Stiff person syndrome (SPS) is characterised by muscle rigidity and episodic spasms involving the axial and limb musculature. The hallmark is co‐contraction of agonist and antagonist muscles, with continuous motor unit firing at rest. An autoimmune pathogenesis is based on the presence of anti‐glutamic acid decarboxylase antibodies (anti‐GAD, rate‐limiting enzyme to synthesise γ‐aminobutyric acid (GABA), in presynaptic GABAergic inhibitory synapses), and association with other autoimmune disorders and autoantibodies, and on response to immunotherapy. There is only a limited number of cases with brain MRI abnormalities. Our patient is the first reported case of SPS with MRI striatal abnormalities.
Case report
A 69‐year‐old woman came in for consultation with an 18‐month history of back pain, stiffness and progressive difficulty at walking. She had developed asymmetrical rigidity in her legs, with the left one being worse, from falls. In one of these falls she broke her left humerus. That limb remains useless, painful and rigid. She had progressive difficulty in turning over in bed and in getting up. In stressful situations and when trying to move, she had painful spasms in the left leg starting distally with toe and ankle extension, and progressing to knee and hip flexion while raising the leg from the bed. She became progressively bedridden. Her medical history was notable for diabetes mellitus (controlled with acarbose) and hypertension (controlled without drugs). A neurological examination showed stiffness and limitation in the range of active and passive movements in the left leg, left arm and, less pronouncedly, in the right leg. Also, there was slowing of finger, hand and arm movements in the left hand. She found it difficult to raise her left leg, which sometimes developed into painful spasms. She had decreased facial expression, cogwheel rigidity in both superior extremities and stony lumbar rigidity. Plantar reflexes were flexor and deep tendon reflexes were symmetrically brisk. She needed help to sit down and was afraid to walk even with aid. On the other hand, her mental status, speech and cranial nerves were normal.
The results of blood tests, including antinuclear antibody, endomysial, gliadin, transglutaminase, mitochondrial, smooth muscle, parietal, anti‐LKM1 (autoimmune hepatitis), thyroid peroxidase and thyroglobulin, were negative. Levels of folic acid, vitamin B12, T4, thyroid‐stimulating hormone, rheumatoid factor, C‐reactive protein, anti‐streptolysin O, immunoglobulins and complement were normal. Serum anti‐GAD level was 14 000 U/ml (radioimmunoassay). Also, the patient tested negative for anti‐Yo, anti‐Hu, anti‐Ri and antiampiphysin. Serum anti‐GAD increased to 60 400 U/ml (N<1 U/ml).
The patient rejected lumbar puncture. Nerve conduction was normal and electromyography showed normal motor unit potentials, an inability to relax any of the muscles tested, and continuous motor unit activity.
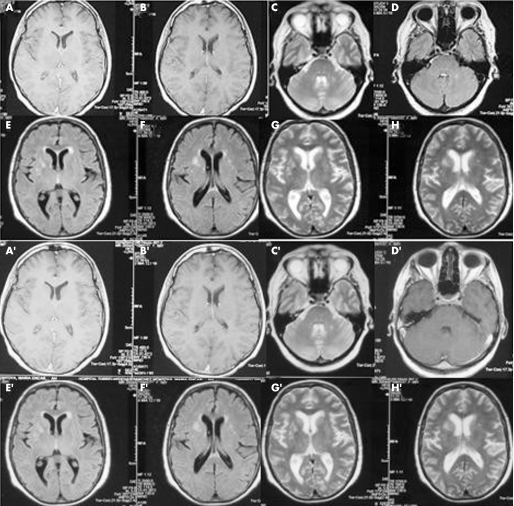
Cervical and lumbar MRI was normal. Cerebral MRI showed bright hyperintense changes on fluid‐attenuated inversion recovery (FLAIR) and T2 in both striatal regions (more intense in the right striatum corresponding to the more symptomatic left side), and a lesion in the left middle cerebellar peduncle on T2, the only one enhancing with gadolinium (fig 1). Treatment was started with L‐dopa without improvement. The patient improved after diazepam (25 mg/day) and a 5‐day course of intravenous immunoglobulin (0.4 mg/kg/day). Spasms and pain disappeared, and she was able to walk without aid after discharge (12 days in hospital). After 6 months, MRI was similar, and on follow‐up her gait was slow but independent. Spasms, rigidity and pain on the legs disappeared. Rigidity and limitation to abduct the left shoulder and to extend the left elbow persisted, and hence another 5‐day course of intravenous immunoglobulin was infused. After 2 weeks, she reported improvement in walking and less rigidity on the left shoulder.
Figure 1 (A,B) Normal post‐gadolinium T1. (C) Left middle cerebellar peduncle T2 lesion, (D) enhancing T1. (E–H) Bilateral striatal hyperintense lesions (right predominant) in fluid‐attenuated inversion recovery (E, F) and T2 (G,H). (A′–H′) MRI after 6 months. The only clear difference is absence of enhancement in the left middle cerebellar peduncle post‐gadolinium T1 (D′).
Discussion
The absence of structural MRI changes in most cases suggests that SPS is a functional rather than a structural disorder. However, here are some MRI findings in patients with SPS.
Cranial T2 MRI hyperintensity in the temporal lobes, hypothalamus and pons in a 71‐year‐old patient with paraneoplastic SPS (amphiphysin+, anti‐GAD−), opsoclonus and encephalopathy.1
Spinal T2 hyperintense lesion (C2–C7) in a patient with SPS with lymphocytic pleocytosis of the cerebrospinal fluid (CSF) and oligoclonal bands. Amphiphysin autoantibodies were detected in serum and CSF. No malignancy occurred during a 3‐year period.2
Atrophy and hyperintense T2 left hippocampus in a 22‐year‐old woman with SPS and seizures.3
Bright signal on FLAIR in the right hippocampus in a 12‐year‐old boy with SPS anti‐GAD+ and a 3‐year history of type 1 diabetes.4
T1‐weighted midline cerebellar atrophy in a 38‐year‐old woman with anti‐GAD+ SPS and eye movement abnormalities without evidence of myasthenia over 12 years.5
There are a few postmortem studies. The relevant findings are: in anti‐GAD+ SPS, reduction of small spinal neurons and changes in larger alpha‐motor neurons, as well as selective depletion of Purkinje cells (GAD‐containing neurones), have taken place; in paraneoplastic SPS (anti‐GAD−, amphiphysin positive), brainstem encephalitis has led to lymphocytic infiltrates, neuronal loss, gliosis and perivascular lymphocytic cuffs.
The pathogenic role of anti‐GAD is controversial. Up to 65% of SPS cases have anti‐GAD, but in paraneoplastic SPS, associated with antibodies to amphiphysin and gephyrin, anti‐GAD are rarely present. Although uncommon, anti‐GAD have been reported in other neurological diseases such as cerebellar ataxia, drug‐resistant epilepsy, tremor of the mouth floor and periodic alternating nystagmus. Anti‐GAD have also been found in diabetes and other autoimmune diseases in a lesser proportion.
In vitro, anti‐GAD inhibit GAD activity; however, in vivo it is uncertain (antibodies circulate extracellularly, but GAD is intracellular).
In SPS, the concentration of GABA is reduced in CSF and assessed by magnetic resonance spectroscopy; GABA is decreased in the sensorimotor cortex. GABA is the major inhibitory neurotransmitter in the striatum and GAD activity was detected in spiny and non‐spiny striatal neurons. Medium spiny projection neurons comprise 90–95% of the striatal neuronal population and are inhibitory via GABA. The basal ganglia inhibit undesirable or competing movements and postural synergies initiated by cerebral cortical mechanisms, with help from the cerebellum.
GABA levels are decreased in the contralateral sensorimotor cortex and lentiform nuclei in focal hand dystonia. In Huntington's disease, striatal neuronal dysfunction would produce an inability to inhibit unwanted movements, resulting in chorea. In our patient with SPS, striatal neuronal dysfunction might have produced inability to inhibit simultaneous co‐contraction of agonist and antagonist muscles. Either functional or structural striatal abnormalities may play a role in SPS, but more studies are needed to confirm this hypothesis.
Footnotes
Competing interests: None.
References
- 1.Wessig C, Klein R, Schneider M F.et al Neuropathology and binding studies in anti‐amphiphysin‐associated stiff‐person syndrome. Neurology 200361195–198. [DOI] [PubMed] [Google Scholar]
- 2.Schmierer K, Valdueza J M, Bender A.et al Atypical stiff‐person syndrome with spinal MRI findings, amphiphysin autoantibodies, and immunosuppression. Neurol Am Acad Neurol 199851250–252. [DOI] [PubMed] [Google Scholar]
- 3.Prevett M C, Brown P, Duncan J S. Improvement of stiff‐man syndrome with vigabatrin. Neurol Am Acad Neurol 1997481133–1134. [DOI] [PubMed] [Google Scholar]
- 4.Mikaeloff Y, Jambaque I, Mayer M.et al Benefit of intravenous immunoglobulin in autoimmune stiff‐person syndrome in a child. J Pediatr 2001139340. [DOI] [PubMed] [Google Scholar]
- 5.Economides J R, Horton J C. Eye movement abnormalities in stiff person syndrome. Neurology 2005651462–1464. [DOI] [PubMed] [Google Scholar]