Abstract
Background
NOGO Receptor 1 (RTN4R) regulates axonal growth, as well as axon regeneration after injury. The gene maps to the 22q11.2 schizophrenia susceptibility locus and is thus a strong functional and positional candidate gene.
Methodology/Principal Findings
We evaluate evidence for genetic association between common RTN4R polymorphisms and schizophrenia in a large family sample of Afrikaner origin and screen the exonic sequence of RTN4R for rare variants in an independent sample from the U.S. We also employ animal model studies to assay a panel of schizophrenia-related behavioral tasks in an Rtn4r-deficient mouse model. We found weak sex-specific evidence for association between common RTN4R polymorphisms and schizophrenia in the Afrikaner patients. In the U.S. sample, we identified two novel non-conservative RTN4R coding variants in two patients with schizophrenia that were absent in 600 control chromosomes. In our complementary mouse model studies, we identified a haploinsufficient effect of Rtn4r on locomotor activity, but normal performance in schizophrenia-related behavioral tasks. We also provide evidence that Rtn4r deficiency can modulate the long-term behavioral effects of transient postnatal N-methyl-D-aspartate (NMDA) receptor hypofunction.
Conclusions
Our results do not support a major role of RTN4R in susceptibility to schizophrenia or the cognitive and behavioral deficits observed in individuals with 22q11 microdeletions. However, they suggest that RTN4R may modulate the genetic risk or clinical expression of schizophrenia in a subset of patients and identify additional studies that will be necessary to clarify the role of RTN4R in psychiatric phenotypes. In addition, our results raise interesting issues about evaluating the significance of rare genetic variants in disease and their role in causation.
Introduction
Axonal growth and regeneration is restricted during the maturation of the central nervous system (CNS) as well as after injury, and several lines of evidence indicate that myelin-associated proteins play a critical role in these processes. Early seminal studies by David and Aguayo, 1981 [Ref. 1] demonstrated that the CNS environment contains inhibitory factors which limit growth, and a number of myelin-associated growth inhibitory factors have since been identified, including oligodendrocyte-myelin glycoprotein (OMgp), myelin-associated glycoprotein (MAG), Nogo-A (RTN4), and most recently, Ephrin-B3. Notably, despite their distinct molecular structures, OMgp, MAG, and RTN4 all share a common receptor, Nogo Receptor 1 (RTN4R) [2]–[4]. RTN4R, a glycosylphosphatidylinositol-linked (GPI-linked) cell surface molecule, forms a heteromeric receptor complex with either LINGO-1 and p75, or LINGO-1 and TROY (a tumor necrosis factor (TNF) receptor family member). Activation of RTN4R initiates a cascade that leads to the activation of RhoA and, ultimately, the inhibition of axonal growth [5]–[7]. Given that it acts as a convergence point for three separate factors, which inhibit neurite outgrowth and regeneration, RTN4R has generated much interest as a target for therapeutic intervention following CNS injury [2]–[4], [8].
The human RTN4R gene is located within the 22q11.2 locus where relatively common hemizygous microdeletions occur at a frequency of 1 in 5000 live births [9]. The majority of these deletions are de novo events and occur on different haplotype backgrounds [10]. The physical phenotype of the 22q11.2 deletion is broad and variable and most frequently includes congenital heart defects, velopharyngeal defects, and thymic hypoplasia. In addition, most patients display a pattern of cognitive impairments and behavioral deficits, including deficits in working memory, conflict monitoring, visuospatial short-term memory, and executive visual attention [11]–[14].
In addition, a range of neuroanatomical abnormalities such as reduced total brain volume, enlarged ventricles, and white matter abnormalities [15], [16] has been described in some 22q11.2 microdeletion carriers. Both syndromic and non-syndromic patients with the deletion also show extremely high frequencies of psychiatric illness, especially schizophrenia: children with the deletion are 25–30 times more likely to develop schizophrenia or schizoaffective disorder by early adulthood [17], [18], and 22q11.2 microdeletions account for ∼2% of schizophrenia cases in Caucasian populations [19]. Children with this microdeletion are also reported to have impaired sensorimotor gating [20], which is considered an endophenotype of several psychiatric disorders including schizophrenia, as well as a high incidence of emotional problems including anxiety, depression, social withdrawal, and obsessive-compulsive behaviors [13].
The possibility that RTN4R deficiency contributes to the psychiatric symptoms associated with the 22q11.2 microdeletion, in particular, is intriguing and supported by some preliminary human genetic and gene expression studies. Although, collectively, the human genetic and animal model studies designed to identify schizophrenia susceptibility genes from the 22q11.2 region have implicated primarily three genes: proline dehydrogenase (PRODH), ZDHHC8 and catechol-O-methyltransferase (COMT) [21]–[25] and their interactions [26], [27], a study by Liu et al. [22] presented suggestive evidence that common variants located at the 3′ end of the RTN4R gene are associated with schizophrenia in patient samples from the U.S. and South Africa. There has been one attempt to replicate these initial findings in the Han Chinese population, with negative results [28]. However, two preliminary reports described sex-specific associations between schizophrenia and common variants in the RTN4R gene [29], as well as its ligand RTN4 [30]. In addition, Sinibaldi et al. [31] reported two rare non-conservative sequence variants in the RTN4R gene in an Italian sample of 120 schizophrenia patients that were absent in a sample of 200 controls. Moreover, alterations in the levels of RTN4R or two RTN4R ligands have been described in postmortem analyses of brains from individuals with schizophrenia. Specifically, microarray expression studies and single-gene quantitative RT-PCR studies indicate a down-regulation of MAG in at least some schizophrenia cohorts [32], [33] and one preliminary study suggested that levels of RTN4 mRNA are also increased in the cortex of some individuals with chronic schizophrenia [34]. Finally, a recent meta-analysis of several expression profiling studies revealed a ∼10% decrease (P = 0.019) of RTN4R transcript levels in brains of individuals with schizophrenia (www.stanleygenomics.org). It is unclear, however, whether the observed changes constitute part of the genetic diathesis in schizophrenia or represent a reactive response. Here, we undertake a comprehensive multi-pronged approach to explore the possibility that RTN4R is a schizophrenia susceptibility gene from the 22q11.2 locus.
Results
Search for rare RTN4R coding variants
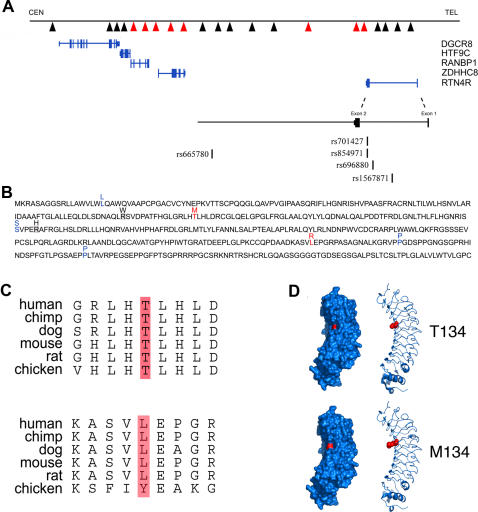
The RTN4R protein is encoded by two exons: exon I, which codes for the first 7 amino acids, and exon II, which encodes amino acids 8–473 ( Fig. 1A ). We sequenced exon II of RTN4R in 208 individuals with schizophrenia from the U.S. (European ancestry) and identified five variants. Of these five variants, three were synonymous changes (L18L, S192S, P394P), and two resulted in non-conservative changes (T134M, L347R) in evolutionary conserved amino acids ( Fig. 1B, C ). Each of these five variants was identified in single individuals except for S192S, which was found in two individuals. We also sequenced exon II of RTN4R in a sample of 300 control individuals from the U.S. and found only two synonymous changes (L18L, S192S). The S192S variant was identified in a single individual, whereas the L18L variant was found in two individuals. Previously, Sinibaldi et al. [31] reported two non-conservative sequence variants in the RTN4R gene (R119W and R196H) in an Italian sample of 120 schizophrenia patients that were absent in 200 controls. In our sample, we did not find these two variants in our patient sample, but we did find the synonymous L18L variant reported in the same study.
Figure 1. Genetic variation at the RTN4R locus.
(A) Location of human RTN4R within chromosomal region 22q11.2. Exons are represented by blue bars. The rs numbers of the SNPs typed in the present study are shown. SNPs genotyped by Liu et al. [22] are indicated by arrowheads (those SNPs that showed significant association with schizophrenia are shown in red arrowheads). The association observed with the three SNPs at the 3′end of RTN4R was independent from the association observed more proximally in the vicinity of the ZDHHC8 gene [22]. (B) Complete amino acid sequence of RTN4R. Rare coding variants identified in the U.S. schizophrenia sample are shown in blue (synonymous) or red (missense). Two missense variants reported by Sinibaldi et al. [31] are shaded in grey. (C) Evolutionary conservation across species of the missense variants identified in this study at amino acid positions 134 (top) and 347 (bottom). (D) Surface (left) and cartoon (right) representation of the crystal structure of the ligand-binding ectodomain of RTN4R. The variant at residue 134, drawn in red, is shown as the wild-type threonine (top) and the mutated methionine (bottom). Prepared using Pymol (DeLano Scientific, LLC, San Francisco, CA).
The RTN4R protein is comprised of two domains: an extracellular N-terminal ectodomain containing eight leucine-rich repeats (LRRs) and a membrane-anchored C-terminal domain containing a glycosylphosphatidylinositol (GPI) anchor sequence [35]. The ectodomain is both necessary and sufficient for binding the RTN4 ligand [35] and the C-terminal domain containing the GPI sequence and the connecting “stalk” is required for interaction with at least one of the co-factors, p75 [36]. The T134M variant we identified in one female patient is located in the concave face of the ligand-binding ectodomain of RTN4R, within the fourth LRR. Based on the crystal structure of the soluble ectodomain [37], [38] the T134 residue is found on the concave face within a negatively charged patch of amino acids including D111, D114, C138, D139, which forms a slight local depression which could be important for ligand or partner interactions [38] ( Fig. 1D ). The other non-synonymous variant we identified in one male patient, L347R, is positioned within the “stalk” of RTN4R that is required for p75 interaction.
Association analysis
Given the difficulties in assessing the significance of association to rare variants, primarily due to a lack of power [39], we sought to obtain additional supportive data by testing for independent association with common variants of RTN4R ( Fig. 1A ) in families with schizophrenia. Previously, we provided weak evidence for an association between RTN4R variation and susceptibility to schizophrenia by identifying three distinct variants located at the 3′ end of the RTN4R gene that were preferentially transmitted in individuals with schizophrenia in two independent, family-based samples from the U.S. (N = 106 triads) and South Africa (N = 93 triads) [22]. Here, we expand the South African sample to 312 families by including an additional 219 families with at least one schizophrenic subject (see Table S1 for study population characteristics). Since it has been suggested that variants in RTN4R may affect schizophrenia susceptibility differently in males and females we conducted both combined and sex-stratified analyses.
To facilitate comparison, three of the five single nucleotide polymorphisms (SNPs) tested in the present study (rs665780, rs701427, and rs696880) were also included in the earlier study [22]. Two of these SNPs previously showed weak evidence for association in that study: rs665780 in the Afrikaner families only (P = 0.04) and rs701427 in the U.S. families only (P = 0.02). Four of the five SNPs in this study are within the RTN4R intron and one is located 3′ of the gene. All SNPs are common with a minor allele frequency of at least 20% (see Table 1 for SNP positions and minor allele frequencies). The average (±SD) R2 and D' between the ten pairs of SNPs are 0.33 (±0.42) and 0.62 (±0.48), respectively. In the HapMap subjects of European descent (CEU), these five SNPs tag the common (minor allele frequency >10%) SNPs in RTN4R with an average R2 (±SD) of 0.75 (±0.39). Three of the five SNPs (rs701427, rs854971, and rs696880) are separated by less than 2-kb and are in high linkage disequilibrium (LD) with each other (R2≥0.90), (see Table 2 for pair-wise values). This group of SNPs, however, shows relatively low LD with the two flanking SNPs (rs665780 and rs1567871).
Table 1. Position and minor allele frequency of SNPs.
| SNP | Position (in Mb) | Position (in Gene) | Major/Minor Alleles | Minor Allele Frequency |
| rs665780 | 18.560 | 3′ | T/C | .21 |
| rs701427 | 18.608 | intronic | C/A | .34 |
| rs854971 | 18.608 | intronic | G/A | .34 |
| rs696880 | 18.610 | intronic | A/G | .36 |
| rs1567871 | 18.616 | intronic | C/T | .24 |
Table 2. Pair-wise LD values.
| rs665780 | rs701427 | rs854971 | rs696880 | rs1567871 | |
| rs665780 | - | <.01 | <.01 | <.01 | <.01 |
| rs701427 | .05 | - | .99 | .90 | .16 |
| rs854971 | .05 | 1.00 | - | .91 | .16 |
| rs696880 | .06 | .99 | 1.00 | - | .17 |
| rs1567871 | .08 | .99 | .99 | .98 | - |
Transmission disequilibrium test (TDT) analysis did not find evidence for unequal allelic transmission ratios at any of the tested variants in the combined sample (data not shown). Application of the more powerful linkage and association modeling in pedigrees (LAMP) analysis ( Table 3 ) on the combined sample also provided no evidence for unequal allelic transmission. In a sex-stratified analysis, SNP rs696880 showed the most significant association in females (Sch1: RRA = 0.74, P = .064; Sch2: RRA = 0.73, P = .046) and non-significant trends were also obtained with the other two linked SNPs, rs701427 and rs854971. In affected males, suggestive evidence for association was observed at rs701427 (Sch1: RRC = 1.19, P = .10; Sch2: RRC = 1.21, P = .019), as well as at the two SNPs (rs696880 and rs854971) in high LD with it. Interestingly, the alleles of rs701427, rs854971, and rs696880 that appear to increase susceptibility to schizophrenia in males show a trend towards decreasing the risk in females, consistent with the observed lack of association in the combined sample. A Bonferroni correction for multiple SNPs and affection statuses results in a P-value threshold of 0.0042. Therefore, after correcting for multiple testing, none of these results are significant.
Table 3. LAMP results for individual SNPs.
| SNP | Allele | Sch1 | Sch2 | |||
| RR | P-Val | RR | P-Val | |||
| All | rs665780 | T | 1.12 | - | 1.10 | - |
| rs701427 | C | 0.97 | - | 0.98 | - | |
| rs854971 | G | 0.97 | - | 0.98 | - | |
| rs696880 | A | 0.96 | - | 0.96 | - | |
| rs1567871 | C | 0.98 | - | 1.01 | - | |
| Females | rs665780 | T | 1.23 | - | 1.23 | - |
| rs701427 | C | 0.73 | .062 | 0.74 | .054 | |
| rs854971 | G | 0.74 | .070 | 0.74 | .064 | |
| rs696880 | A | 0.74 | .064 | 0.73 | .046 | |
| rs1567871 | C | 1.12 | - | 1.18 | - | |
| Males | rs665780 | T | 1.02 | - | 1.00 | - |
| rs701427 | C | 1.19 | - | 1.21 | .019 | |
| rs854971 | G | 1.18 | - | 1.20 | .021 | |
| rs696880 | A | 1.17 | - | 1.18 | .029 | |
| rs1567871 | C | 0.91 | - | 0.91 | - | |
RR = risk ratio; P-Val = P-value of TLD statistic.
Bonferroni corrected P-value cutoff = .0050.
When we estimate the haplotype frequencies in our population using an Expectation Maximization (E-M) algorithm, we observe six common haplotypes (frequency ≥5%) and one additional haplotype with a frequency ≥1%. The TDT analysis reveals a proportionate transmission of all haplotypes (data not shown). Similarly, the LAMP analysis does not detect any association between a haplotype and schizophrenia either in the combined or in the sex-stratified sample (see Table 4 for the results from the LAMP analyses).
Table 4. LAMP results for haplotype analysis (frequency>.05).
| Haplotype* | Freq | Sch1 | Sch2 | |||
| RR | P-Val | RR | P-Val | |||
| All | TCGAC | .34 | 0.94 | - | 0.94 | - |
| TAAGC | .27 | 1.05 | - | 1.01 | - | |
| TCGAT | .17 | 1.10 | - | 1.10 | - | |
| CAAGC | .07 | 0.97 | - | 1.06 | - | |
| CCGAC | .07 | 0.95 | - | 0.98 | - | |
| CCGAT | .06 | 0.89 | - | 0.82 | - | |
| Females | TCGAC | .34 | 0.93 | - | 0.92 | - |
| TAAGC | .27 | 1.28 | .053 | 1.29 | .055 | |
| TCGAT | .17 | 0.89 | - | 0.87 | - | |
| CAAGC | .07 | 1.30 | - | 1.27 | - | |
| CCGAC | .07 | 0.46 | .054 | 0.59 | - | |
| CCGAT | .06 | 0.86 | - | 0.77 | - | |
| Males | TCGAC | .34 | 0.96 | - | 0.98 | - |
| TAAGC | .27 | 0.90 | - | 0.84 | - | |
| TCGAT | .17 | 1.20 | .058 | 1.23 | .061 | |
| CAAGC | .07 | 0.78 | - | 0.90 | - | |
| CCGAC | .07 | 1.32 | .083 | 1.28 | .081 | |
| CCGAT | .06 | 0.94 | - | 0.91 | - | |
Alleles for rs665780, rs701427, rs854971, rs696880, and rs1567871, respectively.
Rare haplotypes (not included in analysis: TCGGC (.018); CCGGC (.007); TCGGT (.001); TAGAC (.001); TAAGT (<.001).
RR = risk ratio; P-Val = P-value of TLD statistic.
At α-level of .05, Bonferroni corrected P-value is .0042.
Generation of Rtn4r-deficient mice
In order to complement our human genetic studies (see Discussion), we generated a mouse model that is deficient in the Rtn4r gene and probed for deficits in phenotypic components, which include endophenotypes associated with schizophrenia that can be modeled reliably in mice [40].
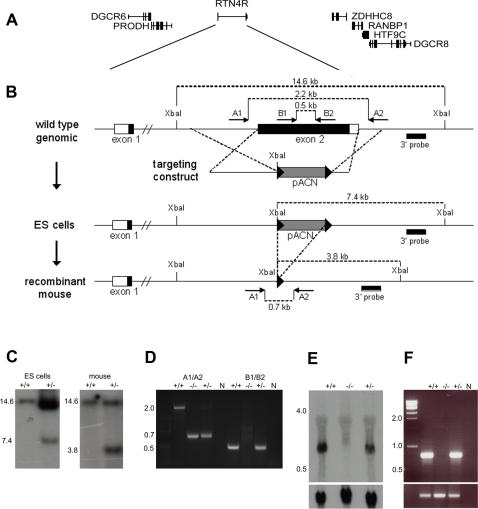
The mouse ortholog of RTN4R is located in the syntenic region of the human 22q11.2 locus that lies on mouse chromosome 16. All human genes except for one are represented in this region, although the order of the genes is different [41]. As a result of this rearrangement, the Rtn4r mouse gene is located between the Prodh gene (∼40-kb proximal) and the Zdhhc8 gene (∼70-kb distal) ( Fig. 2A ), both of which have been implicated by human genetic studies as strong candidate susceptibility genes for schizophrenia [21]–[23]. To minimize the possibility that genetic modification of the Rtn4r locus could affect expression of the two neighboring genes, we designed and implemented a gene targeting strategy ( Fig. 2B–F ) to replace exon II of Rtn4r with the self-excisable selection cassette that includes the neo gene selectable marker (pACN) [40]. Excision of the neo gene, following germline transmission, ensures that any observed phenotype is due to the deletion rather than any long-range transcriptional effects of the selection cassette [42]. Homozygous Rtn4r mutant mice were viable and fertile and have normal brain morphology by gross morphological inspection, as well as finer morphometric calculations of cell densities, laminal thickness and the thickness of the corpus callosum (see Text S1 and Fig. S1 ).
Figure 2. Targeting the Rtn4r locus for homologous recombination.
(A) Gene map of the Rtn4r locus at mouse chromosome 16. (B) Exon II of Rtn4r was replaced by the self-excisable pACN cassette including the neo gene for selection. Genomic DNA was digested with XbaI and the 3′ probe was used for southern blot analysis. A PCR strategy was designed to genotype offspring using primer sets A1/A2 and B1/B2. (C) Genomic southern blot of ES cell DNA and tail biopsies. Wild-type fragments were 14.6-kb, recombinant fragments from ES cells were 7.4-kb, and recombinant fragments from tail biopsies after pACN cassette excision were 3.8-kb. (D) PCR genotyping of tail biopsies using primer sets A1/A2 and B1/B2. Wild-type mice yielded 2.2-kb and 0.5-kb fragments from sets A1/A2 and B1/B2 respectively; homozygotes yielded 0.7-kb and no fragment, respectively; and heterozygotes yielded 0.7-kb and 0.5-kb fragments, respectively. (E) Northern blot of total brain RNA using a Rtn4r 3′UTR probe. (F) RT-PCR of total brain RNA using isoform-specific primers for Rtn4r exon II.
Behavioral characterization of Rtn4r-deficient mice at baseline
We characterized wild-type, heterozygous, and homozygous Rtn4r mutant mice at baseline using tasks designed to assess domains known to be perturbed in schizophrenia, including anxiety, sensorimotor gating, learning and memory, and behavioral despair. Rtn4r-deficient mice demonstrated normal neurological signs and normal pain threshold levels using the hot plate assay (data not shown). In addition to locomotor activity and anxiety-related tasks, we assessed a) sensorimotor gating using the prepulse inhibition (PPI) assay; b) cognitive performance using contextual and cued fear conditioning, as well as a working memory task; and c) a depression-relevant behavior using the tail-suspension assay. For a description of the equipment and procedures used, see Text S2 .
Open field and anxiety-relevant tasks
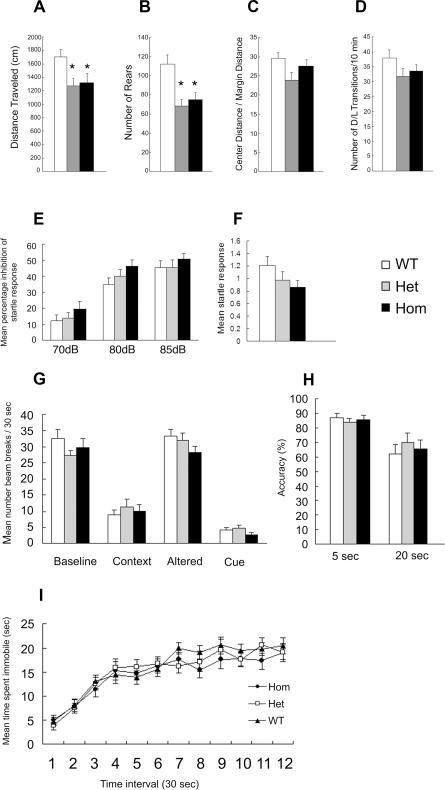
In the open field assay there was a significant effect of genotype on the total distance traveled in the open field [F(2, 60) = 4.192, P<0.02] and number of rears (vertical movements) [F(2, 60) = 9.14, P<0.001] ( Fig. 3A, B ). Interestingly, reductions in distance traveled and rears were observed in both the homozygous mutant and heterozygous mice in comparison to wild-type littermates suggesting that the gene could be haploinsufficient with respect to these behaviors. There were no differences in habituation of locomotor activity in the open field (not shown). Differences in the percentage of distance traveled in the central area of the open field, an index of anxiety-relevant behavior, fell short of significance [F(2, 60) = 2.11, P = 0.071] ( Fig. 3C ). In the light/dark test, which also assays for anxiety-relevant behaviors, we found no genotype or genotype X sex effects on the number of entries into each compartment ( Fig. 3D ) or on the percentage of time animals spent in the light compartment of the light/dark apparatus (data not shown).
Figure 3. Behavioral characterization of Rtn4r-deficient mice.
(A) Total distance traveled, (B) mean number of rears, and (C) ratio of center traveled distance/margin traveled distance in a 20-min open field test. (D) Number of dark-to-light transitions in a 10-min dark/light transition test. (E) PPI using combinations of one startle level (120 dB) and three prepulse levels (70, 80 and 85 dB). (F) Startle response at 120 dB. (G) Contextual and cued fear-conditioning paradigm. Activity (beam breaks) was measured at baseline, after reintroduction into the same context where training occurred (context), at baseline in an altered context (altered), and after presentation of the cued conditioned stimulus in the altered environment (cue). (H) Accuracy in a working memory test after a 5-sec or 20-sec inter-trial delay using a delayed alternation T-maze assay. (I) Mean time spent immobile in a tail suspension test. All results shown are from combined sexes. All data are represented as mean±S.E.M.
Sensorimotor Gating
Sensorimotor gating is typically evaluated using the PPI paradigm, a common measure of pre-attentive processing [40]. Abnormal PPI has been described in individuals with schizophrenia, as well as in children with the 22q11.2 microdeletion syndrome [20]. In this model, the response to a startle-eliciting stimulus, such as a loud tone, is attenuated if the stimulus is preceded within a few hundred milliseconds by a lower-intensity stimulus, or pre-pulse [43], [44]. We evaluated startle response to auditory stimuli as well as inhibition of the startle response elicited by pre-pulses. There were no genotype or genotype X sex differences in inhibition of the startle response at any of the prepulse levels of 70, 80 and 85 dB ( Fig. 3E ). In addition, in control experiments, there were no genotype or genotype X sex effects on acoustic startle response (120 dB) ( Fig. 3F ). Notably, although there was no significant effect of genotype, homozygous mutants tended to show slightly more inhibition of startle response than wild-type littermate control mice, as well as slightly less startle response.
Associative learning and memory
We used the Pavlovian conditioned fear paradigm to assess associative learning and memory in Rtn4r-deficient mice ( Fig. 3G ). We evaluated both contextual conditioning, as measured by suppressed activity in the context where training occurred, and cued conditioning as measured by suppression of activity in a new environment in which the conditioned stimulus (CS) is presented.
Baseline activity
There were no genotype differences or genotype X sex effects in baseline activity during the training phase of fear conditioning. There were also no differences in activity levels after the third presentation of the unconditioned stimulus (US) and tone pairing.
Contextual Conditioning
There were no genotype or genotype X sex effects in activity during contextual conditioning.
Altered Context
There were no genotypic differences in activity levels in the altered context test, which is both amygdala- and hippocampus-dependent [F(2, 60) = 2.11, P = 0.13]; however, there was a significant genotype X sex interaction in activity levels in the altered environment [F(2, 60) = 4.2, P<0.02].
Cued Conditioning
In the cued version of the test, which requires the amygdala but not the hippocampus [45], there were no genotype or genotype X sex effects in the suppression of activity in response to the conditioned stimulus.
Working memory
We used the T-maze delayed alternation task [46], [47] to examine whether deficiency in Rtn4r-mediated signaling activity is associated with changes in spatial working memory. Working memory is defined as the ability to maintain and manipulate information transiently in the service of other cognitive processes to guide behavior [48]. Working memory is frequently impaired in patients with schizophrenia (often prior to or at the onset of the illness), as well as in a portion of their non-schizophrenic, first-degree relatives [49], [50]. Impaired working memory has also been noted in individuals with the 22q11.2 microdeletion syndrome [12]–[14]. Rtn4r-deficient mice learned the 5-sec delay T-maze task during 6 consecutive training days and performed as well as wild-type littermate control mice. In addition, there were no genotypic differences or genotype X sex interactions in their T-maze based working memory performance at both 5-sec and 20-sec delays ( Fig. 3H ).
Behavioral despair
High rates of depressive symptoms have been observed in patients with schizophrenia [51] and children with the 22q11.2 microdeletion [12]. We assayed for behavioral despair, an index of depression-like behavior in rodents, using a tail suspension test [52]. The test is based on the fact that animals subjected to the short-term, inescapable stress of being suspended by their tail will develop an immobile posture. Various antidepressant medications reverse the immobility and promote the occurrence of escape-related behavior [53]. No genotypic differences or genotype X sex interactions in total time spent immobile on the tail suspension task were observed ( Fig. 3I ). A repeated measures ANOVA also showed that there were no genotypic differences or genotype X sex interactions in time spent immobile over intervals of the test session.
Behavioral characterization of Rtn4r-deficient mice following transient postnatal NMDAR blockade
Recent work using an independent animal model suggests that RTN4R may function in the mammalian CNS to stabilize neural circuits in early postnatal development [54]. It is therefore conceivable that RTN4R deficiency facilitates the effect of other schizophrenia susceptibility genes or disease-related pathological processes by destabilizing relevant neural circuits during development. Such a contribution might not be detectable with the study design outlined above but may require additional genetic or pharmacological challenges. Aberrant NMDA receptor-mediated glutamatergic transmission has been implicated in psychiatric disorders such as schizophrenia, and several pharmacological modeling approaches have been established based on this hypothesis [40]. In one of them, transient inhibition of NMDAR signaling during early postnatal development produces long-lasting behavioral deficits including deficits in domains such as cognitive flexibility, working memory and sensorimotor gating [55], [56]. We employed this model to ask whether Rtn4r deficiency modulates the long-term behavioral effects of postnatal NMDA receptor hypofunction. In our experimental paradigm, mouse pups were given injections of the NMDA channel blocker MK-801 during two distinct periods: postnatal days (P) 7–10, which corresponds to a critical period of sensitivity for the NMDAR system, and P11–14, which follows the critical period [57], [58]. Sensorimotor gating, as well as spontaneous alternation (an index of normal working memory) and motoric activity in the Y-maze were then tested in adulthood.
Spontaneous alternation
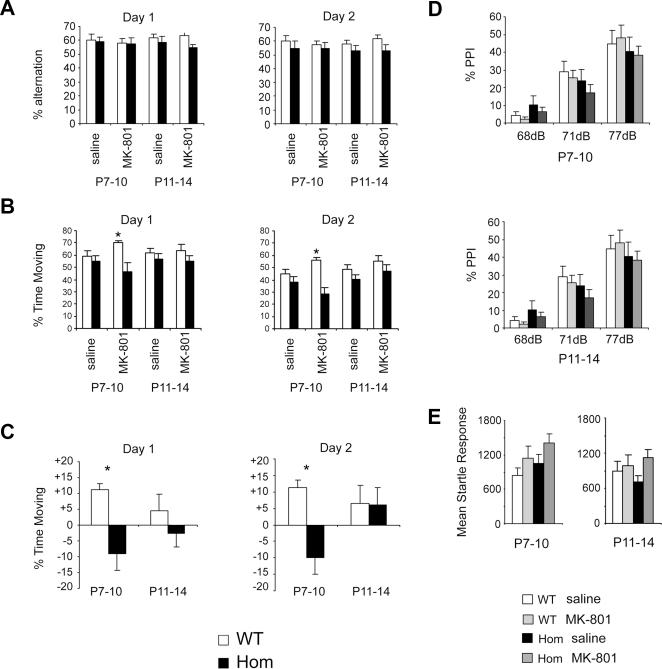
Adult homozygous Rtn4r-deficient mice and their wild-type littermates showed, as expected, a tendency to spontaneously alternate but there were no significant differences in spontaneous alternation in the Y-maze due to genotype or drug treatment ( Fig. 4A ). However, we observed a significant genotype X treatment interaction (P<0.05) in the percentage of time moving, a measure of locomotor activity. Specifically, a significant genotype effect was observed in animals that were injected with MK-801 but not saline at P7–10 (P<0.05). Interestingly, this effect was limited to animals treated at P7–10. Animals that underwent the same treatment at P11–14 showed no significant genotype X treatment interaction ( Fig. 4B ). Further analysis showed that this difference emerged because while neonatal MK-801 treatment at P7–P10 induced an increase in locomotor activity in the wild-type mice in adulthood, it resulted in a decrease in locomotor activity of adult Rtn4r-deficient mice ( Fig. 4C ).
Figure 4. Behavioral characterization of adult Rtn4r-deficient mice following transient postnatal NMDAR blockade.
(A, B) Percentage of alternation (A) and % time moving (B) observed in an 8-min session of a Y-maze spontaneous alternation assay, repeated over 2 days. Animals were injected with MK-801 or saline for 4 consecutive days during either P7–10 or P11–14; behavioral testing was conducted at P62 and P63. (C) Relative effect of MK-801 injection normalized to saline injection on time spent moving. (D) Average percentage of PPI (measured at P91) in animals injected during either P7–10 or P11–14. (E) Mean startle response to a 40 ms, 120 dB acoustic stimulus in animals injected during either P7–10 or P11–14. Startle response was measured at P91.
Acoustic startle and prepulse inhibition
There were no overall significant differences in prepulse inhibition or acoustic startle response due to genotype or drug treatment ( Fig. 4D, E ). Previously, Harris et al. [56] found that neonatal MK-801 administration resulted in a greater decrease in PPI levels in adult females only. However, we found no significant sex effect in both acoustic startle response and prepulse inhibition.
Discussion
Several lines of evidence suggest that oligodendroglial function and myelin maintenance are disturbed in schizophrenia [reviewed in Davis et al. [59]]. These include imaging and neurocytochemical evidence, changes in white matter, myelin-related gene abnormalities, and morphologic abnormalities in the oligodendroglia observed in brains of individuals with schizophrenia. The relation of these findings to the pathogenesis of the disease is still uncertain. Most intriguing is the possibility of a relation between deficits in myelination and function of the prefrontal cortex, a region that modulates many functions impaired in schizophrenia and whose myelination is completed during late adolescence and early adulthood, a period when overt symptomatology of schizophrenia most commonly emerges. In this context, the location of RTN4R within the replicated and highly penetrant 22q11.2 schizophrenia susceptibility locus makes the gene a prime candidate for influencing disease susceptibility.
We tested common SNPs and haplotypes in and around RTN4R in a large sample of Afrikaner families. Given that Afrikaners are a relatively genetically and environmentally homogenous population, we expected higher power in our analysis, making disease susceptibility alleles easier to identify. Our analyses did not identify SNPs or haplotypes that were segregating with the disease in the overall sample. Stratification by sex provided weak evidence for an association between RTN4R and schizophrenia, which did not survive a conservative Bonferroni correction.
We also identified rare non-synonymous changes within the RTN4R gene of schizophrenia patients but not in unaffected controls. This observation appears to lend some support for a role of RTN4R in disease susceptibility. However, the major limitation in conducting association analyses of rare variants is that it is difficult to obtain enough statistical power to unambiguously interpret their results. In our study, the sample of several hundred chromosomes does not have sufficient power for our results to be considered unequivocal in proving the association between rare variants of RTN4R and schizophrenia [39], [60], [61]. Therefore, we cannot exclude the possibility that all four RTN4R non-conservative variants that have been described in patients with schizophrenia (present study and in Sinibaldi et al. [31] study) might actually be rare variants that are neutral in relation to the disease status. In general, interpreting association tests between rare variants and complex traits is an emerging problem in the field [62], [63]. The significance of rare variants such as the ones identified in this study can be addressed by screening thousands of both patient and control chromosomes for the presence of non-synonymous variants [61], by searching for rare non-synonymous variants in extended pedigrees (i.e. ones that show linkage to the 22q11.2 locus) where co-inheritance of rare non-conservative variants with disease status can be strictly evaluated, as well as by using model systems to strengthen a functional link between variation in the gene and the disease.
In the context of suggestive human genetic data, the generation of genetic mouse models that mimic the effect of rare hypomorphic variants can be particularly informative [40]. Our animal model studies, designed to facilitate interpretation of our human genetic results, indicated an effect of Rtn4r on locomotor activity in an open field assay. Furthermore, in heterozygous Rtn4r mice, we found a haploinsufficient effect in the open field assay. However, in schizophrenia-related behavioral tasks, such as PPI and the working memory-dependent T-maze delayed alternation task, our studies revealed an unremarkable behavioral profile with normal performance in these tasks, thus arguing against a major role of RTN4R in susceptibility to schizophrenia. It should be noted that using an independently generated Rtn4r-deficient mouse model, Kim et al. [64] also observed hypoactivity in Rtn4r null mice along with decreased rotarod performance, but normal Basso-Beattie-Bresnahan (BBB) scores, a measure of locomotor function. Schizophrenia-related behavioral tasks were not reported in the Kim et al. [64] study.
Obviously, positive results from accurate genetic mouse models can be instrumental in establishing the “biological plausibility” of disease-associated genetic variants. However, any interpretation based on negative results from behavioral studies in animal models suffers from several caveats that reflect the limitations of behavioral approaches and the complex genetic structure of the disease. For example, the behavioral effect of any given mutation, especially in a gene that could be involved in a complex disorder, could depend on the age and genetic background of the organism. In addition, we cannot ignore the possibility that RTN4R deficiency contributes to other phenotypic components not assayed here. In that respect, future studies designed to examine the effect of Rtn4r deficit in younger animals or in inbred genetic backgrounds including additional hypothesis-driven behavioral assays will be informative.
Behavioral assessment at baseline may also overlook modulatory effects of a gene in a given behavior that may be revealed by additional genetic or pharmacological challenges. NMDA hypofunction is considered one of the leading hypotheses for schizophrenia pathogenesis and has inspired the generation of several pharmacological models of the disease. Indeed, by adapting such a pharmacological model we found that neonatal NMDAR disruption during a presumably critical period for NMDA activity (P7–10), differentially affects wild-type and Rtn4r-deficient mice. During critical periods of development, widespread pharmacological or genetic inhibition of NMDAR signaling has been shown to facilitate terminal axon sprouting and to modulate the effects of activity-based competition on axon growth [58], [65], [66]. Such “wiring” alteration may contribute not only to the behavioral deficits associated with this pharmacological model, but also to the disease pathogenesis. Moreover, Rtn4r may modulate this aspect of NMDA hypofunction on early brain wiring.
Interestingly, and in agreement with our behavioral analysis at baseline, the observed effects were specific to the motor domain. Although it is very difficult to extrapolate from motor deficits in mice to deficits in motor skills in humans, it is worth noting that patients with schizophrenia display motor development delays, as well as motor disturbances [67], [68]. It is also worth noting that motor deficits have been consistently observed in children with the 22q11.2 deletion syndrome including an impairment in fine and gross motor skills that emerges during adolescence [11], [69], [70]. Importantly, a recent study has shown that motoric deficits are more prevalent among 22q11.2-deleted individuals with schizophrenia than without schizophrenia and along with deficits in verbal learning and social cognition can be used to distinguish these two groups of patients [71].
Our results do not support a major role of RTN4R in susceptibility to schizophrenia or in the cognitive and behavioral deficits observed in individuals with 22q11 microdeletions. However, a hypothesis emerging from the findings described here, as well as from previous independent work (see Introduction) is that RTN4R may modulate the genetic risk for psychiatric phenotypes in a subset of patients, at least partly by mediating the developmental effects of NMDAR receptor hypofunction on early brain wiring. Further genetic studies in afflicted families, as well as analysis of the knockout mice in additional behavioral, synaptic and molecular phenotypes are warranted and will be necessary to test this hypothesis and further clarify any role of RTN4R in psychiatric phenotypes.
Materials and Methods
Sample description
All procedures of subject recruitment and evaluation were approved by the Institutional Review Boards at Rockefeller University, New York State Psychiatric Institute and University of Pretoria. Written informed consent was obtained from all participants. The sample used for direct sequencing consisted of 208 individuals with schizophrenia, of European ancestry, recruited from the U.S. and diagnosed by a clinical team specially trained in the use of the Diagnostic Instrument for Genetic Studies (DIGS) [72] and the research application of the Diagnostic and Statistical Manual–4th Edition (DSM-IV) [73]. On the basis of the information gathered in the DIGS, the clinical interviewers assigned appropriate diagnoses according to the DSM-IV. The control sample used for direct sequencing consisted of 60 parents from CEPH trios (residents of Utah with ancestry from Northern and Western Europe) and an additional set of 240 healthy Caucasians collected from the U.S. by us (MK). Sequencing analysis was performed as described in Text S2 .
To evaluate association between the RTN4R gene and susceptibility to schizophrenia, we recruited participants from 312 Afrikaner families with at least one schizophrenic subject. Afrikaners are descendants of mostly Dutch immigrants who settled in South Africa beginning in 1652 [74]. Participants were diagnosed in person by specially trained clinicians again using the DIGS, which had been translated and back-translated into Afrikaans. Patients were classified into two diagnostic categories. The stricter category, Sch1, includes 348 individuals who meet DSM-IV criteria for schizophrenia or depressed-type schizoaffective disorder. Family data suggest that the two diagnoses are alternative expressions of the same genotypes [75] and in the past we have considered them together under one phenotypic liability class, LC I [76]. The broader category, Sch2, includes an additional 52 individuals diagnosed with schizoaffective disorder of mainly affective course. Association analysis was performed as described in Text S2 .
Generation of Rtn4r knockout mice
For the construction of the targeting construct, we replaced an Rtn4r genomic fragment encompassing exon II with the self-excisable pACN cassette including the neo gene selectable marker [77]. Cell culture, embryonic stem (ES) cell electroporation and generation of the chimeric mice were performed essentially as previously described [78]. Approximately 5% of the tested ES cell clones were positive for homologous recombination, and one clone was selected for injection into C57BL/6J blastocysts. Chimeric males were mated with C57BL/6J females, and DNA from tail biopsy samples from F1 agouti-coat pups was genotyped by Southern blotting at the Rtn4r genomic locus (wild-type fragment: 14.6-kb; recombinant fragment after pACN cassette excision: 3.8-kb). We mated F1 heterozygous mice and obtained F2 mice of all three genotypes. The mutation was on a hybrid C57BL/6×129Sv background. All animal procedures were performed according to protocols approved by the appropriate Institutional Animal Care and Use Committee under the federal and state regulations.
Histological analysis
See Text S2.
Behavioral Testing: Equipment and Procedures
See Text S2.
Analysis following transient postnatal NMDAR blockade
See Text S2.
Supporting Information
(0.02 MB DOC)
(0.06 MB DOC)
(0.03 MB DOC)
Brain histology in Rtn4r-deficient mice: Representative microphotographs from Nissl staining of coronal sections through the cerebral cortex (A, B), anterior-dorsal hippocampus (C, D) and corpus callosum (E, F) from 8-wk-old Rtn4r-deficient mice (−/−) and their wild-type littermates (+/+). (G, H) Coronal sections through the cerebral cortex of Rtn4r recombinant mice crossed with Thy1-YFPH expressing transgenics. Scale bars represent 0.4 mm. (I) Average cell density of the retrosplenial agranular cortex at Bregma −2.18 mm, −1.70 mm, and −1.22 mm. (J) Average thickness of the retrosplenial agranular cortex. (K) Average thickness of the corpus callosum. All data are represented as mean±S.E.M.
(9.10 MB TIF)
Acknowledgments
The authors wish to acknowledge Dionne Swor, Megan Sribour and Jessica Pellegrino for technical support and assistance with the mouse colony, Ying-Jiun Chen for help with the generation of the targeting construct, and members of the Gogos and Karayiorgou labs for useful suggestions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported in part by the National Institutes of Health (grant MH67068 to MK and JAG, MH61399 to MK, and U01-MH61971 to Tennessee Mouse Genome Consortium) and by the New York Academy of Sciences (JAG). RH is supported by an HHMI pre-doctoral fellowship. JM is supported by a NARSAD Young Investigator Award. JAG is also the recipient of a McKnight Brain Disorders Award, and a NARSAD Award.
References
- 1.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 2.Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 3.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 4.Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 5.Mi S, Lee X, Shao Z, Thill G, Ji B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 6.Park JB, Yiu G, Kaneko S, Wang J, Chang J, et al. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 7.Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 8.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 9.Botto LD, May K, Fernhoff PM, Correa A, Coleman K, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contributuion to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- 10.Morrow B, Goldberg R, Carlson C, Gupta RD, Sirotkin H, et al. Molecular definition of the 22q11.2 deletions in velo-cardio-facial syndromes. Amer J Hum Genet. 1995;56:1391–1403. [PMC free article] [PubMed] [Google Scholar]
- 11.Antshel KM, Kates WR, Roizen N, Fremont W, Shprintzen RJ. 22q11.2 deletion syndrome: genetics, neuroanatomy and cognitive/behavioral features. Child Neuropsych. 2005;11:5–19. doi: 10.1080/09297040590911185. [DOI] [PubMed] [Google Scholar]
- 12.Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, et al. Neuropsychological characteristics of children with the 22q11.2 deletion syndrome: a descriptive analysis. Child Neuropsychol. 2005;11:39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, et al. Neuropsychological profile of chilren and adolescents with the 22q11.2 microdeletion. Genet Med. 2001;3:34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, et al. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- 15.Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, et al. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 2005;25:169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 16.van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, et al. Structural brain abnormalities associated with deletion at chromosome 22q11.2. Brit J of Psych. 2001;178:412–419. doi: 10.1192/bjp.178.5.412. [DOI] [PubMed] [Google Scholar]
- 17.Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 19.Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11.2. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11.2 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Heath SC, Sobin C, Roos JL, Galke BL, et al. Genetic variation at the 22q11.2 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2002;99:3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, et al. Genetic variation in the 22q11.2 locus and susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2002;99:16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukai J, Liu H, Burt RA, Swor DE, Lai WS, et al. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- 24.Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- 27.Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum Mol Genet. 2006;16:83–91. doi: 10.1093/hmg/ddl443. [DOI] [PubMed] [Google Scholar]
- 28.Meng J, Shi Y, Zhao X, Guo S, Wang H, et al. No association between the genetic polymorphisms in the RTN4R gene and schizophrenia in the Chinese population. J Neural Transm. 2007;114:249–254. doi: 10.1007/s00702-006-0538-y. [DOI] [PubMed] [Google Scholar]
- 29.Fallin MD, Belmonte P, Lasseter VK, Cheng N, Avramopoulos D, et al. Examination of sex and age of onset as important sources of variation for genetic association findings in schizophrenia. Abstract #2076 ASHG Meeting 2006 [Google Scholar]
- 30.Pierce T, Bray N, Williams N, Ivanov D, Owen M, et al. Support for reticulon 4 (Rtn4) as a potential susceptibility gene for schizophrenia. XIV World Congress on Psychiatric Genetics: 179 2006 [Google Scholar]
- 31.Sinibaldi L, De Luca A, Bellacchio E, Conti E, Pasini A, et al. Mutations of the Nogo-66 receptor (RTN4R) gene in schizophrenia. Hum Mutat. 2004;24:534–535. doi: 10.1002/humu.9292. [DOI] [PubMed] [Google Scholar]
- 32.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 34.Novak G, Kim D, Seeman P, Tallerico T. Schizophrenia and Nogo: elevated mRNA in cortex, and high prevalence of a homozygous CAA insert. Brain Res Mol Brain Res. 2002;107:183–189. doi: 10.1016/s0169-328x(02)00492-8. [DOI] [PubMed] [Google Scholar]
- 35.Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG, and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 37.Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He XL, Bazan JF, McDermott G, Park JB, Wang K, et al. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron. 2003;38:177–185. doi: 10.1016/s0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 39.Keen-Kim D, Mathews CA, Reus VI, Lowe TL, Herrera LD, et al. Overrepresentation of rare variants in a specific ethnic group may confuse interpretation of association analyses. Hum Mol Genet. 2006;15:3324–3328. doi: 10.1093/hmg/ddl408. [DOI] [PubMed] [Google Scholar]
- 40.Arguello PA, Gogos JA. Modeling Madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Puech A, Saint-Jore B, Funke B, Gilbert DJ, Sirotkin H, et al. Comparative mapping of the human 22q11.2 chromosomal region and the orthologous region in mice reveals complex changes in gene organization. Proc Natl Acad Sci USA. 1997;94:14608–14613. doi: 10.1073/pnas.94.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson EN, Arnold HH, Rigby PW, Wold BJ. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 43.Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, et al. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 1999;21:434–439. doi: 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- 44.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 45.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 46.Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, et al. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 49.Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Amer J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 51.Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- 53.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 54.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;67:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 56.Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- 57.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, et al. Blockade of NMDA Receptors and Apoptotic Neurodegeneration in the Developing Brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 58.Colonnese MT, Constantine-Paton M. Developmental period for N-methyl-D-aspartate (NMDA) receptor-dependent synapse elimination correlated with visuotopic map refinement. J Comp Neurol. 2006;494:738–751. doi: 10.1002/cne.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell AA, Chakravarti A, Cutler DJ. On the probability that a novel variant is a disease-causing mutation. Genome Res. 2005;15:960–966. doi: 10.1101/gr.3761405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, et al. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 2004;305:869–872. doi: 10.1126/science.1099870. [DOI] [PubMed] [Google Scholar]
- 62.Altshuler D, Hirschhorn JN. MEF2A sequence variants and coronary artery disease: a change of heart? J Clin Invest. 2005;115:831–833. doi: 10.1172/JCI200524715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 64.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005;47:101–114. doi: 10.1016/j.neuron.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 67.Manschreck TC, Ames D. Neurologic features and psychopathology in schizophrenic disorders. Biol Psychiatry. 1984;19:703–719. [PubMed] [Google Scholar]
- 68.Putzhammer A, Klein HE. Quantitative analysis of motor disturbances in schizophrenic patients. Dialogues Clin Neurosci. 2006;8:123–130. doi: 10.31887/DCNS.2006.8.1/aputzhammer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shprintzen RJ. Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Ment Retard Dev Disabil Res Rev. 2000;6:1142–147. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 70.Sobin C, Monk SH, Kiley-Brabeck K, Khuri J, Karayiorgou M. Neuromotor deficits in children with the 22q11 deletion syndrome. Mov Disord. 2006;21:2082–2089. doi: 10.1002/mds.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chow EW, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr Res. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 73.American Psychiatric Association. Washington, DC: American Psychiatric Press; 1994. DSM-IV: diagnostic and statistical manual of mental disorders, 4th ed. [Google Scholar]
- 74.Karayiorgou M, Torrington M, Abecasis GR, Pretorius H, Robertson B, et al. Phenotypic characterization and genealogical tracing in an Afrikaner schizophrenia database. Am J Med Genet B. 2004;124:20–28. doi: 10.1002/ajmg.b.20090. [DOI] [PubMed] [Google Scholar]
- 75.Cloninger CR. Schizophrenia: genetic etiological factors. In: Kaplan HI, Saddock BJ, editors. Comprehensive textbook of psychiatry, 5th ed. Baltimore: William & Wilkins; 1989. [Google Scholar]
- 76.Abecasis GR, Burt RA, Hall D, Bochum S, Doheny KF, et al. Genomewide scan in families with schizophrenia from the founder population of Afrikaners reveals evidence for linkage and uniparental disomy on chromosome 1. Am J Hum Genet. 2004;74:403–417. doi: 10.1086/381713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gogos JA, Osborne J, Nemes A, Mendelsohn M, Axel R. Genetic ablation and restoration of the olfactory topographic map. Cell. 2000;103:609–620. doi: 10.1016/s0092-8674(00)00164-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(0.02 MB DOC)
(0.06 MB DOC)
(0.03 MB DOC)
Brain histology in Rtn4r-deficient mice: Representative microphotographs from Nissl staining of coronal sections through the cerebral cortex (A, B), anterior-dorsal hippocampus (C, D) and corpus callosum (E, F) from 8-wk-old Rtn4r-deficient mice (−/−) and their wild-type littermates (+/+). (G, H) Coronal sections through the cerebral cortex of Rtn4r recombinant mice crossed with Thy1-YFPH expressing transgenics. Scale bars represent 0.4 mm. (I) Average cell density of the retrosplenial agranular cortex at Bregma −2.18 mm, −1.70 mm, and −1.22 mm. (J) Average thickness of the retrosplenial agranular cortex. (K) Average thickness of the corpus callosum. All data are represented as mean±S.E.M.
(9.10 MB TIF)