Tat was increased in HeLa cells infected with Ad-Tat and treated with okadaic acid to inhibit PPP-phosphatases (Ammosova et al., 2006). To analyze if iron chelators affect Tat phosphorylation, HeLa cells were infected with Adeno-Tat and incubated 48 hours post infection to allow expression of Tat, with or without the treatment with iron chelators. As a control, we also transfected cells with CDK2-directed siRNA that we previously showed to block Tat phosphorylation (Ammosova et al., 2006). The cells were pulsed with (32P)-labeled orthophosphate and treated with okadaic acid, Tat was immunoprecipitated from cellular lysates, resolved by SDS-PAGE on 15% Tris-Tricine gel (Schagger et al., 1996) and detected by Phosphor Imager (Fig. 6, lane 2). Inhibition of CDK2 by siRNA reduced the level of Tat phosphorylation (Fig. 6, lane 3). Treatment with 311 or ICL670 also significantly reduced Tat phosphorylation. Taken together, iron chelators inhibit CDK2 activity in cultured cells and also affect Tat phosphorylation.
Figure 6. Iron chelators reduce HIV-1 Tat phosphorylated in cultured cells.
HeLa cells were infected with recombinant adenovirus expressing Flag-tagged Tat as described in Methods (lanes 2–5). Lane 1, control uninfected cells. HeLa cells were transfected with siRNAs targeting CDK2 (lane 3) or treated with 10 μM 311 or 100 μM ICL670. At 48 hours post infection cells were labeled with (32P)-orthophosphate for 2 hours with the addition of 1 μM okadaic acid. Whole cell extracts were prepared and Tat was immunoprecipitated with anti-Flag monoclonal antibodies, resolved on 15% Tris-Tricine SDS-PAGE and detected by Phosphor Imager. Phosphor Imager quantification is shown in the lower panel.
The ICL670 and 311 inhibit association of CDK9 with cyclin T1 and RNA polymerase II phosphorylation
We next analyzed whether iron chelators have an effect on the expression of CDK9, a well-established co-factor in Tat-induced transcription (Wei et al., 1998; Yang et al., 1997; Zhu et al., 1997). CDK9 expression was analyzed by immunoblotting in 293 cells untreated or treated with 100 μM DFO, 10 μM 311 or 100 μM ICL670 for 24 hours. Western blot analysis showed that chelators did not have a significant effect on the protein level of CDK9 (Fig. 7a). Cyclin T1 association with CDK9 is critical for the kinase activity of CDK9 and for its interaction with HIV-1 Tat and TAR RNA (Wei et al., 1998). To analyze association of CDK9 with cyclin T1, we immunoprecipitated Cyclin T1 and analyzed co-precipitation of CDK9 by immunoblotting. CDK9 was efficiently co-precipitated with cyclin T1 in untreated 293 cells (Fig. 7b, lane 3). No reduction in co-precipitation of CDK9 with cyclin T1 was seen in the cells treated with DFO (Fig. 7b, lane 4). In contrast, in the cells treated with 311 or ICL670 there was a significant reduction in CDK9 co-precipitation (Fig. 7b, lanes 5 and 6). To determine whether chelators reduced the enzymatic activity of CDK9, we analyzed the level of RNA Polymerase II (RNAPII) phosphorylation in the treated cells using monoclonal antibodies that recognize Ser-2 phosphorylated residue of the RNAPII C-terminal domain (CTD). RNAPII in the control cells was phosphorylated (Fig. 7c, lane 1). The chelators 311 and ICL670 decreased the level of RNAPII CTD Ser-2 phosphorylation whereas DFO only had a partial effect (Fig. 7c, lanes 2 to 4). Thus, the potent inhibition of HIV-1 transcription by 311 and ICL670 can be explained by a reduction of enzymatic activity of CDK9, for which RNAPII is a substrate in vivo.
Fig. 7. Iron chelators inhibit phosphorylation of RNAPII.
(a) Chelators do not have an effect on expression of CDK9. 293 cells were treated for 24 h with 10 μM 311 or 100 μM of ICL670. The cells were lysed were resolved on 10% SDS-PAGE, and immunoblotted anti-CDK9 antibodies. Lane 1: control untreated cells; lanes 2, 3 and 4: cells treated with DFO, 311 or ICL670. (b) Chelators inhibit association of CDK9 with cyclin T1. The cell lysates prepared as in (a) were subjected to immunoprecipitation with anti-cyclin T1 antibody (lanes 3–6). The immunoprecipitated material was resolved on 10% SDS-PAGE, and immunoblotted anti-CDK9 antibodies. Lane 1: input control untreated cells; lane 2: precipitation of untreated cells with non-specific preimmune IgG, lanes 3–6: immunoprecipitation with anti-cyclin T1 antibodies of lysates from untreated, DFO, 311 and ICL670 treated cells. The precipitated samples were resolved on 10% SDS-PAGE and immunoblotted with antibodies against CDK9. (c) Chelators inhibit phosphorylation of RNAPII. The cell lysates prepared as in (a) were resolved on 7.5% SDS-PAGE, and immunoblotted with the RNAPII CTD phospho-Serine 2 specific antibodies. Lane 1: control untreated cells; lanes 2,3 and 4: cells treated with DFO, 311 or ICL670.
DISCUSSION
Induction of HIV-1 transcription by HIV-1 Tat requires interaction of Tat with CDK9/cyclin T1, TAR RNA (Bieniasz et al., 1998; Garber, Wei, and Jones, 1998; Garber et al., 1998; Wei et al., 1998), and CDK2/cyclin E (Agbottah et al., 2005; Ammosova et al., 2005a; Deng et al., 2002; Nekhai et al., 2002). CDK2/cyclin E regulates G1/S transition (reviewed in (Morgan, 1997)). Tat induces HIV-1 transcription in G1 phase of the cell cycle, whereas HIV-1 transcription in G2 is Tat-independent (Kashanchi et al., 2000; Nekhai et al., 2000b). Tat associates with a protein complex containing CDK2 (Nekhai et al., 2002). CDK2/cyclinE can phosphorylate RNA polymeraseII CTD (Deng et al., 2002). CDK2/cyclin E associates with HIV-1 transcription elongation complex and phosphorylate Tat in vitro and in vivo (Agbottah et al., 2005; Ammosova et al., 2006; Deng et al., 2002). The significance of CDK2 for HIV-1 transcription was verified with the use of Roscovitine and its analog CYC202 that inhibited CDK2/cyclin E kinase activity and prevents replication of wild type and resistant HIV-1 mutants in T-cells, monocytes and PBMCs (Agbottah et al., 2004). Additionally, inhibition of CDK2 by siRNA inhibits Tat-induced transcription from HIV-1 promoter and suppresses viral replication (Ammosova et al., 2005a; Liang et al., 2005). Recent studies showed that CDK2 knock-out mice are viable (Berthet et al., 2003) suggesting that CDK2 is dispensable for proliferation and survival of most cell types. Therefore, CDK2 may present a novel target for anti-HIV-1 therapeutics. Based on these reports, deregulation of CDK2 is likely to affect HIV-1 transcription.
In this study, we examined the effect of iron chelators on Tat-induced activation of HIV-1 transcription and the effect of iron chelators on the expression and activity of CDK2 and CDK9. Using several cell lines containing an integrated HIV-1 promoter or transfected with a reporter under control of HIV-1, we demonstrated that the iron chelators, ICL670 and 311, led to significant inhibition of Tat-induced transcription. The chelators significantly inhibited enzymatic activity of CDK2. The chelators also significantly inhibited the enzymatic activity of CDK9 apparently disrupting the interaction of CDK9 with cyclin T1, without changing the expression level of CDK9. Our findings are consistent with the possibility that iron chelators could conceivably be beneficial in antiretroviral combination therapy.
The mechanism of CDK2 inhibition by iron chelators is not yet clarified. CDK2 is positively regulated by the binding of cyclin E (G1/S transition) or cyclin A (S phase transition) and by the CDK7-mediated phosphorylation of Thr160 (Morgan, 1997). Negative regulation of CDK2 includes its association with p21(CIP1/WAF1) and p27Kip1 inhibitory proteins and phosphorylation of Tyr15 by Wee1 kinase (Coulonval et al., 2003). The p21 is not likely to be involved in the CDk2 inhibition because iron chelators reduce p21 protein levels through the inhibition of translocation of p21 (CIP1/WAF1) mRNA from the nucleus to cytosol and induction of ubiquitin-independent proteasomal degradation of p21 (Fu and Richardson, 2007). Interestingly, DFO markedly increased p27Kip1 expression but not p21(CIP1/WAF1) and the induction of p27Kip1 was accompanied by an increased level of transforming growth factor beta1 (Yoon et al., 2002). Thus it is possible that dramatic decrease of CDK2 activity is related to the inhibition of CDK2 caused by increased p27Kip1 expression. While the effect of 311 on CDK2 was previously described (Gao and Richardson, 2001), this is the first study showing the ICL670 reduces the activity of CDK2. ICL670 is an orally effective iron chelator recently approved for use in humans (Porter, 2006). In clinical trials, transfusion-dependent beta-thalassemia patients tolerated doses up to 80 mg/kg, which resulted in plasma concentrations reaching 250 μM (Galanello et al., 2003). At the dose of 40 mg/kg, the concentration of ICL670 reached 100 μM in plasma (Galanello et al., 2003). In our study, about 80% of Tat-induced transcription in T cells was inhibited by 100 μM ICL670, thus indicating that ICL670 can be potentially investigated in anti-HIV-1 therapeutics. Our findings that 311 and ICL670 decrease CDK9 activity by disrupting the interaction of CDK9 with cyclin T1 indicate that iron chelators can specifically target HIV-1 transcription. One potential mechanism of the inhibition of HIV-1 transcription is deregulation of HIV-1 Tat phosphorylation by CDK2. Our unpublished observations indicate that phosphorylated HIV-1 Tat binds more efficiently to protein phosphatase-1 in vitro (Nekhai, unpublished observation). Because interaction of Tat with PP1 is critical for the HIV-1 transcription (Ammosova et al., 2005b; Ammosova et al., 2003; Nekhai et al., 2007), deregulation of this interaction might be inhibitory for the HIV-1 transcription. Another possibility is that CDK2 directly or indirectly regulates CDK9 activity by controlling the association of CDK9 with cyclin T1. Interaction between Tat and cyclin T1 requires zinc as well as essential cysteine residues in both proteins (Garber et al., 1998). Although chelators clearly had an effect on intracellular iron which is indicated by decreased cellular ferritin levels, chelation of zinc might affect the interaction of Tat with cyclin T and inhibit HIV-1 transcription. That ICL670 inhibited cell proliferation only slightly while HIV-1 transcription was potently inhibited further adds to the attractiveness of considering this agent in HIV therapeutics. Our findings suggest further evaluation of iron chelators as potential CDK2 inhibitors, and perhaps design of novel chelators with higher specificity.
Materials and methods
Materials
Recombinant CDK2/cyclin E was expressed and purified as described (Deng et al., 2002). Histone-H1 was purchased from Upstate Cell Signaling Solutions (Charlottesville, VA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich Chemical Co. (St Louis, MO). All other inorganic reagents were purchased from Fisher Scientific (Fair Lawn, NJ) or Sigma Chemical (St Louis, MO). Anti-Flag monoclonal antibodies, protein (G) and protein (A) agarose, and okadaic acid were purchased from Sigma (Atlanta, GA). All radioactive reagents were purchased from GE Health Care Life Sciences. Antibodies for CDK2 and CDK9 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against RNAPII were from Covance Research Products (Cambridge, MA). 293T cells and 293 cells were purchased from ATCC (Manassas, VA). Horseradish peroxidase (HRP)-conjugated F(ab)2 fragment was purchased from Amersham Biosciences (Piscataway, NJ). HIV-1 Tat was expressed in Escherichia coli and purified on Aquapore RP-300 column (Applied Biosystems, Foster City, CA) by reverse-phase chromatography as we described (Deng et al., 2002).
Plasmids
The HIV-1 reporter contained HIV-1 LTR (−138 to +82) followed by a nuclear localization signal (NLS) and the LacZ reporter gene (courtesy of Dr. Michael Emmerman, Fred Hutchinson Cancer Institute, Seattle, WA) (Kimpton and Emerman, 1992). The HIV-1 reporter plasmid without TAR contained a deletion of +19 to +87 nucleotides of LTR introduced by restriction digestion with BglII (Ammosova et al., 2003). The CMV-EGFP was cloned into the Adenovirus shuttle vector. The SIN vector containing phosphoglycerol kinase (PGK) promoter followed by EGFP (Brenner and Malech, 2003) was a gift from Dr. John Tisdale (NIDDK, NIH). An HIV-1 genomic plasmid pNL4-3.Luc.R-E- containing firefly luciferase gene in place of nef and frame shifts in the Env and Vpr genes were obtained from the NIH AIDS Research and Reference Reagent Program (courtesy of Dr. Nathaniel Landau, Aaron Diamond AIDS Research Center, The Rockefeller University) (He et al., 1995).
Iron chelator solutions and chelator-Fe(III) complexes
The aroylhydrazone iron chelator, 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone, (311) is a tridentate aroylhydrazone acid ligand with high affinity for Fe3+ and it was synthesized as previously described (Richardson and Bernhardt, 1999). The oral iron chelator 4-[3,5-bis-(hydroxyphenyl) -1,2,4-triazol-1-yl]-benzoic acid (ICL670, deferasirox) which forms a very stable 2:1 complex with Fe3+ was a generous gift from Dr. Anna Suter, Novartis, Pharma AG, Ltd., Basel, Switzerland. Where indicated, the chelators were complexed to ferric ammonium citrate at equimolar concentrations.
Cell Culture
293T and 293 cells (purchased from ATCC, Manassas, VA) were grown and maintained in Dulbecco’s Minimal Essential Medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco-BRL) and 1% glutamine (Gibco-BRL). CEM–HIV-1 LTR-GFP cells (CEM-GFP; obtained from the NIH AIDS Research and Reference Reagent Program, courtesy of Dr. Jacques Corbeil) were cultured and maintained in RPMI Medium 1640 containing 10% FBS, with 1% antibiotic solution and 500 μg/ml G418 (Invitrogen). HeLa-CD4-LTR-β-gal cells (MAGI; obtained from the NIH AIDS Research and Reference Reagent Program, courtesy of Dr. Michael Emerman) (Kimpton and Emerman, 1992) were cultured and maintained in DMEM containing 10% fetal bovine serum (FBS) (Gibco-BRL) and 1% glutamine (Gibco-BRL) with 1% antibiotic solution (penicillin and streptomycin; Gibco-BRL) and 200 μg/ml G418 and 100 ng/ml Hygromycin B (Invitrogen). The viability of CEM-GFP cells was determined by trypan blue exclusion assay. HeLa MAGI cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye conversion assay, which has a stable endpoint. If not indicated, the cell cultures were treated with the chelators for 24 hours.
Induction of HIV-1 transcription with Ad-Tat
The E1-deleted recombinant Adeno virus carrying Tat was generated as previously described (Ammosova et al., 2003). CEM-GFP cells were infected with MOI 100 Pfu per cell in a 96-well plate containing 400,000 cells per well. After 24 hours of incubation, the photographs of the cells were taken using Olympus CKX41 fluorescent microscope with blue filter and Kodak DC120 digital camera. Then 10 μl aliquots were removed, supplemented with trypan blue and counted to determine the cellular viability. The remaining cells were precipitated by centrifugation, lysed for 20 min at room temperature in 50 μl of lysis buffer, containing 20 mM HEPES at pH 7.9, 0.1% NP-40 and 5 mM EDTA transferred into 150 μl of PBS. The fluorescence was measured with 480 nm excitation and 510 nm emission on Luminescence Spectrometer LS50B (Perkin-Elmer) equipped with the robotic 96-well scanner. HeLa-MAGI cells were infected with MOI 10 Pfu per cell in a 96-well plate containing 10,000 cells per well. After 24 hours of culturing, half of the HeLa cells were incubated with MTT to determine cell viability and the second half were analyzed for the level of β-galactosidase expression. To determine the activity of β-galactosidase, media was removed and the cells were lysed for 20 min at room temperature in 50μl of lysis buffer, containing 20 mM HEPES at pH 7.9, 0.1% NP-40 and 5 mM EDTA. Subsequently, 100 μl of o-nitrophenyl-β-D-galactopyranoside (ONPG) solution (72 mM Na2 PO4 at pH 7.5, 1 mg/ml ONPG, 12 mM MgCl2, 180 mM 2-mercaptoethanol) was added and incubated at room temperature until a yellow color was developed. The reactions were timed and stopped by addition of 100 μl of 1M Na2CO3. The color intensity of the reaction in the 96-well plate was analyzed using a micro plate reader at a wavelength of 414 nm (Lab Systems Multiscan MS). The MTT reactions were analyzed at a wavelength of 620 nm.
Induction of HIV-1 transcription with recombinant Tat
HIV-1 Tat was expressed in Escherichia coli using the pGEM2 Tat bacterial expression vector (obtained from the NIH AIDS Research and Reference Reagent Program, courtesy of Dr. Richard Gaynor) and purified on Aquapore RP-300 column (Applied Biosystems, Foster City, CA) by reversed-phase chromatography, as described. Purified Tat was dissolved in RPMI media and added to CEM-GFP cells at 3 μg/400,000 cells in the presence of 10 mM chloroquine (Frankel and Pabo, 1988). At 24 hours incubation fluorescence was measured as described above.
Luciferase Assay
293T cells were grown in 96-well plate and transfected with pNL4-3.Luc.R-E- plasmid to measure HIV-1 transcription and with CMV-EGFP to control for the efficiency of transfection. At 48 hours posttransfection the cells were washed with PBS 3 times and then 100 μl of PBS was added to each well. Then 100 ml of reconstituted luclite buffer (luclite kit, Perkin Elmer) was added to each well and after 10 min incubation the lysates were transferred into the white plates (Perkin Elmer) and luminescence was measured on Labsystems Luminoscan RT (Perkin Elmer). The fluorescence was measured with 480 nm excitation and 510 nm emission on Luminescence Spectrometer LS50B (Perkin-Elmer) equipped with the robotic 96-well scanner.
Immunoblots and kinase assay
Cells were washed with PBS, and whole-cell lysates were prepared from control untreated and chelator-treated cells using whole cell lysis buffer (50 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 1% NP-40, 0.1% SDS) supplemented with protease cocktail (Sigma). Protein concentration of lysates was determined by Bradford assay (Bio-Rad) and 50 μg of total protein was subjected to electrophoresis on 10% SDS-PAGE. The gels were transferred onto PVDF membrane (Millipore, Allen, TX), and analyzed for CDK9. The blots were developed and quantified using ChemiDoc XRS Station (Bio-Rad). CDK2 immunoprecipitation was carried out with 100 μg of the cell lysate and 400 ng of anti-CDK2 rabbit polyclonal antibodies combined with 40 μl of 50% slurry of protein A agarose for 2 h at 4°C in a TNN Buffer containing 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, and 1% NP-40. The agarose beads were precipitated, washed with TNN buffer and divided into two parts, and used for the kinase assay and western blotting. Kinase assay was performed at 30°C for 30 min in a kinase assay buffer (50 mM HEPES-KOH, pH 7.9, 10 mM MgCl2, 6 mM EGTA, 2.5 mM DTT) containing 1 μg of histone H1 as a substrate, 200 μM cold ATP and 5 μCi of [γ-P32]ATP. Recombinant CDK2/cyclin E was used as a control. Reaction was stopped with SDS-loading buffer and resolved on 10% PAGE. Dried gel was exposed to Phosphor Imager screen. In parallel, the immunoprecipitated CDK2 was resolved on 10% Tris-Glycine SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Allen, TX) and immunoblotted with anti-CDK2 antibodies.
LDH dehydrogenase cytotoxicity assay
Lactate dehydrogenase (LDH) release, used as indicator of cell membrane damage was measured in the culture medium using an LDH assay kit (LDH-Cytotoxicity Assay Kit, BioVision, Mountain View, CA). To measure the cytotoxicity of the iron chelators, the procedure was followed as described in the kit. Briefly, the media was collected and mixed with 100 μl of reaction mixture provided by the manufacturer and incubated for 30 min at room temperature protected from light. High control was generated by treating cells with 1% Triton X-100 for 2 hours prior to the beginning of the assay. The colorimetric change was measured with a microtiter reader (Labsystem Multiskan MS) at 450 nm. Percentage of cytotoxicity was calculated using the formula [(Absorbance of the treated samples – Absorbance of control untreated cells)/(Absorbance of high control – Absorbance of control)] *100.
Measurement of cellular ferritin
CEM-GFP cells were grown in 6-well plates and treated with the indicated concentrations of iron chelators and ferric ammonium citrate. After 24 hours of treatment cytoplasmic extracts were prepared as we previously described (Nekhai et al., 2000a). In brief, the cells were lysed for 10 min at 4°C in a cell homogenizing buffer containing 10 mM Tris-HCl (pH 7.5), 6mM MgCl2, 80 mM KCl, 2mM DTT, 250 mM Sucrose, 0.1mM EDTA and 1% Triton. Lysates were span at 10,000 g for 15 min to precipitate nuclear material and organelles. The S-10 cytoplasmic extracts were used to measure ferritin using Spectro Ferritin Elisa kit (Ramco Laboratories, TX).
Tat phosphorylation in cultured cells
HeLa cells were infected with recombinant Ad-Tat. At 48 hours post infection the media was changed for 1 hour to a phosphate-free DMEM media (Life Technologies, Rockville, MD) containing no serum. Then the media was changed to phosphate-free DMEM supplemented with 0.5 mCi/ml of (32P)-orthophosphate and cells were further incubated for 2 hours at 37°C. About 1 μM okadaic acid (Sigma) was added to block cellular PPP-phosphatases. Cells were washed with PBS and lysed in whole cell lysis buffer (50 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 1% NP-40, 0.1% SDS) supplemented with protease cocktail (Sigma). After 10 min on ice, cellular material was scraped and then centrifuged at 14,000 rpm, 4°C for 30 min. The supernatant was recovered and immediately used for immunoprecipitation. Tat was precipitated with anti-Flag monoclonal antibodies coupled to protein G agarose for 2 h at 4°C in a TNN Buffer containing 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, and 1% NP-40. The immunoprecipitated Tat was recovered by heating for 2 min at 100°C in Tricine SDS-loading buffer, resolved on 15% Tris-Tricine SDS-PAGE (Schagger et al., 1996) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Allen, TX). The membrane was analyzed with anti-Tat monoclonal antibodies using 3,3′-Diaminobenzidine enhancer system (Sigma) and was also exposed to Phosphor Imager screen (Packard Instruments, Wellesley, MA). CDK2 expression was inhibited with CDK2-directed siRNA pool (M-003236-03-005) purchased from Dharmacon (Dallas, TX). The siRNAs were transfected at final concentration of 100 nM using Lipofectamin reagent (Invitrogen) according to the manufacturer’s recommendations. The siRNA were incubated with cells for 2 days before cells were labeled with 32P.
Supplementary Material
Supplemental Fig. 1. Chemical structures of iron chelators used in the study. A, 311, 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone, a tridentate aroylhydrazone ligand with high affinity for Fe3+. B, ICL670, 4-[3,5-bis-(hydroxyphenyl) -1,2,4-triazol-1-yl]-benzoic acid (ICL670), an achiral, tridentates iron-selective synthetic chelator of the bis-hydroxyphenyl-triazole class of compounds that forms a very stable 2:1 complex with ferric iron.
Supplemental Fig. 2. Inhibition of HIV-1 transcription is not due to the inhibition of cell proliferation. (a) HeLa-MAGI cells were infected with indicated amounts of the Ad-Tat or Ad-GFP. At 24 hours post infection the cells were lysed and analyzed for β-galactosidase activity with ONPG, and Tat transactivation was calculated. (b–d) HeLa-MAGI cells grown in 96-well plate were infected with Ad-Tat and treated with various concentrations of ICL670 (b), 311 (c), or DFO (d). Cell cultures were continued for 24 hours, and then the cells were either lysed and analyzed for β-galactosidase activity with ONPG, or treated with the MTT dye to determine cell proliferation. Open symbols, β-galactosidase activity, closed symbols – MTT assay. The IC50 of each chelators and MTT assays calculated with Prism Software are indicated. Data are presented as percent of a control without chelator.
Supplemental Fig. 3. Iron chelators precomplexed with Fe do not affect cell proliferation and HIV-1 transcription. (a and b) HeLa-MAGI cells grown in 96-well plate were treated with various concentrations 311 (a) or ICL670 (b) alone or preincubated with equimolar concentrations of ferric ammonium citrate. Cell cultures were continued for 24 hours, and then the cells were treated with the MTT dye to determine cell viability. Open symbols, chelators, closed symbols – chelators precoupled with Fe. (c) HeLa-MAGI cells were grown in 96-well plate, infected with Ad-Tat (lane 1) and treated with 5 μM 311 or 30 μM ICL670 without iron (lanes 2 and 4) or with ferric ammonium citrate (lanes 3 and 5). Cell cultures were continued for 24 hours, and then the cells were lysed and β-galactosidase activity was determined.
Acknowledgments
The authors thank Dr. Anna Suter (Novartis, Pharma AG, Ltd., Basel) for the gift of ICL670. We also thank Dr. Michael Emmerman (Fred Hutchinson Cancer Institute, Seattle, WA) for the HIV-1 LTR LacZ expression vector. We thank Dr. John Tisdale (NIDDK, NIH) for the gift of PGK-EGFP reporter vector. We thank the NIH AIDS Research and Reference Reagent Program for pNL4-3.Luc.R-E- plasmid (courtesy of Dr. Nathaniel Landau), CEM–HIV-1 LTR-GFP cells (courtesy of Dr. Jacques Corbeil), HeLa-CD4-LTR-β-gal cells (courtesy of Dr. Michael Emerman), and pGEM2 Tat bacterial expression vector (courtesy of Dr. Richard Gaynor). We thank Dr. Anna Suter (Novartis, Pharma AG, Ltd., Basel, Switzerland) for the gift of ICL670. This project was supported by NIH Research Grant 2 R25 HL003679-08 funded by the National Heart, Lung, and Blood Institute and The Office of Research on Minority Health; by Howard University General Clinical Research Center grant from the NIH No.2MO1 RR10284, by NIH Grant R21 AI 156973-01 (to S. N.) and by Grants RO1 DK49419 and RO1 HL55605 (to P. E. R.). The authors would like to thank members of Dr. Victor Gordeuk’s laboratory at the Center for Sickle Cell Disease, Howard University for valuable discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agbottah E, de la Fuente C, Nekhai S, Barnett A, Gianella-Borradori A, Pumfery A, Kashanchi F. Antiviral activity of CYC202 in HIV-1 infected cells. J Biol Chem. 2004 doi: 10.1074/jbc.M406435200. [DOI] [PubMed] [Google Scholar]
- Agbottah E, de La Fuente C, Nekhai S, Barnett A, Gianella-Borradori A, Pumfery A, Kashanchi F. Antiviral activity of CYC202 in HIV-1-infected cells. J Biol Chem. 2005;280(4):3029–42. doi: 10.1074/jbc.M406435200. [DOI] [PubMed] [Google Scholar]
- Ammosova T, Berro R, Jerebtsova M, Jackson A, Charles S, Klase Z, Southerland W, Gordeuk VR, Kashanchi F, Nekhai S. Phosphorylation of HIV-1 Tat by CDK2 in HIV-1 transcription. Retrovirology. 2006;3:78. doi: 10.1186/1742-4690-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammosova T, Berro R, Kashanchi F, Nekhai S. RNA interference directed to CDK2 inhibits HIV-1 transcription. Virology. 2005a;341(2):171–8. doi: 10.1016/j.virol.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Ammosova T, Jerebtsova M, Beullens M, Lesage B, Jackson A, Kashanchi F, Southerland W, Gordeuk VR, Bollen M, Nekhai S. Nuclear targeting of protein phosphatase-1 by HIV-1 Tat protein. J Biol Chem. 2005b;280(43):36364–71. doi: 10.1074/jbc.M503673200. [DOI] [PubMed] [Google Scholar]
- Ammosova T, Jerebtsova M, Beullens M, Voloshin Y, Ray PE, Kumar A, Bollen M, Nekhai S. Nuclear protein phosphatase-1 regulates HIV-1 transcription. J Biol Chem. 2003;278(34):32189–94. doi: 10.1074/jbc.M300521200. [DOI] [PubMed] [Google Scholar]
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13(20):1775–85. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD, Grdina TA, Bogerd HP, Cullen BR. Recruitment of a protein complex containing Tat and cyclin T1 to TAR governs the species specificity of HIV-1 Tat. Embo J. 1998;17(23):7056–65. doi: 10.1093/emboj/17.23.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S, Malech HL. Current developments in the design of onco-retrovirus and lentivirus vector systems for hematopoietic cell gene therapy. Biochim Biophys Acta. 2003;1640(1):1–24. doi: 10.1016/s0167-4889(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Coulonval K, Bockstaele L, Paternot S, Roger PP. Phosphorylations of cyclin-dependent kinase 2 revisited using two-dimensional gel electrophoresis. J Biol Chem. 2003;278(52):52052–60. doi: 10.1074/jbc.M307012200. [DOI] [PubMed] [Google Scholar]
- Deng L, Ammosova T, Pumfery A, Kashanchi F, Nekhai S. HIV-1 Tat interaction with RNA polymerase II C-terminal domain (CTD) and a dynamic association with CDK2 induce CTD phosphorylation and transcription from HIV-1 promoter. J Biol Chem. 2002;277(37):33922–9. doi: 10.1074/jbc.M111349200. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Fu D, Richardson DR. Iron chelation and regulation of the cell-cycle: two mechanisms of post-transcriptional regulation of the universal cyclin-dependent kinase inhibitor p21CIP1/WAF1 by iron depletion. Blood. 2007 doi: 10.1182/blood-2007-03-076737. [DOI] [PubMed] [Google Scholar]
- Galanello R, Piga A, Alberti D, Rouan MC, Bigler H, Sechaud R. Safety, tolerability, and pharmacokinetics of ICL670, a new orally active iron-chelating agent in patients with transfusion-dependent iron overload due to beta-thalassemia. J Clin Pharmacol. 2003;43(6):565–72. [PubMed] [Google Scholar]
- Gao J, Richardson DR. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents, IV: The mechanisms involved in inhibiting cell-cycle progression. Blood. 2001;98(3):842–50. doi: 10.1182/blood.v98.3.842. [DOI] [PubMed] [Google Scholar]
- Garber ME, Wei P, Jones KA. HIV-1 Tat interacts with cyclin T1 to direct the P-TEFb CTD kinase complex to TAR RNA. Cold Spring Harb Symp Quant Biol. 1998;63:371–80. doi: 10.1101/sqb.1998.63.371. [DOI] [PubMed] [Google Scholar]
- Garber ME, Wei P, KewalRamani VN, Mayall TP, Herrmann CH, Rice AP, Littman DR, Jones KA. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998;12(22):3512–27. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou NA, van der Bruggen T, Oudshoorn M, Hider RC, Marx JJ, van Asbeck BS. Human immunodeficiency virus type 1 replication inhibition by the bidentate iron chelators CP502 and CP511 is caused by proliferation inhibition and the onset of apoptosis. Eur J Clin Invest. 2002;32(Suppl 1):91–6. doi: 10.1046/j.1365-2362.2002.0320s1091.x. [DOI] [PubMed] [Google Scholar]
- Georgiou NA, van der Bruggen T, Oudshoorn M, Nottet HS, Marx JJ, van Asbeck BS. Inhibition of human immunodeficiency virus type 1 replication in human mononuclear blood cells by the iron chelators deferoxamine, deferiprone, and bleomycin. J Infect Dis. 2000;181(2):484–90. doi: 10.1086/315223. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Delanghe JR, Langlois MR, Boelaert JR. Iron status and the outcome of HIV infection: an overview. J Clin Virol. 2001;20(3):111–5. doi: 10.1016/s1386-6532(00)00134-7. [DOI] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69(11):6705–11. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanchi F, Agbottah ET, Pise-Masison CA, Mahieux R, Duvall J, Kumar A, Brady JN. Cell cycle-regulated transcription by the human immunodeficiency virus type 1 Tat transactivator. J Virol. 2000;74(2):652–60. doi: 10.1128/jvi.74.2.652-660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66(4):2232–9. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WS, Maddukuri A, Teslovich TM, de la Fuente C, Agbottah E, Dadgar S, Kehn K, Hautaniemi S, Pumfery A, Stephan DA, Kashanchi F. Therapeutic targets for HIV-1 infection in the host proteome. Retrovirology. 2005;2(1):20. doi: 10.1186/1742-4690-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Nekhai S, Bhat UG, Ammosova T, Radhakrishnan SK, Jerebtsova M, Niu X, Foster A, Layden TJ, Gartel AL. A novel anticancer agent ARC antagonizes HIV-1 and HCV. Oncogene. 2006 doi: 10.1038/sj.onc.1210158. [DOI] [PubMed] [Google Scholar]
- Nekhai S, Bottaro DP, Woldehawariat G, Spellerberg A, Petryshyn R. A cell-permeable peptide inhibits activation of PKR and enhances cell proliferation. Peptides. 2000a;21(10):1449–56. doi: 10.1016/s0196-9781(00)00297-7. [DOI] [PubMed] [Google Scholar]
- Nekhai S, Jerebtsova M, Jackson A, Southerland W. Regulation of HIV-1 transcription by protein phosphatase 1. Curr HIV Res. 2007;5(1):3–9. doi: 10.2174/157016207779316279. [DOI] [PubMed] [Google Scholar]
- Nekhai S, Shukla RR, Fernandez A, Kumar A, Lamb NJ. Cell cycle-dependent stimulation of the HIV-1 promoter by Tat-associated CAK activator. Virology. 2000b;266(2):246–56. doi: 10.1006/viro.1999.0035. [DOI] [PubMed] [Google Scholar]
- Nekhai S, Zhou M, Fernandez A, Lane WS, Lamb NJ, Brady J, Kumar A. HIV-1 Tat-associated RNA polymerase C-terminal domain kinase, CDK2, phosphorylates CDK7 and stimulates Tat-mediated transcription. Biochem J. 2002;364(Pt 3):649–57. doi: 10.1042/BJ20011191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JB. Deferasirox: An effective once-daily orally active iron chelator. Drugs Today (Barc) 2006;42(10):623–37. doi: 10.1358/dot.2006.42.10.1009901. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Bernhardt PV. Crystal and molecular structure of 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone (NIH) and its iron(III) complex: an iron chelator with anti-tumour activity. J Biol Inorg Chem. 1999;4(3):266–73. doi: 10.1007/s007750050312. [DOI] [PubMed] [Google Scholar]
- Schagger H, Bentlage H, Ruitenbeek W, Pfeiffer K, Rotter S, Rother C, Bottcher-Purkl A, Lodemann E. Electrophoretic separation of multiprotein complexes from blood platelets and cell lines: technique for the analysis of diseases with defects in oxidative phosphorylation. Electrophoresis. 1996;17(4):709–14. doi: 10.1002/elps.1150170415. [DOI] [PubMed] [Google Scholar]
- Traore HN, Meyer D. The effect of iron overload on in vitro HIV-1 infection. J Clin Virol. 2004;31(Suppl 1):S92–8. doi: 10.1016/j.jcv.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92(4):451–62. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- Yang X, Gold MO, Tang DN, Lewis DE, Aguilar-Cordova E, Rice AP, Herrmann CH. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci U S A. 1997;94(23):12331–6. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Kim HJ, Yoon YS, Cho H, Lim IK, Lee JH. Iron chelation-induced senescence-like growth arrest in hepatocyte cell lines: association of transforming growth factor beta1 (TGF-beta1)-mediated p27Kip1 expression. Biochem J. 2002;366(Pt 2):613–21. doi: 10.1042/BJ20011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews MB, Price DH. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11(20):2622–32. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Chemical structures of iron chelators used in the study. A, 311, 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone, a tridentate aroylhydrazone ligand with high affinity for Fe3+. B, ICL670, 4-[3,5-bis-(hydroxyphenyl) -1,2,4-triazol-1-yl]-benzoic acid (ICL670), an achiral, tridentates iron-selective synthetic chelator of the bis-hydroxyphenyl-triazole class of compounds that forms a very stable 2:1 complex with ferric iron.
Supplemental Fig. 2. Inhibition of HIV-1 transcription is not due to the inhibition of cell proliferation. (a) HeLa-MAGI cells were infected with indicated amounts of the Ad-Tat or Ad-GFP. At 24 hours post infection the cells were lysed and analyzed for β-galactosidase activity with ONPG, and Tat transactivation was calculated. (b–d) HeLa-MAGI cells grown in 96-well plate were infected with Ad-Tat and treated with various concentrations of ICL670 (b), 311 (c), or DFO (d). Cell cultures were continued for 24 hours, and then the cells were either lysed and analyzed for β-galactosidase activity with ONPG, or treated with the MTT dye to determine cell proliferation. Open symbols, β-galactosidase activity, closed symbols – MTT assay. The IC50 of each chelators and MTT assays calculated with Prism Software are indicated. Data are presented as percent of a control without chelator.
Supplemental Fig. 3. Iron chelators precomplexed with Fe do not affect cell proliferation and HIV-1 transcription. (a and b) HeLa-MAGI cells grown in 96-well plate were treated with various concentrations 311 (a) or ICL670 (b) alone or preincubated with equimolar concentrations of ferric ammonium citrate. Cell cultures were continued for 24 hours, and then the cells were treated with the MTT dye to determine cell viability. Open symbols, chelators, closed symbols – chelators precoupled with Fe. (c) HeLa-MAGI cells were grown in 96-well plate, infected with Ad-Tat (lane 1) and treated with 5 μM 311 or 30 μM ICL670 without iron (lanes 2 and 4) or with ferric ammonium citrate (lanes 3 and 5). Cell cultures were continued for 24 hours, and then the cells were lysed and β-galactosidase activity was determined.