Abstract
Background
Patients with alcohol addiction show a number of transient or persistent neurological and psychiatric deficits. The complexity of these brain alterations suggests that several brain areas are involved, although the definition of the brain alteration patterns is not yet accomplished.
Aim
To determine brain atrophy patterns in patients with alcohol dependence.
Methods
Voxel‐based morphometry (VBM) of grey matter (GM) and white matter (WM) was performed in 22 patients with alcohol dependence and in 22 healthy controls matched for age and sex.
Results
In patients with alcohol dependence, VBM of GM revealed a significant decrease in density (p<0.001) in the precentral gyrus, middle frontal gyrus, insular cortex, dorsal hippocampus, anterior thalamus and cerebellum compared with controls. Reduced density of WM was found in the periventricular area, pons and cerebellar pedunculi in patients with alcohol addiction.
Conclusions
Our findings provide evidence that alcohol addiction is associated with altered density of GM and WM of specific brain regions. This supports the assumption that alcohol dependence is associated with both local GM dysfunction and altered brain connectivity. Also, VBM is an effective tool for in vivo investigation of cerebral atrophy in patients with alcohol addiction.
Alcoholism can affect the brain and behaviour in a variety of ways, and multiple factors can influence these effects. A key goal of brain imaging in the research of alcoholism is to detect changes in specific brain regions. Previous studies using different imaging techniques have revealed a general reduction of brain sizes as well as a consistent association between heavy alcohol consumption and regional brain damage. Various cortical regions and parts of the cerebellum have been suggested to be predominantly involved in alcohol‐associated brain atrophy. Several neuroimaging studies have recently described global and regional brain atrophy in patients with alcohol dependence in both cross‐sectional and longitudinal imaging studies.1,2,3,4,5 Neuropathological studies conducted on the brains of deceased patients as well as findings derived from neuroimaging studies of the brains of living patients, point to increased susceptibility of frontal brain systems to alcoholism‐related damage.6,7 Neuropathological studies have also demonstrated substantial changes in different brain regions1 such as parts of cerebral cortex,8 basal forebrain,9 thalamus10 and hypothalamus.11
Since previous imaging studies applying conventional volumetry focused on preselected brain regions,12,13 the particular pattern of alcohol‐associated brain tissue alterations is not completely established.8
Voxel‐based morphometry (VBM) is a recently introduced automated method of indirect volumetry, which allows the investigation of the entire brain without restriction to a priori defined regions of interest.14 In recent years, VBM has been successfully applied in characterising structural brain differences in a variety of diseases including schizophrenia,15 autism,16 Alzheimer's disease17 and dementia with Lewy bodies.18
The purpose of this study was to investigate the altered density of grey matter (GM) and white matter (WM) in the whole brain of patients with alcohol addiction and to reveal the atrophy pattern and alteration of different brain regions induced by chronic alcohol consumption in order to provide evidence for a preferential vulnerability of some brain regions with respect to the toxic effects of alcohol.
Materials and methods
Subjects
Patients (n = 22; mean age 53.6 years, range 31–69 years; 14 men and 8 women) with alcohol addiction who were admitted to the Department of Psychiatry, Innsbruck University Hospital, Innsbruck, Austria, were included in this study. The following exclusion criteria were applied: history of illicit drug misuse or dependency, history of severe benzodiazepine misuse, liver cirrhosis, major psychiatric disorders (other than alcohol addiction) as defined by the International classification of diseases, 10th revision, history of severe brain injury, neoplastic brain processes, history of vascular brain alterations, Wernicke encephalopathy as defined by clinical operational criteria19 and general contraindications for magnetic resonance investigation. In all, 22 age‐ and sex‐matched healthy subjects (mean age 53.7 years, range 31–73 years; 14 men and 8 women) without a history of alcohol misuse served as controls. Patients were investigated after alcohol abstinence of at least 10 days. All patients included in this study had a drinking history of >10 years. The range of daily alcohol consumption was between 180 and 310 g/day and the number of smokers was 17 of 22. The level of education was not significantly different between both groups (patients, mean (SD) 9.7 (2.6) years; controls, 10.1 (2.3) years).
Alcohol addiction in patients was assessed according to the International classification of diseases, 10th revision, diagnostic criteria. Patients underwent neurological and general medical examination and laboratory testing to exclude other causes of possible brain alterations. The investigation included chest radiography, ECG, chemistry profile, complete blood count, thyroid function tests, vitamin B12 level, folic acid level and syphilis serology. Patients were scanned within the framework of their routine diagnostic investigation and controls gave their informed consent for this research project. The MRI data acquisition protocol was approved by the local ethics committee of the Medical University Hospital Innsbruck, Innsbruck, Austria.
Data acquisition, pre‐processing and analysis
All participants were scanned on the same 1.5 T Siemens Symphony MRI scanner using a T1‐weighted fast low‐angle shot three‐dimensional sequence with a repetition time of 9.7 ms, an echo time of 4 ms, a matrix size of 256×256 and a field of view of 230 mm, yielding sagittal slices with a thickness of 1.5 mm and an in‐plane resolution of 0.98×0.98 mm. These raw images were pre‐processed using the optimised protocol described by Good et al20 and analysed using SPM2 software (Welcome Department of Neurology, London, UK) implemented in Matlab V.6.5 (Mathworks, Sherborn, Massachusetts, USA).
Customised template creation
The study group‐specific template was created to minimise the scanner‐specific bias by averaging all images from the study‐specific subject group, after being normalised using an affine‐only procedure. Probability maps were obtained by segmenting the individual normalised images into GM, WM and cerebrospinal fluid (CSF), averaging and smoothing with an isotropic Gaussian kernel of 8 mm full‐width at half‐maximum.
Segmentation
The optimised VBM protocol20 includes two segmentation steps: (1) segmentation was performed in native space and non‐brain tissue removed automatically by modulation with an individually derived brain‐tissue mask; and (2) segmentation was performed after applying the normalisation parameters to the original whole‐brain images, including, once again, removing of non‐brain tissue followed by reslicing onto a voxel size of 1×1×1 mm.
Normalisation
The spatial normalisation parameters were estimated by matching the native spaced individual GM image with the study‐specific GM template.
Modulation
Voxel values of the segmented images were multiplied with the Jacobian determinants to convert the GM segments into measures of absolute GM volume, as opposed to relative GM volume following spatial normalisation.
Smoothing
Finally, all modulated images were smoothed with a 10 mm full‐width at half‐maximum Gaussian kernel.
Statistical analyses were performed with SPM2 using the general linear model‐based on the Gaussian field theory. The global mean voxel values and the total intracranial volumes (obtained by summing up GM, WM and CSF voxels) were used as confounding covariates in an analysis of covariance to focus on the regional differences in GM. The significance level was set at p<0.05 false discovery rate corrected for multiple comparisons across the entire brain volume.
Results
Patients with alcohol addiction demonstrated lower volumes of GM (patients: mean (SD) 569.4 (63.5) ml; controls: mean (SD) 631.9 (62.75) ml; p = 0.002) and WM (patients: mean (SD) 435.5 (61.2) ml; controls: mean (SD) 470.1 (68.9) ml; p = 0.085), as well as increased CSF volumes (patients: mean (SD) 712.7 (137.7) ml; controls: mean (SD) 565.7 (93.2) ml; p = 0.001). Total intracranial volumes were equal in both groups (patients: mean (SD) 1717.6 (205.6) ml; controls: mean (SD) 1667.7 (202.8) ml; p = 0.423).
Table 1 presents GM statistics for different brain regions. Significantly reduced GM volumes (p<0.001) in patients with alcohol addiction have been found in the right and left anterior and dorsal parts of the thalamic region as well as in the left thalamic nucleus medialis as compared with controls (table 1, fig 1). Right and left dorsal hippocampus (p = 0.005 and p<0.001, respectively) and cerebellum (p = 0.003) also showed significantly reduced GM volumes in patients as compared with controls (table 1). In patients, precentral gyrus (Brodmann area 6) and middle frontal gyrus (Brodmann area 9) were significantly (p<0.001) altered in both hemispheres. Furthermore, area 46 (middle frontal gyrus) showed reduced volume in the left hemisphere (p<0.001). The insular region (Brodmann area 13) was found to be altered in the right hemisphere (p<0.001) as compared with controls.
Table 1 Reduced grey matter volumes in patients with alcohol addiction: anatomical locations, Brodmann areas, z scores and p values (false discovery rate corrected for multiple comparisons across the entire volume).
| Location | BA | Peak coordinates (mm) | Cluster size | z Value | p Value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Thalamus | |||||||
| Right | 6 | −13 | 15 | 8232 | 6.27 | <0.001 | |
| Left | −7 | −13 | 15 | 8232 | 6.27 | <0.001 | |
| Dorsal hippocampus | |||||||
| Right | 32 | −34 | −5 | 8232 | 3.84 | 0.005 | |
| Left | −28 | −45 | −4 | 8232 | 4.74 | <0.001 | |
| Precentral gyrus | |||||||
| Right | 6 | 57 | −7 | 34 | 3845 | 6.21 | <0.001 |
| Left | 6 | −57 | −11 | 31 | 276 | 5.13 | <0.001 |
| Middle frontal gyrus | |||||||
| Right | 9 | 45 | 12 | 32 | 3845 | 5.17 | <0.001 |
| Left | 9 | −41 | 16 | 28 | 889 | 5.31 | <0.001 |
| Left | 46 | −44 | 27 | 25 | 967 | 4.68 | <0.001 |
| Insula | |||||||
| Right | 13 | 42 | −19 | 10 | 4870 | 4.74 | <0.001 |
| Left | 13 | −37 | 20 | 7 | 518 | 3.82 | 0.005 |
| Cerebellum | |||||||
| Right | 40 | −47 | −51 | 2048 | 4.04 | 0.003 | |
| Left | −42 | −48 | −51 | 275 | 4.02 | 0.003 | |
BA, Brodmann areas; MNI, Montreal Neurological Institute.
Peak coordinates are given in MNI space (http://www.bic.mni.mcgill.ca).
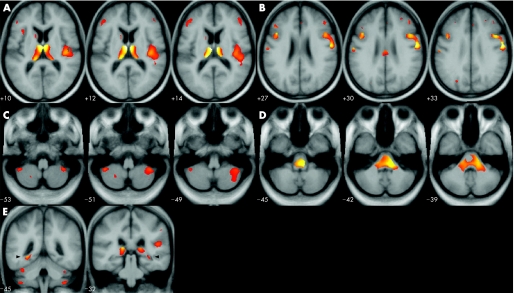
Figure 1 Areas of significant grey and white matter decrease in patients with alcohol addiction relative to healthy controls. Results are illustrated as statistical parametric map blobs superimposed on the slices of a T1‐weighted mean picture in standard stereotactic space from all 44 study participants. The left side of the figure is the left side of the brain. Threshold was set at p>3.31 (peak). (A) Thalamus, insula; (B) middle frontal gyrus, precentral gyrus; (C) cerebellum; (D) brainstem; (E) dorsal hippocampus.
Table 2 presents the VBM results of WM. Patients with alcohol addiction showed significant volume loss (p = 0.001) in the entire periventricular WM (anterior, central and posterior parts) and reduced volume of the pons (p<0.001) and cerebellar pedunculi (p<0.001).
Table 2 Reduced white matter volumes in patients with alcohol addiction: anatomical locations, Brodmann areas, z scores and p values (false discovery rate corrected for multiple comparisons across the entire volume).
| Location | BA | Peak coordinates (mm) | z Value | p Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Pons | 2 | −35 | −48 | 5.11 | <0.001 | |
| Cerebellum | ||||||
| Pedunculi mediales/inferiores | ||||||
| Right | 6 | 57 | −7 | 34 | 6.21 | <0.001 |
| Left | 6 | −57 | −11 | 31 | 5.13 | <0.001 |
BA, Brodmann areas; MNI, Montreal Neurological Institute.
Peak coordinates are given in MNI space (http://www.bic.mni.mcgill.ca).
An additional analysis focusing on gender differences was also performed despite the smaller number of female participants. Age and gender were included as confounding covariates. At a p<0.05 level after correction for multiple comparisons, no significant regional differences were detected.
Discussion
In this study, we investigated the distribution patterns of brain atrophy in patients with alcohol addiction using VBM, which allows the analysis of regional GM and WM partitions without predefining regions of interest. This method provides the possibility to analyse complex patterns of brain atrophy also in those brain regions that are difficult to investigate using anatomically based methods of volumetry. Previous studies have shown that VBM is more sensitive in detecting subtle changes in brain volume than conventional methods of volumetry.14,16,20 To minimise methodological bias, we used an optimised protocol based on the creation of study‐specific templates and the modulation of the segmented GM partitions to compensate for volume changes in brain normalisation.20
One of the most intriguing findings of this study is the pronounced decrease in GM volumes in the thalamus of patients with alcohol addiction. These data are consistent with several previous reports on the involvement of thalamic neuronal circuits in different behavioural changes in patients with alcohol dependence.8 On the other hand, our data contradict those of a previous study demonstrating reduced thalamic volume only in subjects with Korsakoff's syndrome but not in subjects with chronic alcoholism using conventional MRI volumetry.4 In contrast with that, George et al21 have shown that patients with alcohol addiction, when exposed to alcohol cues, have increased brain activity in the prefrontal cortex and anterior thalamic regions, which are associated with regulation of emotions, attention and appetitive behaviour. The most recent study on this issue has shown a significant role of the thalamus in processing cue‐related information and in controlling alcohol‐related behaviour.22 The reduction of GM volumes found in this study may suggest functional insufficiency of thalamic regions that are responsible for altered behavioural patterns occurring in patients with alcohol addiction.
The analysis of our data suggests alcohol‐induced alterations of the posterior hippocampus as well. Although previous neuropathological studies failed to prove alcohol‐associated neurodegeneration of the hippocampus in human brains,11 animal models have demonstrated that binge drinking of ethanol can produce necrotic neurodegeneration in the areas of the brain most closely associated with the hippocampus.23 Our findings support the hypothesis of involvement of the hippocampus in the brain of patients with alcohol addiction. There exists ample evidence of possible mechanisms underlying the effect of alcohol on the hippocampus. The hippocampus is the area with the greatest increase in lipofuscin deposition in neurons as a result of chronic alcohol consumption.24 Further, the fatty acid ethyl esters produced in the brain from ethanol are known to be particularly damaging to the hippocampus.25
Our data are further consistent with the previous findings on involvement of frontal cortical areas in the brain of patients with alcohol addiction. Numerous neuropsychological studies demonstrated substantial deficits in frontal executive functions in patients with alcohol dependence.6,26,27 Our results suggest substantial volume reduction in the middle frontal gyrus and precentral gyrus, although no changes were detected in other frontal regions. These findings support previous reports on decreased glucose metabolic rates in middle frontal regions in patients with alcohol addiction 28 and a reduction of γ aminobutyric acid A/benzodiazepine receptors in superior medial parts of the frontal lobes.29
A significant decrease in WM volumes in the pons and cerebellum in the our study is consistent with the previous results that have shown the alcohol‐associated degeneration of pontine and cerebellar WM in patients with alcohol addiction,7,30,31 whereas in healthy subjects these regions have been shown to remain stable across the entire age span in both men and women.32 The significant decrease in periventricular WM found in our study is also consistent with previous data; however, the analysis and interpretation of these changes in VBM studies is difficult because of the possible bias due to partial volume effects.
Even low‐to‐moderate consumption of alcohol was associated with brain atrophy in a study of middle‐aged men.5 Ethanol can increase the release of arachidonic acid from cell membranes and cause oxidative stress in the brain by increased cyclo‐oxygenase activity. Furthermore, hydroxyethyl free radicals derived directly from ethanol are nearly as damaging as hydroxyl radicals.33 There is also evidence from animal studies that alcohol causes cell death. Rats fed a liquid diet containing moderate amounts of ethanol for 6 weeks had a 66.3% decrease in the number of new neurons and a 227–279% increase in cell death in the dentate gyrus as compared with rats fed an alcohol‐free diet.34
In general, our data support the previous assumption that the regional reduction of GM volumes may result from alcohol‐induced neuronal loss, whereas global brain shrinkage might be caused by loss of WM.1 Furthermore, our results support previous findings on alteration of selected regions of the frontal cortex and cerebellum in patients with alcohol addiction and suggest the involvement of the anterior thalamus, posterior hippocampus, insular cortex and periventricular WM in alcohol‐associated brain damage. A causal relationship between alcohol consumption and regional brain atrophy still demands further research, whereas VBM seems to represent a tool of choice for in vivo detection of the brain areas predisposed to alcohol‐induced damage.
Abbreviations
CSF - cerebrospinal fluid
GM - grey matter
VBM - voxel‐based morphometry
WM - white matter
Footnotes
Competing interests: None declared.
References
- 1.Harper C. The neuropathology of alcohol‐specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol 199857101–110. [DOI] [PubMed] [Google Scholar]
- 2.Pfefferbaum A, Rosenbloom M, Deshmukh A.et al Sex differences in the effects of alcohol on brain structure. Am J Psychiatry 2001158188–197. [DOI] [PubMed] [Google Scholar]
- 3.Pfefferbaum A, Sullivan E V, Mathalon D H.et al Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 199721521–529. [DOI] [PubMed] [Google Scholar]
- 4.Visser P J, Krabbendam L, Verhey F R.et al Brain correlates of memory dysfunction in alcoholic Korsakoff's syndrome. J Neurol Neurosurg Psychiatry 199967774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding J, Eigenbrodt M L, Mosley T H., Jret al Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community‐based population of middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke 20043516–21. [DOI] [PubMed] [Google Scholar]
- 6.Moselhy H F, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 200136357–368. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan E V, Pfefferbaum A. Magnetic resonance relaxometry reveals central pontine abnormalities in clinically asymptomatic alcoholic men. Alcohol Clin Exp Res 2001251206–1212. [PubMed] [Google Scholar]
- 8.Kril J J, Halliday G M. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol 199958381–387. [DOI] [PubMed] [Google Scholar]
- 9.Cullen K M, Halliday G M, Caine D.et al The nucleus basalis (Ch4) in the alcoholic Wernicke‐Korsakoff syndrome: reduced cell number in both amnesic and non‐amnesic patients. J Neurol Neurosurg Psychiatry 199763315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding A, Halliday G, Caine D.et al Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 2000123(Pt 1)141–154. [DOI] [PubMed] [Google Scholar]
- 11.Harding A J, Wong A, Svoboda M.et al Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 1997778–87. [DOI] [PubMed] [Google Scholar]
- 12.Mann K, Widmann U. The neurobiology of alcoholism. Neuropathology and CT/NMR findings. Fortschr Neurol Psychiatr 199563238–247. [DOI] [PubMed] [Google Scholar]
- 13.Bloomer C W, Langleben D D, Meyerhoff D J. Magnetic resonance detects brainstem changes in chronic, active heavy drinkers. Psychiatry Res 2004132209–218. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner J, Friston K J. Voxel‐based morphometry—the methods. Neuroimage 200011805–821. [DOI] [PubMed] [Google Scholar]
- 15.Kubicki M, Shenton M E, Salisbury D F.et al Voxel‐based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002171711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abell F, Krams M, Ashburner J.et al The neuroanatomy of autism: a voxel‐based whole brain analysis of structural scans. Neuroreport 1999101647–1651. [DOI] [PubMed] [Google Scholar]
- 17.Karas G B, Scheltens P, Rombouts S A.et al Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage 200423708–716. [DOI] [PubMed] [Google Scholar]
- 18.Brenneis C, Wenning G K, Egger K E.et al Basal forebrain atrophy is a distinctive pattern in dementia with Lewy bodies. Neuroreport 2004151711–1714. [DOI] [PubMed] [Google Scholar]
- 19.Caine D, Halliday G M, Kril J J.et al Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry 19976251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 21.George M S, Anton R F, Bloomer C.et al Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol‐specific cues. Arch Gen Psychiatry 200158345–352. [DOI] [PubMed] [Google Scholar]
- 22.Hermann D, Smolka M N, Wrase J.et al Blockade of cue‐induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 2006301349–1354. [DOI] [PubMed] [Google Scholar]
- 23.Obernier J A, Bouldin T W, Crews F T. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res 200226547–557. [PubMed] [Google Scholar]
- 24.Borges M M, Paula‐Barbosa M M, Volk B. Chronic alcohol consumption induces lipofuscin deposition in the rat hippocampus. Neurobiol Aging 19867347–355. [DOI] [PubMed] [Google Scholar]
- 25.Gubitosi‐Klug R A, Gross R W. Fatty acid ethyl esters, nonoxidative metabolites of ethanol, accelerate the kinetics of activation of the human brain delayed rectifier K+ channel, Kv1.1. J Biol Chem 199627132519–32522. [DOI] [PubMed] [Google Scholar]
- 26.Beatty W W, Hames K A, Blanco C R.et al Visuospatial perception, construction and memory in alcoholism. J Stud Alcohol 199657136–143. [DOI] [PubMed] [Google Scholar]
- 27.Nixon K, Crews F T. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 2002831087–1093. [DOI] [PubMed] [Google Scholar]
- 28.Adams K M, Gilman S, Koeppe R A.et al Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res 199317205–210. [DOI] [PubMed] [Google Scholar]
- 29.Gilman S, Koeppe R A, Adams K.et al Positron emission tomographic studies of cerebral benzodiazepine‐receptor binding in chronic alcoholics. Ann Neurol 199640163–171. [DOI] [PubMed] [Google Scholar]
- 30.Pfefferbaum A, Rosenbloom M, Serventi K L.et al Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin Exp Res 200226400–406. [PubMed] [Google Scholar]
- 31.Pfefferbaum A, Sullivan E V. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 200215708–718. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan E V, Rosenbloom M, Serventi K L.et al Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 200425185–192. [DOI] [PubMed] [Google Scholar]
- 33.Sun A Y, Simonyi A, Sun G Y. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med 200232314–318. [DOI] [PubMed] [Google Scholar]
- 34.Herrera D G, Yague A G, Johnsen‐Soriano S.et al Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA 20031007919–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]