Abstract
The development of GABAergic inhibitory circuits is shaped by neural activity, but the underlying mechanisms are unclear. we demonstrate a novel function of GABA in regulating GABAergic innervation in the adolescent brain, when GABA is mainly known as an inhibitory transmitter. Conditional knockdown of the rate-limiting synthetic enzyme GAD67 in basket interneurons in adolescent visual cortex resulted in cell autonomous deficits in axon branching, perisomatic synapse formation around pyramidal neurons, and complexity of the innervation fields; the same manipulation had little influence on the subsequent maintenance of perisomatic synapses. These effects of GABA deficiency were rescued by suppressing GABA re-uptake and by GABA receptor agonists. Germ-line knockdown of GAD67 but not GAD65 showed similar deficits, suggesting a specific role of GAD67 in the maturation of perisomatic innervation. Since intracellular GABA levels are modulated by neuronal activity, our results implicate GAD67-mediated GABA synthesis in activity-dependent regulation of inhibitory innervation patterns.
Introduction
The development of neural networks requires precise and activity-dependent regulation of axon growth, branching, and synapse formation (Hua and Smith, 2004; Zhang and Poo, 2001). As key mediators of neural activity, neurotransmitters seem particularly well suited to sculpt connections with the appropriate spatial and temporal precision, and to couple transmission with synaptic morphogenesis and refinement. Indeed, signaling through glutamate, the major excitatory neurotransmitter in the vertebrate brain, has been shown to regulate nearly all aspects of synapse development (Tashiro et al., 2003; Zhang and Poo, 2001; Zheng et al., 1994) and lies at the heart of the emerging rules underlying activity-dependent plasticity (Malinow and Malenka, 2002; Zhang and Poo, 2001). Therefore, excitatory neurotransmitters also function as developmental signals for synapse formation, maturation, and plasticity, in addition to their classic role in mediating transmission at mature synapses. The mechanisms regulating the development and plasticity of GABAergic inhibitory synapses are much less understood.
Initially discovered as an inhibitory neurotransmitter, GABA has since been implicated in multiple processes of neural development (reviewed by(Owens and Kriegstein, 2002b), from cell proliferation (LoTurco et al., 1995), neurite growth (Spoerri, 1988), to adult neurogenesis (Ge et al., 2006). Indeed, GABA synthesis and signaling begin during mid-gestation in rodents, long before the establishment of synaptic communications. The potent trophic effects of GABA on neuronal migration and neurite growth during embryonic and perinatal period are largely explained by its depolarizing action in immature neurons (Ben-Ari, 2002), which triggers calcium influx and signaling. In addition, GABA-mediated signaling in neonatal mammalian forebrain represents the first wave of synaptic communication, which precedes and promotes the formation of glutamatergic synapses (Ben-Ari et al., 2004). On the other hand, the development of GABAergic innervation patterns (Tamas et al., 1997) and functionally potent inhibitory transmission (Morales et al., 2002) in neocortex is a protracted postnatal process, extending well into adolescence (Chattopadhyaya et al., 2004; Miller, 1986). For example, a highly characteristic feature of GABAergic innervation is its local exuberance: a single basket interneuron in the mature visual cortex innervates hundreds of neurons in its vicinity, and forms multiple, clustered synapses onto the soma and proximal dendrites of target neurons (Tamas et al., 1997). Such a pattern of perisomatic innervation is only achieved during the fourth postnatal week in rodents and it is regulated by neural activity and visual inputs (Chattopadhyaya et al., 2004). The cellular and molecular mechanisms underlying the activity-dependent development of inhibitory synaptic innervation are poorly understood; in particular, it is not known whether GABA, thought to act as a classic inhibitory transmitter in the adolescent and adult brain, might play a role.
Receptor antagonists have been instrumental tools in discovering the multifaceted role of glutamate in the formation and plasticity of excitatory synapses, and also were key in revealing the depolarizing action and the morphogenic effects of GABA in embryonic and neonatal brain (Ben-Ari et al., 1989; Chen et al., 1996). In the adolescent and adult brain however, the epileptogenic effects of GABA receptor blockers have largely precluded their use in studying the role of GABA in the morphogenesis of inhibitory synapes. Here we disrupt GABA signaling by genetic manipulation of the GABA synthetic enzymes. GABA is synthesized by two glutamic acid decarboxylases: GAD67 and GAD65 (encoded by the Gad1 and Gad2 genes, respectively) (Pinal and Tobin, 1998). GABA synthesis is the rate-limiting step in GABA metabolism (Soghomonian and Martin, 1998), which readily influences the cellular and vesicular GABA content (Engel et al., 2001; Murphy et al., 1998). Between the two isoforms, GAD67 is responsible for over 90% of basal GABA synthesis and is produced at limiting levels in the brain (Asada et al., 1997; Kash et al., 1997). GAD67 and GAD65 show striking differences in their developmental expression (Kiser et al., 1998), subcellular localization (Dupuy and Houser, 1996), enzymatic activity (Battaglioli et al., 2003), and gene regulation (Feldblum et al., 1993; Pinal and Tobin, 1998); but the functional significance of these differences has been elusive. Here we used germ-line and conditional mutants of Gad1 and Gad2 to knock down GABA synthesis at defined stages of inhibitory circuit development. We reveal a novel and specific role of GAD67-mediated GABA synthesis and signaling in regulating the maturation of inhibitory innervation pattern in the adolescent mouse visual cortex.
Results
Cell Autonomous Regulation of Axon Branching and Synapse Density of Basket Interneurons by GAD67
The basic features of perisomatic innervation of pyramidal cells by basket interneurons mature in visual cortical organotypic cultures (Chattopadhyaya et al., 2004; Di Cristo et al., 2004; Klostermann and Wahle, 1999), but is retarded by the suppression of neuronal activity (e.g. by TTX treatment). To explore the role of GABA in regulating perisomatic innervation, we first examined whether GABA levels are influenced by changes in neuronal activity. Cortical organotypic cultures develop a considerable level of synaptically driven spontaneous activity in a well-balanced state of excitation and inhibition (Echevarria and Albus, 2000; Klostermann and Wahle, 1999), although the activity levels in our slice culture system is yet to be characterized. We have previously shown that TTX (1 μM) application from EP18-24 (EP18: equivalent postnatal day18 = P3 pups+15 days in vitro) resulted in profound reduction of perisomatic synapse density, size, and terminal branching (Chattopadhyaya et al., 2004). Here we show that the same treatment resulted in a significant decrease in the number of GABA positive cells as well as in the intensity of GABA immunofluorescence in cell somata (57% and 42% reduction, respectively, Sppl Fig. 1). Activity blockade reduced GABA levels without affecting neuronal survival because we found no change in the density of NeuN positive cells. This result is consistent with previous in vitro (Rutherford et al., 1997) and in vivo (Benevento et al., 1995; Micheva and Beaulieu, 1995) studies, demonstrating activity-dependent regulation of GABA levels in visual and somatosensory cortex. To examine whether GABA plays a more direct role in regulating the maturation of perisomatic innervation, we reduced GABA levels in basket interneurons by conditional knockdown and knockout of its major synthetic enzyme GAD67.
Germ-line homozygous deletion of the Gad1 gene results in a ~90% reduction of the brain GABA content and perinatal death due to severe cleft palate, but show no gross defects in brain morphology (Asada et al., 1997). To examine the role of GABA in the postnatal maturation of GABAergic inhibitory circuits in visual cortex, we generated a conditional allele of Gad1 (see Material and Methods; Sppl Fig. 2), which allows cell type and developmental stage restricted knockdown of GABA synthesis. In this floxed-Gad1 allele (Gad1lox), Cre-mediated recombination results in splicing from exon 1 to 3, generating an mRNA that is out of frame for translation. Mice with 2 inactive Gad1−/− alleles generated by breeding Gad1lox/lox mice with the Mox2-Cre mice (Tallquist and Soriano, 2000) die at birth, with phenotypes identical to germ line Gad1-null mice (Asada et al., 1997).
To reduce GABA synthesis in basket interneurons and simultaneously labeled their axons and synapses, we used a previously characterized promoter region (PG67; (Chattopadhyaya et al., 2004) to express Cre recombinase and EGFP (PG67-GFP-ires-Cre) in parvalbumin-positive basket interneurons in organotypic cultures from the visual cortex of Gad1lox/lox mice. PG67-GFP-ires-Cre transfection resulted in significant GABA deficiency in basket cells (Figure 1). In control slice cultures from either Gad1lox/lox mice transfected with PG67-GFP or from wild-type mice transfected with PG67-GFP-ires-Cre at EP16 or EP26, more than 95% of the basket cell somata showed GABA immunofluorescence (Figure 1B1; in Gad1lox/lox cultures: 28 out of 30 cells transfected from EP16-24, and 29 out of 30 cells from EP26-32; in wild-type cultures: 29 out of 30 cells from EP16-24). On the other hand, in Gad1lox/lox slice cultures transfected with PG67-GFP-ires-Cre, there was no detectable GABA in basket cell somata (Figure 1B2, 0 out of 30 cells in the above two time windows). GABA deficiency was apparent 48 h following transfection (29 out of 30 cellssomata showed undetectable GABA staining two days after transfection at EP16, Figure 1C), and likely resulted from inactivation of both Gad1 alleles. These results are consistent with the finding that GAD67 contributes to over 90% of GABA synthesis in rodent neocortex (Asada et al., 1997) and has a half-life of only a few hours (Pinal and Tobin, 1998). Because typically less than 10 isolated basket cells were transfected in a cortical slice, and Gad2 gene was left intact, the overall activity levels in the slices were unlikely to be affected.
Figure 1. Gad1 deficiency in single basket interneuron reduces GABA levels.
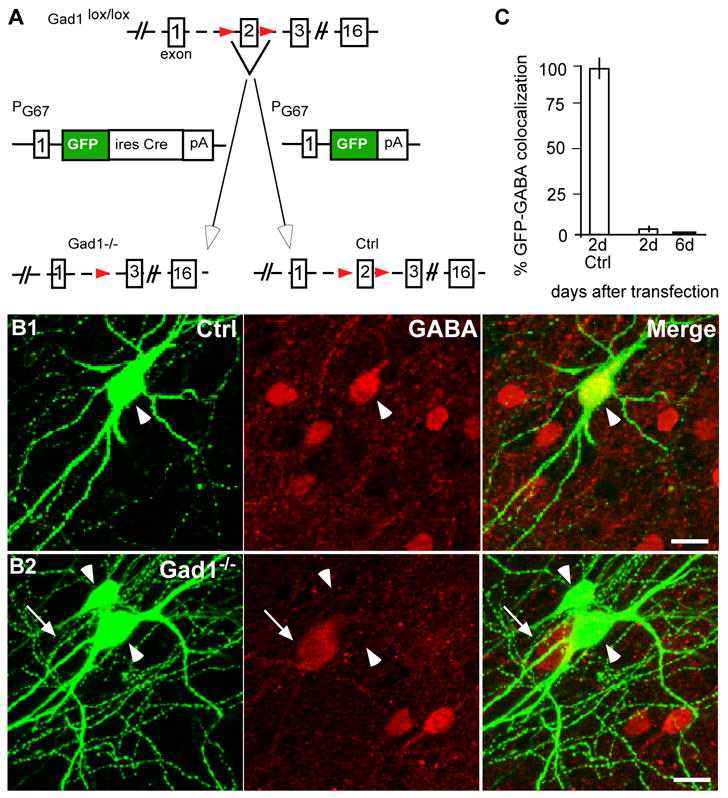
A, Exon 2 of the Gad1 gene was flanked with loxP sites to create a conditional Gad1lox allele. Cortical organotypic cultures were transfected with biolistics with PG67-GFP-ires-Cre to generate GAD67-null (Gad1−/−) or PG67-GFP for control (Ctrl) basket cells. B1, A control basket cell (green, arrowhead) with prominent GABA immunostaining (red) in soma. B2, Two GAD67-null basket cells (arrowheads) with no detectable GABA staining in their somata while neighboring untransfected interneurons (arrow) show prominent GABA staining. Scale bar: 10 μm. C, Only 4% of PG67-GFP-ires-Cre-transfected basket cells show detectable levels of GABA 2 days after transfection as compared to 98% of control cells (2d-Ctrl); none of PG67-GFP-ires-Cre-transfected basket interneurons show detectable levels of GABA 6 days after transfection. Bars represent the average from 3 experiments±SEM.
In organotypic cultures, basket interneurons started out with very sparse and simple axons, which developed into highly elaborate arbors in the subsequent 4 weeks (Chattopadhyaya et al., 2004). We focused our study in the third and fourth week when there was significant and stereotyped maturation of perisomatic innervation. Two sets of control basket cells were used - those from wild-type cultures transfected with PG67-GFP-ires-Cre and those from Gad1lox/lox cultures transfected with PG67-GFP. These two groups were similar in all the major parameters analyzed (terminal branching, bouton density and size around pyramidal cell somata, Mann Whitney test p>0.1) and were pooled. Between EP16-24, control basket axons continued to grow, branch, contact more pyramidal cell somata, and form perisomatic synapses (Figure 2A). By EP24, basket cell axons typically innervated hundreds of pyramidal cells (Figure 2A1, 5A) and extended two or more characteristic “terminal branches” bearing multiple and clustered boutons around each pyramidal cell soma (Figure 2A3, 2D, 2F). This is a highly stereotyped feature of perisomatic synapses throughout the innervation field, independent of the distance of a terminal branch from its “parent” basket cell soma (Sppl Fig 3). Three lines of evidence indicate that a vast majority of GFP-labeled boutons most likely represent presynaptic terminals. First, 96% of GFP-positive boutons contained the GABAergic synaptic marker GAD65 (100 boutons from 3 basket cells at EP24; also see (Chattopadhyaya et al., 2004). GAD65 is highly concentrated in mature presynaptic terminals (Dupuy and Houser, 1996; Huang et al., 1999), where it is physically associated with the vesicular GABA transporter (Jin et al., 2003). Second, we co-transfected basket cells with PG67-tdtomato and PG67-synaptophysin-GFP (syn-GFP) to simultaneously visualize axon-bouton morphology with a synaptic marker. We found that 95.3% of tdtomato-labeled boutons contained syn-GFP at EP18 (273 boutons from 3 cells) and 98.5% at EP24 (486 boutons from 3 cells; Sppl Fig 4A). Finally, we performed immuno-EM to examine the ultrastructure of GFP-labeled boutons at EP24. GFP-labeled boutons showed typical features of presynaptic inhibitory terminals making symmetric contacts with postsynaptic targets, with clear evidence of synaptic cleft, vesicle clustering, mitochondria, and thickening of postsynaptic membrane (Figure 2A4; Sppl Fig 4B; also see Chattopadhyaya et al., 2004). 21 out of 21 GFP-labeled boutons showed such characteristics of symmetric synapses. These studies demonstrate that GFP-positive boutons are presynaptic component of symmetric synapses.
Figure 2. GAD67-mediated GABA synthesis is limiting for perisomatic synapse maturation but not its maintenance.
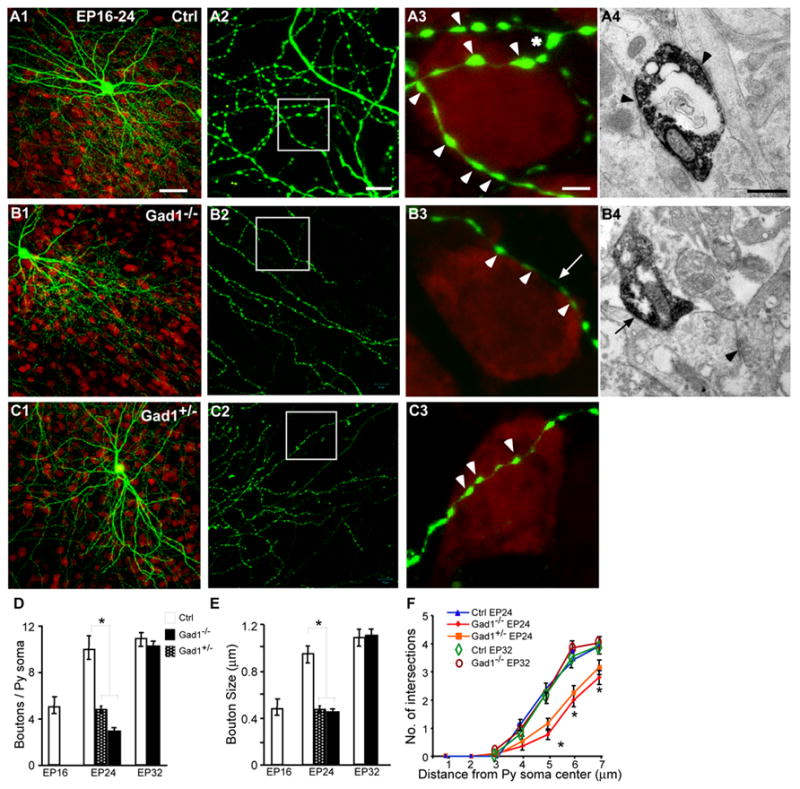
A1, Control basket cell (green) at EP24 with exuberant innervation field characterized by extensive branching, dense boutons along axons (A2), and terminal branches (asterisks, A3) with prominent and clustered boutons (arrowheads) around pyramidal cell somata (NeuN immunostaining, red). B, Gad1−/− basket cell show overall similar axon arbor size and morphology (B1) but reduced axon branching (B2), terminal branching, bouton size and density (B3, arrow indicates larger inter-bouton intervals). C, Inactivation of only one Gad1 allele in basket cells yields a phenotype comparable to of Gad1−/− cells. A3, B3, C3 are from boxed regions in A2, B2, C2. A4, Immuno-EM of a typical GFP-labeled axonal bouton in a control basket cell, which forms symmetric synaptic contacts (arrowheads) with synaptic clefts, vesicle clustering, and thickening of the postsynaptic membrane. In Gad1−/− basket cells (B4), it is difficult to identify vesicles within the bouton-like structures and evidence of synaptic specialization throughout the bouton (arrow). Note that a neighboring GFP-negative symmetric synapse (arrowhead) from a Gad1+/+ cell appears entirely normal. Scale bar: A1, B1, C1: 50 μm; A2, B2, C2: 10 μm; A3, B3, C3: 5 μm; A4, B4: 0.5 μm. D–E, When transfected from EP16-24, bouton density and size are significantly reduced in Gad1−/− cells (7 basket cells and 50 pyramidal somata) and Gad1+/− cells (7 basket cells and 45 pyramidal somata) compared to aged-matched controls (8 basket cells and 50 pyramidal somata; One-way Anova, posthoc Dunn’s test p<0.05; also see Sppl Fig 3). When transfected from EP26-32, however, both parameters are unaffected in Gad1−/− cells (7 basket cells and 50 pyramidal somata) as compared to controls (7 basket cells and 45 pyramidal somata; Mann-Whitney U test, p>0.1; also see images in Sppl Fig 4). F, When transfected from EP16-24, Gad1−/− and Gad1+/− cells show reduced terminal branching compared to controls quantified as in Chattopadhyaya et al., 2004 (One-way Anova, posthoc Dunn’s test, p<0.05). When transfected from EP26-32, however, terminal branch complexity is maintained in Gad1−/− cells (Mann-Whitney U test, P>0.1).
Figure 5. GAD67-mediated GABA synthesis regulates basket cell axon density, branching, target coverage and innervation field.
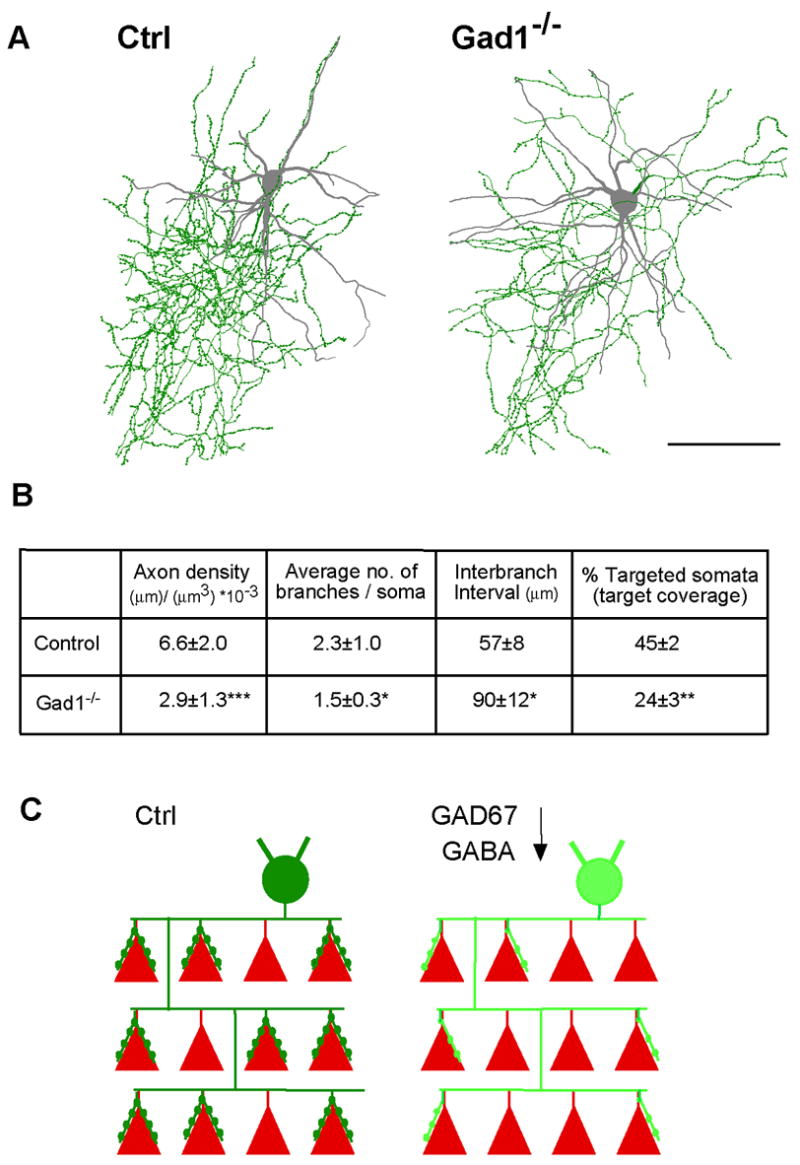
A, Reconstruction of local axon arbor of control and Gad1−/− basket cells at EP24 (axons: green, soma and dendrite: gray, dots on axon: boutons). Gad1−/− basket cells show reduced axon density, branching, and bouton density. Scale bar: 100 μm. B, Quantitative analysis of local axon arbor and innervation field from Gad1−/− (n=6) and control (n=5) basket cells. Mann Whitney U test, * p<0.01; ** p<0.001; *** p<0.0001. C, Schematic summarizing the effects of reduced GAD67 and GABA levels in a basket cell, on its axon branching, target coverage, synapse density and innervation field. Red: pyramidal cell somata; green, basket cell.
In age-matched basket cells from Gad1lox/lox cultures transfected with PG67-GFP-ires-Cre (referred as Gad1−/− cells), the shape and gross branching patterns of axon arbors appeared similar to that of control cells (Figure 2B1, 5B). However, Gad1−/− axons appeared thinner and showed reduced number of finer branches, especially terminal branches (Figure 2B, F, Figure 5A, B; Mann Whitney test p<0.05). These terminal branches also bore fewer and smaller boutons around pyramidal cell somata throughout the innervation field (Figure 2B3, D, E; Sppl Fig 3; boutons/soma±SEM= 2.7±0.4 for Gad1−/− vs 8.9±0.7 for control cells, Mann-Whitney test p<0.001). Since the vast majority of GFP-labeled boutons represent presynaptic terminals in WT basket cells, the reduced density and size of boutons along Gad1−/− axons suggest reduced and deficient synaptic contact. Indeed, immuno-EM revealed that, along GFP-labeled Gad1−/− basket axons, it was often difficult to discern vesicles throughout “bouton-like structures” and to detect evidence of synaptic specialization; yet normal synaptic contacts can be clearly found in close vicinity from wildtype (Gad1 lox/lox) GABAergic terminals (Figure 2B4). Together, these results suggest that GAD67-mediated GABA synthesis regulates basket cell axon branching and perisomatic synapse formation in a cell autonomous manner during the maturation of inhibitory circuits, when GABA transmission in the perisomatic region has already become hyperpolarizing (Ikeda et al., 2003). Residual GABA synthesis by GAD65 within basket cells was insufficient to sustain the maturation of perisomatic innervation.
GAD67 Levels in Basket Interneurons Birectionally Regulate Axon Branching and Perisomatic Bouton Formation
GAD67 and GABA levels in rodent brain are highly dependent on Gad1 gene dosage. Deletion of one Gad1 allele results in ~35% reduction of GABA contents in neonatal brain and less pronounced but significant reduction in adult brain (Asada et al., 1997), suggesting that GAD67 level is limiting for GABA synthesis. To explore whether the level of GAD67 in a basket cell is limiting for the maturation of its axon arbor and perisomatic boutons, we inactivated only one Gad1 allele in basket interneurons using slice cultures from Gad1+/lox mice. Gad1+/− basket cells (EP16-24) showed significant reduction of terminal branch complexity, bouton size and bouton number around pyramidal cell somata (Figure 2C3, D, E, F; boutons/soma±SEM =5.0±0.3 for Gad1+/− vs 8.9±0.4 for control, One-way Anova, posthoc Dunn’s test p<0.05). The reduction of perisomatic innervation in Gad1+/− basket cells was less severe compared to Gad1−/− cells even though the difference between the two groups did not reach statistical significance (Figure 2B, C, D; One-way Anova, posthoc Dunn’s test, p>0.05). These results suggest that even a modest reduction of GABA level in basket interneurons can impair axon growth and bouton formation during the maturation of perisomatic innervation. The mechanism underlying the GABA regulation of perisomatic innervation is likely to act locally at or near the site of release since ambient GABA or GABA spill-over, if any, from neighboring Gad+/+ axons is insufficient to rescue the deficits in perisomatic synapses of Gad1+/− basket cells.
Since Gad1 transcription is up-regulated during postnatal development and by neural activity (Kiser et al., 1998, Liang et al., 1996), we also examined whether GAD67 overexpression could accelerate the maturation of perisomatic innervation. Slice cultures from wild type mice were co-transfected with PG67-GFP and PG67-GAD67 (Gad1over, a full length rat gad1 cDNA driven by the PG67 promoter) or PG67-GFP alone (control) from EP12-18. In comparison to controls, Gad1over basket cells showed significantly increased perisomatic innervation (Figure 3B1–3 vs Figure 3A1–3). Both bouton density (Figure 3A3, 3B3, 3C; boutons/soma±SEM = 7.1±0.2 for Gad1over cells vs 3.55±0.2 for controls, Mann-Whitney test p<0.001) and terminal branch complexity (Figure 3A3, 3B3, 3E; Mann-Whitney test, p<0.001) were significantly higher than for control cells; and bouton size remained unchanged (Figure 3D; bouton size = 0.52±0.02 μm for Gad1over cells vs 0.58±0.03 μm for controls, Mann-Whitney test, p>0.1). Therefore, an increase of GAD67 expression, which likely results in elevated cellular GABA contents, can accelerate the maturation of perisomatic innervation.
Figure 3. Overexpression of GAD67 increases basket cell axon branching and perisomatic innervation.
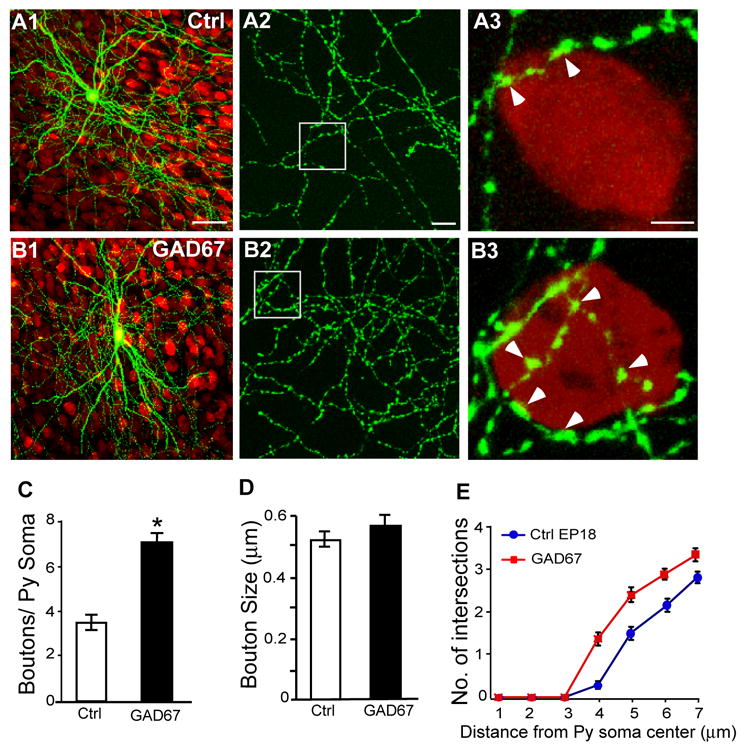
A, At EP18, perisomatic synapses in control basket cells is immature (A2), with a single terminal branch and only a few putative boutons (A3, arrowheads) around each pyramidal soma (NeuN staining, red). B, A GAD67-overexpressing basket cell from EP12-18 shows increased axon branching (B2), terminal branching and bouton density around pyramidal cell soma (B3). A3, B3, C3 are from boxed regions in A2, B2, C2. Scale bar: A1, B1, C1: 50 μm; A2, B2, C2: 10 μm; A3, B3, C3: 2 μm. When transfected from EP12-18, bouton density (C) and terminal branching (E) are significantly increased in GAD67-overexpressing basket cells (7 basket cells and 88 pyramidal somata) compared to controls (5 basket cells and 48 pyramidal somata; Mann-Whitney U test, p<0.05), while bouton size is unaffected (Mann-Whitney U test, p>0.1).
Extracellular GABA Signaling Regulates Basket Cell Axon Branching and Perisomatic Bouton Formation Through GABAA and GABAB Receptors
To examine whether GAD67 regulates the maturation of perisomatic innervation through extracellular GABA signaling, we investigated whether an increase of extracellular GABA by the blockade of GABA reuptake was able to rescue the phenotype of Gad1+/−basket cells. GABA reuptake by transporters is a major mechanism to terminate transmission and replenish GABA content in nerve terminals. GAT1 is the major neuronal GABA transporter, highly concentrated at GABAergic axons and synaptic terminals (Chiu et al., 2002; Jensen et al., 2003). NO711 is a specific inhibitor of GAT1, which elevates ambient GABA levels in brain slice (Jensen et al., 2003) and leads to tonic activation of GABA receptors (Overstreet et al., 2000). We therefore included NO711 (10 μM) in the culture medium when Gad1+/lox slice cultures were transfected with PG67-GFP-ires-Cre between EP16-24. While NO711 treatment of control basket cells did not affect the basic characteristics of perisomatic synapses (Figure 4A; boutons/soma±SEM=8.8±0.3 for NO711-treated vs 8.9±0.2 for untreated Gad1+/− basket cells; Mann Whitney test, p>0.1), it rescued the perisomatic bouton formation in Gad1+/− basket cells (Figure 4A, Sppl Fig 6). The complexity of terminal branches (Figure 4A3, One-Way Anova, posthoc Dunn’s test, p<0.05), bouton density (Figure 4A1; boutons/soma±SEM= 7.9±0.2 for NO711-treated Gad1+/− vs. 3.4±0.2 for control basket cells; One-way Anova, posthoc Dunn’s test, p<0.05) and size of perisomatic boutons (Figure 4A2; bouton size = 0.88±0.02 μm for NO711-treated Gad1+/− cells vs 0.45±0.01 μm for controls; One-way Anova, posthoc Dunn’s test, p<0.05) in NO711-treated Gad1+/− were increased compared to untreated Gad1+/− basket cells. These data suggested that extracellular concentration of GABA, regulated by GAD67, is a critical factor for basket cell axon branching and perisomatic bouton formation.
Figure 4. GABA signaling regulates basket cell axon branching and perisomatic synapse formation through GABAA and GABAB receptors.
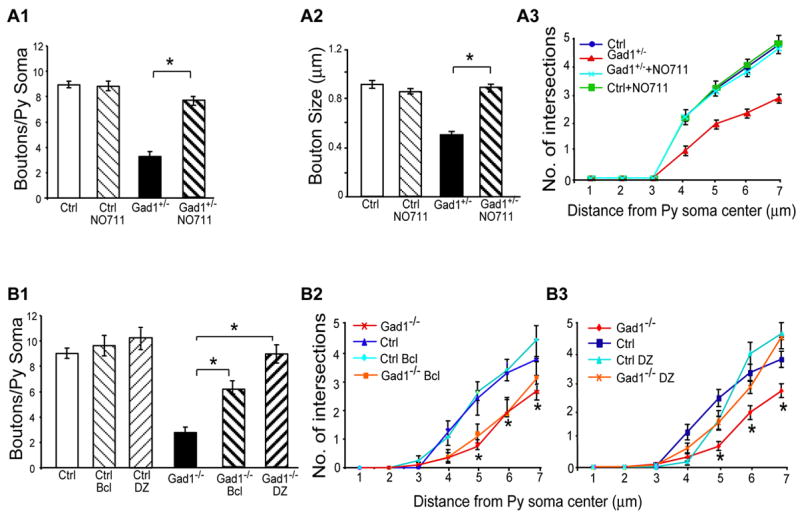
A, GAT1 antagonist rescues bouton formation, bouton size and terminal branching in Gad1+/−cells. NO711-treated (EP16-24) Gad1+/− basket cells (7 cells and 51 pyramidal somata) show increased bouton density (A1), bouton size (A2) and terminal branching (A3) compared to untreated Gad+/− basket cells (5 cells and 54 pyramidal somata; one-way Anova, posthoc Dunn’s test, p< 0.05). Control basket cells treated with NO711 (3 cells and 36 pyramidal somata) show no changes in these parameters compared to untreated controls (6 cells and 58 pyramidal somata; one-way Anova, posthoc Dunn’s test, p> 0.05; See also Sppl Fig 6). B, Diazepam (DZ) or Baclofen (Bcl) treatment (EP16-24) rescued different aspects of perisomatic innervation in Gad1−/− basket cells. DZ-treated Gad1−/− basket cells (7 cells and 50 pyramidal somata) show bouton density (B1) and terminal branching (B3) similar to control cells (7 cells and 44 pyramidal somata; one-way Anova, posthoc Dunn’s test, p> 0.05). Bcl-treated Gad1−/− basket cells (6 cells and 45 pyramidal somata) show higher bouton density compared to untreated Gad1−/− cells (7 cells and 50 pyramidal somata, B1; One-way Anova, posthoc Dunn’s test p<0.05), but poor terminal branching similar to untreated Gad1−/− (B2; One-way Anova, posthoc Dunn’s test p>0.05). Control basket cells treated with Bcl (6 cells and 45 pyramidal somata) or DZ (7 cells and 50 pyramidal somata) show no significant changes in bouton density (B1) or terminal branching (B2, B3; Mann Whitney test, p>0.1; see also Sppl Fig 7).
We next examined whether the effects of GABA signaling on perisomatic innervation can be rescued by the extracellular activation of GABA receptor signaling. To test the role of GABAAreceptors, we included the GABAA agonist, diazepam (10 μM), in the culture medium when Gad1lox/lox slice cultures were transfected with PG67-GFP-ires-Cre between EP16-24. Diazepam is an allosteric modulator of GABAA receptors, which discriminates between activated and non-activated receptor states, and enhances the endogenous activity of GABA. Diazepam infusion in visual cortex enhances GABA transmission and accelerates the onset of the ocular dominance plasticity (Hensch et al 1998), but does not grossly alter neuronal response properties. While diazepam treatment of control basket cells did not affect the basic characteristics of perisomatic synapses (Figure 4B1; boutons/soma±SEM=10.1±0.8 for diazepam-treated vs 8.90.4 for untreated controls; Mann Whitney test, p>0.1; Sppl Fig 7A, E), diazepam-treated Gad1−/− basket cells showed much less pronounced defects in their perisomatic synapses compared to those of untreated mutant cells (Sppl Fig 7A, B, F). Both the complexity of terminal branches and the density of perisomatic synapses were rescued to levels comparable to control cells (Figure 4B1, 4B3; boutons/soma±SEM= 8.8±0.7 for diazepam-treated Gad1−/− vs. 8.9±0.4 for control basket cells; One-way Anova, posthoc Dunn’s test, p>0.05), although the size and shape of diazepam-treated boutons appeared notably more variable (Sppl Fig 7F).
The GABAB receptor agonist baclofen (15 μM) was less potent in rescuing the effects of GABA deficiency. Terminal branches of baclofen-treated Gad1−/− basket cells remained as underdeveloped as those of untreated Gad1−/− cells (Figure 4B2, One-way Anova, posthoc Dunn’s test p>0.05; Sppl Fig 7D). However, bouton density around pyramidal cell somata was significantly but not completely rescued (Figure 4B1, boutons/soma±SEM= 6.1±0.5 for baclofen-treated vs 2.7±0.4 for untreated Gad1−/− cells, and 8.9±0.4 for controls; One-way Anova, posthoc Dunn’s test, p<0.05; Sppl Fig 7D). This partial rescue may be due to the reduced terminal branching in baclofen-treated Gad1−/− basket cells because bouton density along the remaining terminal branches was similar to that of control axons, and significantly higher than that of untreated Gad1−/− basket cells (boutons/micron terminal branch= 0.36 for baclofen-treated Gad1−/− cells, 0.33 for control cells, and 0.16 for untreated Gad1−/− cells; One-way Anova, posthoc Dunn’s test, p<0.05). Baclofen treatment of control cells did not affect the basic characteristics of perisomatic synapses (Figure 4B1; boutons/soma ±SEM=9.6±0.8 for baclofen-treated vs 8.9±0.4 for untreated controls; Mann Whitney test, p>0.1; Sppl Fig 7C). These results suggest that GAD67-mediated GABA synthesis regulates basket cell axon growth and synapse formation through both GABAA and GABAB receptors, which may promote different aspects of perisomatic innervation.
GAD67-mediated GABA Synthesis Is Not Necessary for the Maintenance of Perisomatic Innervation
To examine whether GAD67-mediated GABA synthesis was continuously required to maintain the extensive basket cell axon arbors and perisomatic innervation, we inactivated both Gad1 alleles in basket interneurons from EP26-32, after the mature innervation pattern had been established (Chattopadhyaya et al., 2004). Comparison between Gad1lox/lox cultures transfected with PG67-GFP and PG67-GFP-ires-Cre showed no differences in overall axon morphology, terminal axon branching, bouton size or bouton number around pyramidal cell somata (Figure 2D, E, F, Sppl Fig 5; boutons/soma±SEM= 9.8±0.5 for Gad1−/− vs 10.2± 0.7 for control, Mann-Whitney test p>0.1), suggesting that GAD67-mediated GABA synthesis is not necessary for the structural maintenance of perisomatic innervation. It is possible that the increased synaptic localization and activity of GAD65 in the fourth postnatal week might produce sufficient GABA to maintain perisomatic innervation. Alternatively, the structural maintenance of mature perisomatic synapses may not require GABA signaling, at least in the relatively short period we examined.
GAD67-mediated GABA Synthesis Regulates the Innervation Field of Basket Interneurons
To examine the effect of GABA deficiency on the innervation field of basket cells, we locally reconstructed basket axon arbors within a radius of 300-μm from the basket cell soma along with all the pyramidal cell somata, from confocal image stacks. We then quantified basket cell axon density, interbranch intervals, and the fraction of innervated pyramidal cell somata (Figure 5). Compared to wild-type controls, Gad1−/− basket cells (EP16-24) showed a significant reduction of both axon density and percentage of targeted neuronal somata (56% and 47% reduction, respectively; Mann-Whitney test, p<0.01; Figure 5B). Because basket axons also innervate the proximal dendrites of pyramidal cells, which were not detected in our analysis, the fraction of innervated pyramidal cells might be somewhat underestimated. On the other hand, such underestimation, if it exists, should equally affect wildtype and Gad1−/− basket cells. Together with the significant reduction of axon density, these results suggest that GAD67-mediated GABA synthesis in a basket cell not only influences the number of its perisomatic synapses around individual target neurons, but also the number of targeted neurons within its axon arbor. GAD67-mediated GABA synthesis therefore may confer to basket interneurons a cell-wide mechanism in regulating their innervation field.
GAD67-mediated GABA Synthesis Regulates Perisomatic Innervation in Adolescent Visual Cortex
To examine whether GAD67-mediated GABA synthesis regulates the maturation of perisomatic synapses in adolescent visual cortex in vivo, we used a strain of adeno-associated virus expressing Cre and GFP (AAV-GFP-ires-Cre) to inactivate Gad1 in Gad1lox/lox mice (Figure 6A). When injected in visual cortex after P12, AAV selectively infected neurons but few astrocytes (Di Cristo & Huang, unpublished data). Two methods were used to demonstrate Cre-mediated recombination. First, AAV-GFP-ires-Cre was injected into the visual cortex of the floxed-Rosa26-lacZ reporter mice (Soriano, 1999). Recombination-activated lacZ expression was prominent 15 days post-infection, even in neurons in which GFP expression was below detection (Sppl Fig 8). Second, GABA levels in AAV-infected basket interneuron somata were quantified 7 days after injection at P13 in the visual cortex of Gad1lox/lox mice. In the uninjected hemisphere, 98% of the basket interneurons, identified by parvalbumin (Pv) immunoreactivity, also expressed GABA; while in the injected hemisphere only 32% of GFP positive basket interneurons showed detectable levels of GABA at the injection site (Figure 6B). Similar reduction of GABA levels in AAV-infected basket cells was found at P32 (data not shown). To examine the effect of reduced GAD67 and GABA levels on the development of perisomatic innervation, Gad1lox/lox mice and their wild-type littermates were infected at P13 and perfused at P32. Although AAV infected both glutamatergic and GABAergic neurons, perisomatic synapses from infected basket interneurons could be readily identified by the co-expression of GFP and parvalbumin (Figure 6C). In wild-type mice, GFP-labeled basket axon terminals surrounded NeuN-positive pyramidal cell somata with distinct perisomatic boutons at rather regular inter-bouton intervals (Figure 6C1). In Gad1lox/lox mice infected with AAV-GFP-ires-Cre, basket cell axon terminals appeared thinner and frequently had stretches without boutons around pyramidal cell somata (Figure 6C2). The most consistent and quantifiable effect was the significantly smaller size of perisomatic boutons (a 40% average reduction in bouton diamater; Mann Whitney test, p<0.001; Figure 6D), a result consistent with those obtained in organotypic cultures (Figure 2E).
Figure 6. Deficiency of GAD67-mediated GABA synthesis in basket interneurons affects perisomatic synapse maturation in primary visual cortex of adolescent mice.
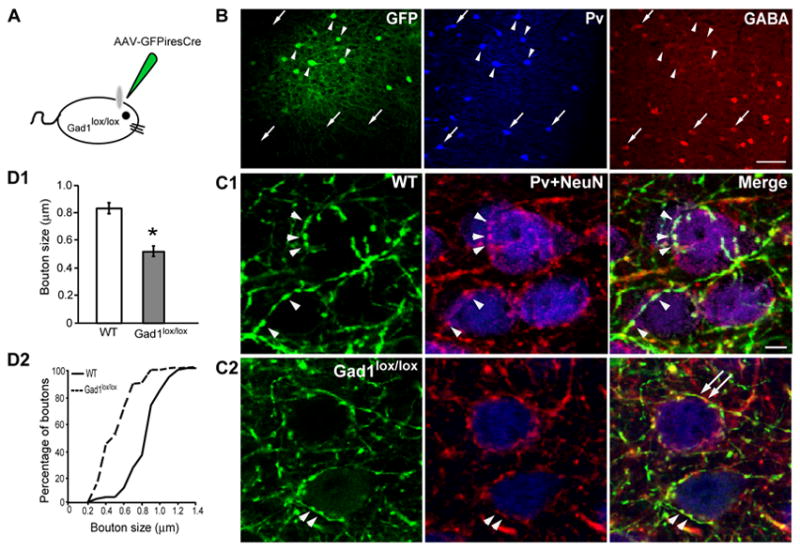
A, Visual cortex of Gad1lox/lox mice was injected with AAV-GFP-ires-Cre virus at P13 and analyzed at P20 or P32. B, Infected basket interneurons [arrowheads; blue: parvalbumin (Pv) immunostaining], show reduction of GABA immunostaining (red) 7 days after injection compared to neighboring untransfected basket cells (arrows). Scale bar: 50 μm. C, At P32, infected basket cell axons, identified by the colocalization of GFP (green) and Pv (red), have smaller perisomatic boutons (arrowheads) around pyramidal cell somata (NeuN, blue) in Gad1lox/lox mice (C2) as compared to those in wild-type mice (C1). Terminal branches from infected Gad1lox/lox basket cell axons frequently have large intervals without boutons (arrows). Scale bar: 5 μm. D1, Perisomatic boutons of infected basket cells from Gad1lox/lox mice (n=4) are significantly smaller compared to those from wild-type mice (n=4, Mann-Whitney test, p<0.001). Bars represent average ± SEM from 120 boutons in each genotype. D2, Cumulative distribution of perisomatic bouton size from infected basket cells in Gad1lox/lox mice is shifted towards lower values compared to those from wild type mice (K-S test, p<0.001).
Deficient Perisomatic GABAergic Innervation in the Visual Cortex of Gad1+/− mice
In germ-line Gad1+/− mice, significant GABA deficiency persists in embryonic, neonatal, and adult brain (Asada et al., 1997; Ji et al., 1999), yet no overt anatomical and behavioral deficits have been detected. The generation (Tamamaki et al., 2003), tangential migration (Tanaka et al., 2003), and differentiation (Tamamaki et al., 2003) of different classes of GABAergic neurons all appear normal, suggesting that these earlier developmental processes can proceed with significant reduction of GABA levels. However, our results from the conditional inactivation of one Gad1 allele in basket cells (Gad1+/−cells) in organotypic cultures indicate that the development of perisomatic inhibitory synapses is particularly sensitive to Gad1 dosage (Figure 2). We therefore examined perisomatic innervation in Gad1+/− mice, first by labeling GABAergic synapses using GAD65 immunofluorescence. GAD65 is highly restricted to axon terminals and boutons in mature cortex and has been used as a reliable presynaptic marker for GABAergic synapses (Huang et al., 1999). We focused our analysis on perisomatic synapses surrounding neuronal somata, which are largely derived from the parvalbumin-containing basket interneurons (Pv, Figure 7E). In visual cortex of postnatal day 28 (P28) wild-type mice, GAD65 immunofluorescence around neuronal somata appears as distinct puncta, which form highly characteristic ring-like structures (Figure 7A; (Huang et al., 1999). In visual cortex of P28 Gad1+/− mice, on the other hand, the number of GAD65 puncta around single neuronal somata was reduced by 36% compared to wild-type littermates (Figure 7C, Mann Whitney test, p<0.05), and GAD65 puncta often did not form ring-like structures (compare right and left panels of Figures 7A and 7B). This reduction of perisomatic GAD65 puncta was not due to an overall decrease of GAD65 expression in Gad1+/− mice because quantification of GAD65 protein levels in visual cortex by western blotting showed no difference between Gad1+/− and Gad1+/+ mice (Figure 7D; n=3 for each genotype, t-test p>0.05). On the other hand, GAD67 protein level was reduced by 40% in Gad1+/− visual cortex (Figure 7D, t-test p<0.05), a result consistent with previous reports (e.g. (Asada et al., 1997). Together, these results suggest that perisomatic GABAergic innervation, largely derived from presynaptic boutons of parvalbumin-positive basket interneurons (Figure 7E), is significantly reduced in visual cortex of Gad1+/− mice.
Figure 7. Perisomatic GAD65-positive bouton density is reduced in germ-line Gad1+/− mice A, B.
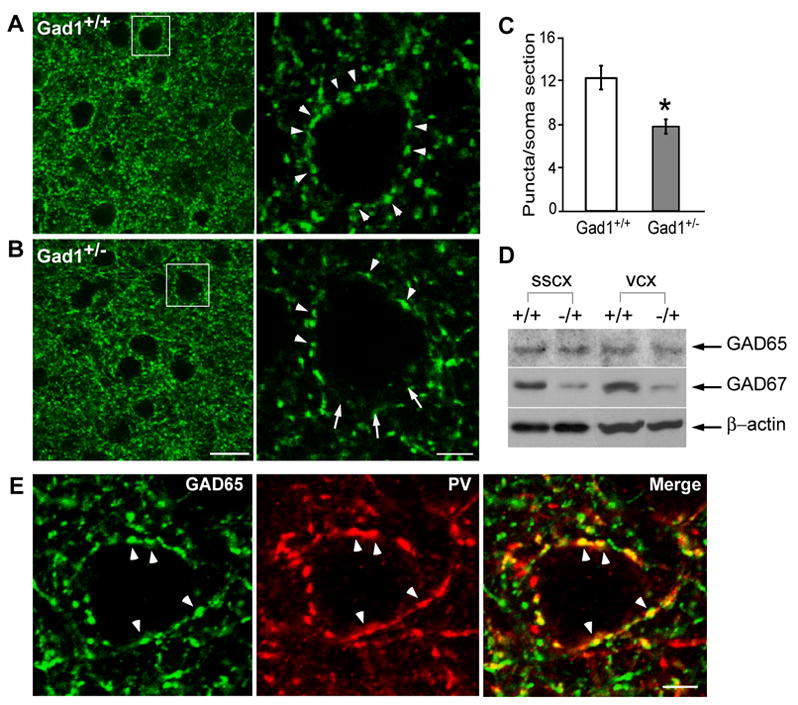
Low magnification images in left panels show similar levels of GAD65 expression (green) in P28 visual cortex of a Gad1+/− mouse and its Gad1+/+ littermate. Right panels are from boxed regions. GAD65 puncta always form distinct ring-like structures around neuronal somata in Gad1+/+ mice (arrowheads) but often not in Gad1+/− mice, Scale bar: left panels, 20 μm; right panels, 5 μm. C, The density of perisomatic GAD65 puncta was reduced in Gad1+/−mice (n=8) compared to Gad1+/+ (n=5) littermates (t-test, p<0.01; data from two different litters were not significantly different and were pooled). D, Western blot analysis of somatosensory (SSCX) and visual (VCX) cortex using GAD65, GAD67, and β-actin antibodies showed that GAD65 levels were very similar in Gad1+/− (+/−) compared to their Gad1+/+ (+/+) littermates. Note that GAD67 levels were reduced by 40% in Gad1+/− samples (see text). E, Perisomatic GAD65 positive puncta (green) colocalize with Pv immunostaining (red), suggesting that these puncta represent presynaptic boutons of basket interneurons. Scale bar: 5 μm.
We further analyzed perisomatic synapses formed by single basket cells in organotypic cultures of Gad1+/− mice. At EP27, when perisomatic innervation was largely mature (Chattopadhyaya et al., 2004), basket axons achieved highly exuberant innervation pattern characterized by extensive local axon branches innervating hundreds of pyramidal cells (Figure 8A). In age-matched basket cells from Gad1+/− cultures, the shape and gross branching patterns of axon arbors appeared similar to that of control cells (Figure 8B1); however, these cells showed significant reduction in the number of terminal branches (Figures 8B3, C3; Mann Whitney test p<0.05), and the density and size of boutons around pyramidal cell somata (Figures 8B3, C1, C2; boutons/soma±SEM= 4.2±0.2 for basket cells from Gad1+/− cultures vs 9.8±0.3 for basket cells from Gad1+/+ littermates, Mann-Whitney test p<0.001). These defects are consistent with the reduction of perisomatic GAD65 puncta in the visual cortex of Gad1+/− mice. Therefore, although the development of many aspects of GABAergic circuits can proceed with reduced GABA levels, the maturation of GABAergic innervation patterns, such as perisomatic innervation by parvalbumin-positive basket interneurons, is exquisitely sensitive to GAD67-mediated GABA synthesis.
Figure 8. Perisomatic innervation is deficient in Gad1+/−but not Gad2−/− cortical organotypic cultures.
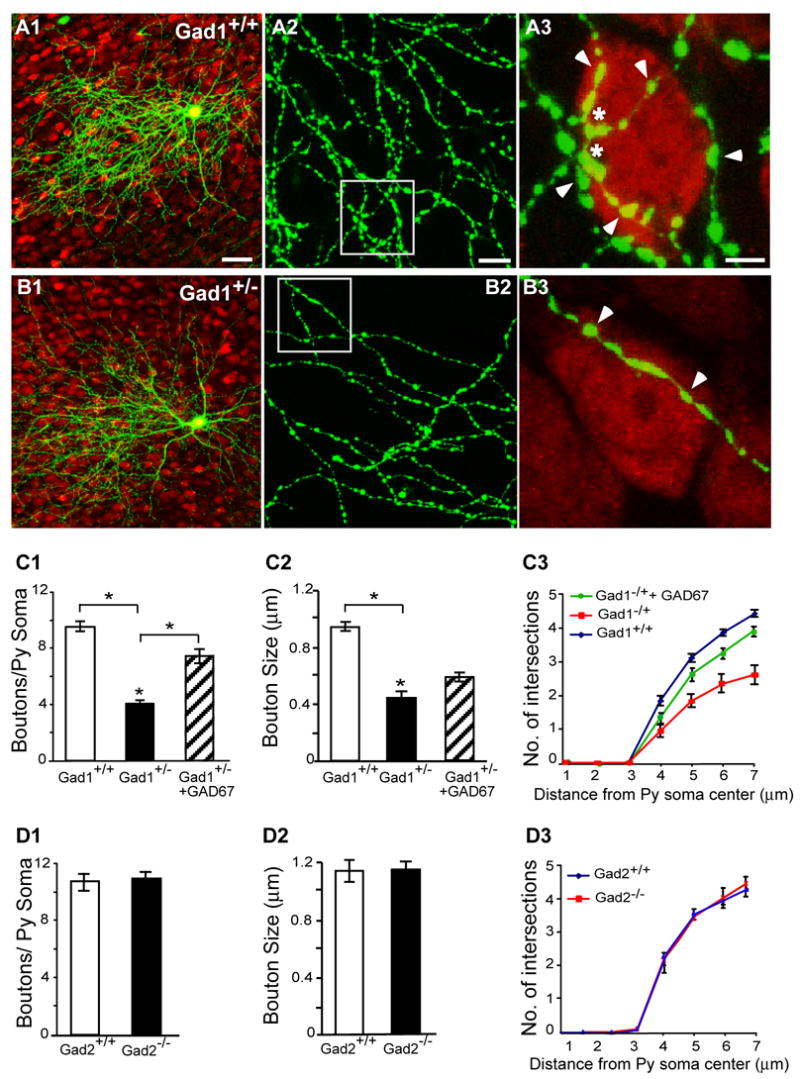
A, B, At EP27, basket interneurons in organotypic cultures from Gad1+/− mice were not different from those of wild-type littermates in their axon morphology and size (A1-B1), but show reduced axon branching (A2-B2), terminal branches, synapse size and density (A3-B3, red: NeuN immunostaining). A3 and B3 are from boxed region in A2, B2. Arrowheads: boutons, asterisk: terminal branching. Scale bar: A1, B1: 50 μm; A2, B2: 10 μm; A3, B3: 5 μm. C, bouton density (C1), size (C2) and terminal branching complexity (C3) are significantly reduced in basket cells from Gad1+/− mice (6 basket cells and 50 pyramidal somata) compared to aged-matched control littermates (6 basket cells and 60 pyramidal somata; Mann-Whitney U test p<0.001). Transfection with P67-GAD67 from EP16-24 increased bouton density (C1) and terminal branching (C3) of basket cells from Gad1+/− mice (6 basket cells and 57 pyramidal somata) compared to basket cells transfected with PG67-GFP alone (Mann-Whitney U test p<0.01). D, basket cells from GAD65-null mice are not significantly different from those of wild type littermate in their bouton density (D1), size (D2) and axon terminal branching complexity (D3) (Mann Whitney U test, p>0.1; Gad2−/− mice: 7 basket cells and 50 pyramidal somata; control littermates: 7 basket cells and 50 pyramidal somata; also see Sppl Fig 9).
Interestingly, the deficits in perisomatic synapses in single Gad1+/− basket cells from EP16 were very similar to those in basket cells from germ-line Gad1+/− mice. Since a single pyramidal neuron receives perisomatic innervation from multiple basket interneurons, this result implies that the effects of GAD67 deficiency on Gad1+/− basket cells are cell autonomous and are unlikely due to a loss of competition in GABA-mediated signaling with neighboring Gad1+/+ interneurons. To further support our interpretation, we examined whether an overexpression of GAD67 in Gad1+/− cells could rescue perisomatic innervation. Basket interneurons in cortical organotypic cultures from Gad1+/− mice were transfected with either PG67-GAD67/PG67-GFP or with PG67-GFP. Basket cells transfected with PG67-GAD67 shows significantly higher bouton density (Figure 8C1; boutons/soma±SEM = 6.92±0.2 for Gad1+/− + GAD67 vs 4.0±0.2 for Gad1+/− controls, One-way Anova, posthoc Dunn’s test, p<0.05) and terminal branch complexity (Figure 8C3; One-way Anova, posthoc Dunn’s test, p<0.05) as compared to Gad1+/− controls. These results further support the hypothesis that the maturation of perisomatic GABAergic innervation in neocortex is sensitive to GAD67-mediated GABA synthesis.
Normal Perisomatic Innervation in the Visual Cortex of Gad2−/− Mice
GAD65 significantly contributes to GABA levels in the adolescent mouse visual cortex (P18-19) (Hensch et al., 1998) but not in adult cortex (Asada et al., 1996; Kash et al., 1997). Gad2−/− mice have no discernable abnormalities in cortical morphology, but show defects in cortical plasticity (Hensch et al., 1998) and, in contrast to Gad1+/− mice, are prone to seizure (Kash et al., 1997). To examine whether GAD65 might also play a role in the maturation of perisomatic innervation, we transfected slice cultures from Gad2−/− mice and their wild-type littermates with PG67-GFP and examined single basket cells at EP27. Basket cell axon branching and perisomatic synapses were indistinguishable between these two genotypes, suggesting that GAD65-mediated GABA synthesis does not play a major role in the maturation of perisomatic innervation (Figure 8D, Sppl Fig 9; boutons/soma±SEM =11.5±0.5 for Gad2−/− vs 11.0± 0.7 for control Gad2+/+; Mann Whitney test, p>0.1). The lack of a deficit in perisomatic innervation in Gad2−/− mice is unlikely due to compensation by GAD67-mediated GABA synthesis because the deficit in ocular dominance plasticity in these mice cannot be compensated and persists into adulthood. It remains possible that GAD65 might regulate more “subtle” aspect, such as the rate or stability of perisomatic synapse formation. Altogether, our results suggest that GAD67-mediated GABA synthesis plays a major role in the development of perisomatic innervation.
Discussion
First discovered as a principal inhibitory neurotransmitter, GABA has since been implicated in multiple aspects of neural development (Owens and Kriegstein, 2002b). The early trophic role of GABA in cell proliferation (LoTurco et al., 1995), migration (Behar et al., 2000), neurite growth (Spoerri, 1988), and synapse formation (Wolff et al., 1978) can largely be explained by its depolarizing action in the embryonic and neonatal brain (Ben-Ari, 2002). Here we demonstrate yet another facet of GABA function in regulating inhibitory synaptic innervation in the adolescent brain, when GABA is thought to have assumed its classic role as an inhibitory transmitter. Our analysis of germ-line Gad1+/− mice revealed that different aspects of GABAergic development are differentially sensitive to GABA levels. Although earlier steps of GABAergic development, such as cell migration (Tanaka et al., 2003) and differentiation (Tamamaki et al., 2003), proceed normally, the maturation of perisomatic synapses in the adolescent visual cortex is significantly deficient, suggesting a more stringent requirement for GABA signaling in shaping the fine architecture of GABAergic circuits. In addition, we show that GABA synthesis by GAD67, but not GAD65, regulates interneuron axon branching and synapse formation during the maturation of inhibitory innervation. Since intracellular GABA levels (Sppl. Fig 1; (Benevento et al., 1995; Micheva and Beaulieu, 1995;Rutherford et al., 1997) and its rate-limiting enzyme GAD67 (Liang et al., 1996; Patz et al., 2003) are regulated by neuronal activity, our results implicate GAD67-mediated GABA synthesis as an activity-dependent mechanism to sculpt inhibitory innervation patterns.
GABA Signaling Regulates Interneuron Axonal Morphogenesis
The mechanism by which GABA regulates perisomatic innervation is probably through the activation of extracellular GABA receptors. Although GABA synthesis is related to the oxidative metabolism of carbohydrates as an alternative energy source, this GABA shunt is of subtle metabolic significance (Soghomonian and Martin, 1998). A minor disturbance of the GABA shunt is unlikely to impact the development of GABAergic neurons and perisomatic innervation because: 1) many aspects of GABAergic development proceed normally in germ line Gad1+/− mice with ~35% decrease of GABA levels in the brain (Tamamaki et al., 2003; Tanaka et al., 2003); 2) abnormalities in perisomatic boutons of GABA-deficient basket cells can be partially rescued by an inhibitor of the GABA transporter (GAT-1) and by agonists of GABA receptors (Figure 4). Together, our results suggest that in addition to mediating inhibitory transmission, GABA signaling through both GABAA and GABAB receptors also regulate GABAergic axonal and synaptic morphogenesis during the maturation of inhibitory circuits.
GABA is known to activate presynaptic GABAB autoreceptors, which modulate Ca2+ channels and GABA release (Dunlap and Fischbach, 1981; Gonchar et al., 2001). Such cell autonomous regulation of presynaptic Ca2+ dynamics in developing interneurons might influence growth cone motility and bouton stability (Henley and Poo, 2004). On the other hand, GABA signaling through postsynaptic or glial (Kang et al., 1998; Nilsson et al., 1993) GABAA and GABAB receptors may trigger the release of retrograde factors, which in turn promote axon branching and synapse formation. Such transmitter-mediated neuronal morphogenesis has been well demonstrated at glutamatergic synapses, where glutamate receptor signaling coordinates both pre- and postsynaptic development and plasticity (De Paola et al., 2003; Tashiro et al., 2003).
GABAA and GABAB receptors are localized not only at synaptic junctions, but also at extrasynaptic, and non-synaptic sites (Mody and Pearce, 2004). In addition, the subunit composition of GABA receptors is developmentally regulated (Laurie et al., 1992), likely in a cell type and subcellular location-dependent manner, which may confer different mode of signaling (Owens and Kriegstein, 2002b). It is therefore possible that GABA receptors involved in regulating synaptic morphogenesis in the adolescent circuits may have different subunit composition, subcellular localization, or signaling properties compared to those involved in transmission at mature synapses.
Distinct Mode of GABA Synthesis by GAD67 and GAD65 Confer Different Signaling and Function
GAD67 and GAD65 show striking differences in subcellular localization, enzyme activity, and gene regulation, and likely contribute to distinct as well as overlaping pools of GABA synthesis (Soghomonian and Martin, 1998). GAD67 is widely distributed in synapses, axons, and dendrites while GAD65 is highly concentrated in nerve terminals (Dupuy and Houser, 1996) and directly associates with the vesicular GABA transporter (vGAT) (Jin et al., 2003). Although both isoforms require the cofactor pyridoxal 5′ phosphate (PLP) for their enzymatic activity, most GAD67 exists as a constitutively active holoenzyme (PLP-bound) and is responsible for basal GABA synthesis; whereas GAD65 is primarily present as an inactive apoenzyme (PLP-free), which can be activated by influx of PLP especially during enhanced synaptic activity (Battaglioli et al., 2003). Here we demonstrate a clear functional divergence between GAD67 and GAD65: although GABA contents were significantly reduced in the adolescent cerebral cortex of both GAD65-knockout mice (Hensch et al., 1998) and GAD67-heterozygote mice (Asada et al., 1997), perisomatic innervation in visual cortex was deficient only in GAD67 heterozygotes but not in GAD65 knockouts. This result suggests that GAD67-mediated GABA synthesis is specifically involved in regulating GABAergic axonal and synaptic morphogenesis.
The effect of GABA on basket cell axon terminal branching is likely mediated by GABA signaling prior to the formation of mature synapses. Indeed, GABA release has been detected from growth cones of developing axons, prior to synaptic contact (Gao and van den Pol, 2000). Since GAD65 is specifically concentrated in more mature nerve terminals, a GAD67-synthesized GABA pool may be involved in release from axonal sites and growth cones, and influences filopodia dynamics, and subsequent synapse formation and maturation. GABA can be secreted by action potential-evoked release, spontaneous release, or non-vesicular release. A recent study in dissociated hippocampal culture reports that reduction of spiking in single GABAergic neurons does not seems to decrease synaptic output, although spiking activity was reduced at neonatal stage when GABA was still depolarizing, and different classes of GABAergic synapses were not distinguished (Hartman et al., 2006). It is possible that spontaneous and/or non-vesicular release (e.g. reversal of a GABA transporter) (Demarque et al., 2002; Owens and Kriegstein, 2002a) also contribute to GABA regulation of axonal growth.
Activity-regulated GABA Synthesis as a Cell-wide Mechanism in Shaping Inhibitory Innervation Patterns
The role of excitatory transmitters in regulating synapse development has been well established, for example, at glutamatergic synapses in the CNS (Malinow and Malenka, 2002) and at the neuromuscular junction (Brandon et al., 2003; Misgeld et al., 2002). Our studies demonstrate an interesting parallel at the inhibitory connections, where GABA signaling likely plays an important role in regulating inhibitory axon and synapse development. Much remains to be explored regarding the mechanisms linking neural activity to GABAergic axonal and synaptic morphogenesis. We have shown that TTX treatment in slice cultures retards the maturation of perisomatic innervation (Chattopadhyaya et al., 2004). Activity reduction in pyramidal neurons may decrease the production and signaling of trophic factors, such as BDNF, which promote GABAergic synapse formation (Huang et al., 1999; Rutherford et al., 1997). Our current data shows that activity deprivation decreases GABA levels, likely through down-regulation of GAD67 levels and/or enzyme activity, and may lead to reduced GABA signaling, and deficits in basket cell axons and synapses.
A major focus in studying activity-regulated synapse development has been on the mechanisms by which neurotransmitters direct local morphogenesis at or near the site of synaptic contact. Our discovery of a crucial role for GAD67-mediated GABA synthesis in the maturation of perisomatic innervation implies an additional, cell-wide mechanism in activity-dependent regulation of inhibitory connections. Unlike glutamate, which is both the product and precursor in multiple biogenic processes, GABA can only be synthesized from glutamate by the two GADs (Soghomonian and Martin, 1998). In most brain regions, GAD67 is the rate-limiting enzyme and is produced at a limiting level (Asada et al., 1997); thus alterations in GAD67 levels and enzyme activity may readily influence cellular and vesicular GABA contents (Engel et al., 2001; Murphy et al., 1998). A major step in the physiological control of GAD67 activity is Gad1 transcription (Pinal and Tobin, 1998; Soghomonian and Martin, 1998), which is dynamically regulated during development (Kiser et al., 1998), by neural activity (Patz et al., 2003) and experience (Benevento et al., 1995; Benson et al., 1989; Gierdalski et al., 2001; Kobori and Dash, 2006; Liang et al., 1996). GAD67 activity may also be regulated by post-translational mechanisms (Rimvall and Martin, 1994; Wei et al., 2004). Activity-dependent regulation of GAD67 may thus result in “on-line” adjustment of intracellular GABA pool for release. Here we show that alterations in GAD67 and GABA levels profoundly influence interneuron axon growth and synapse formation during the development of inhibitory circuits. Therefore, neuronal activity may shape the pattern of inhibitory synaptic innervation through GAD67-mediated GABA synthesis and signaling. Such activity-dependent and likely cell-wide regulation of a “transmitter resource” suggests a novel logic for the maturation and plasticity of GABAergic synapses and circuits.
GAD67 and GABA deficiency have been implicated in a variety of neurodevelopmental disorders. Reduced Gad1 mRNA expression in the dorsal lateral prefrontal cortex (DLPFC) is one of the most consistent molecular pathology in individuals with schizophrenia (Lewis et al., 2005); and SNPs in the 5′ regulatory regions of Gad1 are associated with childhood-onset schizophrenia (Addington et al., 2005). In addition, the methyl-CpG-binding protein 2 (MeCP2), implicated in the Rett syndrome of the autism spectrum disorders, has been shown to repress the promoter region of Dlx5 (Horike et al., 2005), a homeodomain transcription factor which stimulates Gad1 expression (Stuhmer et al., 2002). Our results suggest that GAD67 deficiency may perturb the maturation and plasticity of specific classes of inhibitory synapses and innervation patterns, and contributes to aberrant circuit connectivity and function in neurodevelopmental disorders.
Experimental Procedures
Mutant Mice and DNA Constructs
To generate Gad1lox/lox mice, exon 2 (the first coding exon) of the Gad1 gene was flanked by loxP sites using gene targeting in ES cells. Splicing from exon 1 to 3 is predicted to generate a frame-shift mutation (Sppl Fig 1). After removing the Sv-NeoR selectable gene by the action of FLP recombinase, the resulting Gad1lox/+ mice were bred with each other to generate Gad1lox/lox mice, which were phenotypically normal. Some of these mice were bred with Mox2-Cre mice to delete exon 2 in the germline (Gad1D/+). Gad1D/+ mice were bred with each other, but no mice survived beyond the perinatal period, as was observed for with Gad1-null mice (Asada et al., 1997). Gad2−/− mice (Kash et al., 1997) and floxed-Rosa26-LacZ mice (Soriano, 1999) were obtained from Jackson Laboratory. PG67-GFP was generated by subcloning of a 10 kb region of Gad1 gene promoter by gap repair in front of the GFP coding region in pEGFP (Clontech) as described (Chattopadhyaya et al., 2004). The EGFP coding region was substituted with DNA fragment containing GFP-ires-Cre (a gift of Dr. Guoping Feng) to generate PG67- GFP-ires-Cre. . PG67-GAD67 was generated by cloning a full length rat GAD67 cDNA (gift from Alan Tobin) into the PG67 construct. PG67-tdtomato and PG67-synaptophysin-GFP were generated by cloning cDNAs coding for tdtomato and synaptophysin-GFP into the PG67 construct, respectively.
Analysis of Perisomatic Innervation in Organotypic Slices
Slice culture and biolistic transfection
Slice culture preparation has been described elsewhere (Chattopadhyaya et al., 2004). Constructs were transfected using Gene Gun system (BioRad). For pharmacological experiments, 1 μM of TTX(Sigma), 10 μM of diazepam (Tocris), 15 μM of baclofen (Tocris) or 10 μM of NO711 (Sigma) were added to the culture medium during the specified time window.
Immunohistochemistry, imaging and analysis
Slices were fixed, freeze-thawed and immunostained as described (Chattopadhyaya et al., 2004). Primary antibodies were used at following concentrations: NeuN (mouse monoclonal, 1:400, Chemicon), Parvalbumin (rabbit polyclonal, 1:1000, Sigma), GABA (rabbit polyclonal, 1:1000, Sigma). Confocal imaging and data analysis were performed as described (Chattopadhyaya et al., 2004). For further details and EM analysis see Sppl Methods.
Analysis of Local Axon Arbor and Innervation Field of Basket Interneurons
See Sppl Methods.
Analysis of Perisomatic Innervation in Visual Cortex of Gad1 lox/lox Mice Injected with AAV-GFP-ires-Cre
Virus construction and injection
The GFP-ires-Cre cassette was cloned into the AAV vector pCMV-MCS (Stratagene), and viruses were prepared by VIRAPUR (San Diego, CA) at 1012 virus particles/ml. The AAV-GFP virus was a gift from Dr. Brian Kaspar (Salk Institute). Both constructs were under the control of the CMV promoter. For cortical injections, P13 Gad1lox/lox or wild-type pups were anesthetized with ketamine 0.56 mg/g; xylazine, 0.03 mg/g body weight. After incision of the skin overlying the skull, a small hole was made directly over the left hemisphere in the visual cortex. A micropipette attached to a Picospritzer was inserted to a depth of 0.5 mm below the pia. A total of 1.0 μl of the concentrated AAV suspension was injected in at least 4 different sites with 15 psi at a frequency of 0.3 Hz. Data analysis: Confocal imaging and analysis of perisomatic innervation were performed as described (Chattopadhyaya et al., 2004). For details see Sppl.Methods.
Analysis of Perisomatic Innervation in Visual Cortex of Germ-line Gad1 +/− Mice
See Sppl.Methods.
Western Blotting
See Sppl.Methods.
Immuno Electron Microscopy
See Sppl.Methods.
Supplementary Material
Acknowledgments
We thank Priscilla Wu for technical assistance and Drs. Rachel Wong and Holly Cline for critical reading of the manuscript. This work was supported by NIH-RO1 EY 13564-01 to ZJH. GDC has a NARSAD Young Investigator Award founded by the Forrest C. Lattner Foundation and a EMBO Long Term Fellowship. ZJH is a Pew and McKnight Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglioli G, Liu H, Martin DL. Kinetic differences between the isoforms of glutamate decarboxylase: implications for the regulation of GABA synthesis. J Neurochem. 2003;86:879–887. doi: 10.1046/j.1471-4159.2003.01910.x. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS, Port JD. gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Benson DL, Isackson PJ, Hendry SH, Jones EG. Expression of glutamic acid decarboxylase mRNA in normal and monocularly deprived cat visual cortex. Brain Res Mol Brain Res. 1989;5:279–287. doi: 10.1016/0169-328x(89)90062-4. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Lin W, D’Amour KA, Pizzo DP, Dominguez B, Sugiura Y, Thode S, Ko CP, Thal LJ, Gage FH, Lee KF. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J Neurosci. 2003;23:539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol . 1996;494(Pt 2):451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CS, Jensen K, Sokolova I, Wang D, Li M, Deshpande P, Davidson N, Mody I, Quick MW, Quake SR, Lester HA. Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1-green fluorescent protein fusions. J Neurosci. 2002;22:10251–10266. doi: 10.1523/JNEUROSCI.22-23-10251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Arber S, Caroni P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci. 2003;6:491–500. doi: 10.1038/nn1046. [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy ST, Houser CR. Prominent expression of two forms of glutamate decarboxylase in the embryonic and early postnatal rat hippocampal formation. J Neurosci. 1996;16:6919–6932. doi: 10.1523/JNEUROSCI.16-21-06919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria D, Albus K. Activity-dependent development of spontaneous bioelectric activity in organotypic cultures of rat occipital cortex. Brain Res Dev Brain Res. 2000;123:151–164. doi: 10.1016/s0165-3806(00)00089-4. [DOI] [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A. Plasticity of rat central inhibitory synapses through GABA metabolism. J Physiol. 2001;535:473–482. doi: 10.1111/j.1469-7793.2001.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA release from mouse axonal growth cones. J Physiol . 2000;523(Pt 3):629–637. doi: 10.1111/j.1469-7793.2000.t01-1-00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M. Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex. 2001;11:806–815. doi: 10.1093/cercor/11.9.806. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Pang L, Malitschek B, Bettler B, Burkhalter A. Subcellular localization of GABA(B) receptor subunits in rat visual cortex. J Comp Neurol. 2001;431:182–197. doi: 10.1002/1096-9861(20010305)431:2<182::aid-cne1064>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet. 2005;37:31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Toyoda H, Yamada J, Okabe A, Sato K, Hotta Y, Fukuda A. Differential development of cation-chloride cotransporters and Cl-homeostasis contributes to differential GABAergic actions between developing rat visual cortex and dorsal lateral geniculate nucleus. Brain Res. 2003;984:149–159. doi: 10.1016/s0006-8993(03)03126-3. [DOI] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Ji F, Kanbara N, Obata K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci Res. 1999;33:187–194. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PJ, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- Klostermann O, Wahle P. Patterns of spontaneous activity and morphology of interneuron types in organotypic cortex and thalamus-cortex cultures. Neuroscience. 1999;92:1243–1259. doi: 10.1016/s0306-4522(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Kobori N, Dash PK. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J Neurosci. 2006;26:4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liang F, Isackson PJ, Jones EG. Stimulus-dependent, reciprocal up- and downregulation of glutamic acid decarboxylase and Ca2+/calmodulin-dependent protein kinase II gene expression in rat cerebral cortex. Exp Brain Res. 1996;110:163–174. doi: 10.1007/BF00228548. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci U S A. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Maturation of rat visual cortex. III. Postnatal morphogenesis and synaptogenesis of local circuit neurons. Brain Res. 1986;390:271–285. doi: 10.1016/s0006-8993(86)80236-0. [DOI] [PubMed] [Google Scholar]
- Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR, Greenberg ME. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Eriksson PS, Ronnback L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993;54:605–614. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Jones MV, Westbrook GL. Slow desensitization regulates the availability of synaptic GABA(A) receptors. J Neurosci. 2000;20:7914–7921. doi: 10.1523/JNEUROSCI.20-21-07914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002a;36:989–991. doi: 10.1016/s0896-6273(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002b;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P. Neuronal activity and neurotrophic factors regulate GAD-65/67 mRNA and protein expression in organotypic cultures of rat visual cortex. Eur J Neurosci. 2003;18:1–12. doi: 10.1046/j.1460-9568.2003.02702.x. [DOI] [PubMed] [Google Scholar]
- Pinal CS, Tobin AJ. Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol. 1998;5:109–118. [PubMed] [Google Scholar]
- Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spoerri PE. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse. 1988;2:11–22. doi: 10.1002/syn.890020104. [DOI] [PubMed] [Google Scholar]
- Stuhmer T, Anderson SA, Ekker M, Rubenstein JL. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 2002;129:245–252. doi: 10.1242/dev.129.1.245. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol. 1997;500:715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2003;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron. 2003;38:773–784. doi: 10.1016/s0896-6273(03)00299-x. [DOI] [PubMed] [Google Scholar]
- Wei J, Davis KM, Wu H, Wu JY. Protein phosphorylation of human brain glutamic acid decarboxylase (GAD)65 and GAD67 and its physiological implications. Biochemistry. 2004;43:6182–6189. doi: 10.1021/bi0496992. [DOI] [PubMed] [Google Scholar]
- Wolff JR, Joo F, Dames W. Plasticity in dendrites shown by continuous GABA administration in superior cervical ganglion of adult rat. Nature. 1978;274:72–74. doi: 10.1038/274072a0. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci . 2001;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


