Abstract
Background
Progressive supranuclear palsy (PSP) is a progressive neurodegenerative disorder involving motor and cognitive dysfunction. Currently, there is no effective treatment either for symptomatic relief or disease modification. This relates, in part, to a lack of knowledge of the underlying neurochemical abnormalities, including cholinergic receptor status in the basal ganglia.
Aim
To measure muscarinic M2 and M4 receptors in the basal ganglia in PSP.
Methods
The muscarinic M2 (presynaptic) and M4 (postsynaptic) receptors in the striatum, pallidum and adjacent insular cortex were autoradiographically measured in pathologically confirmed cases of PSP (n = 18), and compared with cases of Lewy body dementias (LBDs; n = 45), Alzheimer's disease (AD; n = 39) and controls (n = 50).
Results
In cases of PSP, there was a reduction in M2 and M4 receptors in the posterior caudate and putamen compared to controls, but no significant changes in the pallidum. Cases with AD showed lower M2 receptors in the posterior striatum. Groups with LBD and AD showed higher M2 binding in the insular cortex compared with controls.
Conclusions
The results suggest loss of posterior striatal cholinergic interneurones in PSP, and reduction in medium spiny projection neurones bearing M4 receptors. These results should be taken in the context of more widespread pathology in PSP, but may have implications for future trials of cholinergic treatments.
Progressive supranuclear palsy (PSP) was first described by Steele et al in 19641, as a disease causing vertical gaze and pseudobulbar palsy, early falls, parkinsonism and dementia. Patients typically present in their 60s and the average time from symptom‐onset to death ranges from 5 to 8.6 years.2,3,4,5
Neuropathological hallmarks of PSP include neurofibrillar tangles, neuropil threads and tufted astrocytes which are found predominantly within the basal ganglia, midbrain, pontine reticular formations and to a lesser extent, the thalamus.6 The pathological inclusions comprise insoluble aggregates of four‐repeat τ phosphoprotein. The basal ganglia, in particular the striatum (caudate and putamen) and its connections, are involved in motor and cognitive aspects of PSP.
Neurochemical abnormalities are found within the dopaminergic, cholinergic and possibly GABAergic systems.7,8 Cholinergic deficits are thought to be responsible for the early postural instability and cognitive impairment commonly found in PSP. Trials of a muscarinic M1/M2 cholinergic agonist9 and cholinesterase inhibitors10,11 have, however, failed to show improvement in motor function, quality of life or cognitive impairment.
In cases of PSP, there are abnormalities within the cholinergic system based on pathology in known cholinergic nuclei and reduced markers for the synthesis and release of acetylcholine (ACh) but there is a dearth of knowledge about the status of cholinergic receptors.
M1, M2 and M4 receptors are the predominant muscarinic receptors found in the striatum. We have recently demonstrated no significant difference in the M1 receptors in the striatum of patients with PSP compared with controls.12 This suggests preservation of the medium spiny neurones bearing these receptors, which are predominantly involved in the indirect pathway (projecting to the external globus pallidus (GPe)). M2 receptors are found presynaptically on striatal cholinergic interneurones, and also on corticostriatal and thalamostriatal afferents. Their activation inhibits the release of neurotransmitter and, therefore, through the cholinergic interneurones they provide a negative feedback mechanism for the release of ACh. M4 receptors are predominantly located on the postsynaptic membranes of striatal medium spiny projection neurones expressing D1 receptors, which form part of the direct pathway, projecting to the internal globus pallidus (GPi). When activated, M2 and M4 receptors inhibit adenylyl cyclase by a complex cascade of events through a G protein.
We aimed to define the status of the muscarinic M2 and M4 receptors in the striatum and pallidum to provide a more detailed understanding of the basal ganglia cholinergic neurochemistry of PSP. Cases with Lewy body dementia (LBD, a group comprising dementia with Lewy bodies (DLB) and Parkinson's disease with dementia (PDD)) and Alzheimer's disease (AD) were chosen as disease controls as they share some cliniconeurochemical similarities with PSP. Notably, cholinesterase inhibitors improve cognitive and neuropsychiatric symptoms in patients with DLB, PDD and AD13,14,15 but are not beneficial in PSP, showing no demonstrable improvement in cognition and a possibly detrimental effect on motor symptoms.11,16
Methods
Subjects
Eighteen pathologically confirmed cases of PSP, from which anterior striatal sections were available in 16 and posterior in 12, were acquired from the Newcastle Brain Bank, Newcastle upon Tyne, UK and the Sara Koe PSP Research Centre, Institute of Neurology, University College London, London, UK. They were compared with age‐matched controls (n = 50, 27 anterior striatum, 33 posterior striatum) and patients with LBD (n = 45 (27 DLB and 18 PDD), 37 anterior striatum, 34 posterior striatum) and AD (n = 39, 27 anterior striatum, 27 posterior striatum). Clinical information for patients with PSP was obtained where possible, through a retrospective notes review. Patients with DLB, PDD and AD were prospectively assessed annually. Table 1 shows the patient demographics. One patient with LBD was taking a cholinesterase inhibitor, and three were taking anticholinergics. None of the groups with PSP or AD had taken cholinergic drugs. There were no significant intergroup differences in postmortem delay. Table 2 gives a summary of the pathological findings in cases of PSP in relevant areas. Consent for brain donation was obtained either from the patient, or/and the next of kin, in accordance with the local research ethics committee (Newcastle and North Tyneside) and the London Multi‐Centre Research Ethics Committee.
Table 1 Demographic details of study groups.
| Control (n = 50) | PSP (n = 18) | LBD (n = 45) | AD (n = 39) | |
|---|---|---|---|---|
| Mean (SD) age at death (years) | 79.6 (8.8) | 76.0 (8.2) | 79.3 (7.0) | 83.9 (6.1)* |
| Male/Female | 17/33 | 11/7 | 25/20 | 19/20 |
| Mean (SD), disease duration (years) | – | 6.1 (3.5) | 7.8 (5.6) | 5.5 (2.9) |
| PM delay (hours) | 38.4 (22.5) | 45.7 (23.4) | 37.4 (21.2) | 44.9 (23.8) |
AD, Alzheimer's disease; LBD, Lewy body dementia; PSP, progressive supranuclear palsy; PM, post mortem.
*AD age higher vs controls and LBD p<0.05, and vs PSP p<0.01.
Table 2 Range of progressive supranuclear palsy pathology found in 18 cases.
| Caudate (anterior and posterior levels) | Putamen (anterior and posterior levels) | GPe | GPi | ||||
|---|---|---|---|---|---|---|---|
| NL | NFT | NL | NFT | NL | NFT | NL | NFT |
| 0/+ | ++ to +++ | + | + to ++ | +++ | ++ to +++ | ++ | ++ to +++ |
+ = mild, ++ = moderate, +++ = severely affected. GPe, external globus pallidus; GPi, internal globus pallidus; NL, neuronal loss; NFT, neurofibrillary tangles.
Tissue preparation
The right cerebral hemisphere was fixed and used for pathological diagnosis and the left cerebral hemisphere was sliced into 1 cm thick coronal blocks and rapidly frozen in liquid Arcton cooled over liquid nitrogen and stored at −70°C. Cases acquired in the past 3 years have been quickly frozen on copper plates maintained at −80°C. There is no difference in tissue quality between the two freezing methods. Frozen tissue sections containing basal ganglia at a level including the striatum, globus pallidus and insular cortex were subdissected. Anterior striatum was up to, and including, coronal level 13, and posterior from coronal level 14, that is rostral and caudal of the anterior commissure.17 For autoradiography, 20 μm cryostat sections were cut and dried onto Vectabond‐coated (Vector labs, Peterborough, UK) slides before storage at –70°C. Prior to assay, slides were taken to room temperature and air dried for 1–2 h.
Autoradiographic ligand binding assays
The total and selective binding was determined in triplicate contiguous sections and non‐specific binding established in one section by the addition of 2 μM atropine. At room temperature, sections were prewashed in a buffer (10 mM KH2PO4, 10 mM Na2HPO4 pH 7.4) for 15 min to remove any endogenous ligand, for example acetylcholine or residual drugs. M2 and M4 combined receptor density was measured using 3H AFDX 384, which labels both receptors (total binding) and, in adjacent sections, selectively blocking M4 binding with dicyclomine. After prewashing with a buffer, 4.8 nM 3H AFDX 384 alone, with dicyclomine (10 nM) or with atropine was added and incubated for 1 h at room temperature. Sections were washed in the buffer two times for 2 min before being dipped in ice‐cold water, dried and apposed to tritium‐sensitive hyperfilm (Amersham, GE Healthcare, Little Chalfont, UK) for 7 weeks.
Developing and analysis
Films were developed after warming to room temperature (2 h) before development using 500 ml D19 for 5 min, stopped using 500 ml 1% aqueous acetic acid for 1 min, fixed using 500 ml 25% Unifix (H.A. West, Gateshead, UK) for 6 min and washed for 20 min in running water. Films were dried and binding was assessed by comparison to 3H autoradiographic microscale standards (Amersham) using MCID M5+ image analysis (Imaging Research GE Healthcare, Interfocus, Linton, UK) to give binding in fmols/mg. The specific binding for combined M2 and M4 receptors was calculated by subtracting the non‐specific binding from the total. Similarly, M2 was determined by subtracting non‐specific binding from total plus dicyclomine. M4 was then determined by taking the M2‐binding value away from the total.
Statistical analysis
Statistical analysis was performed using MINITAB V.13. For the neurochemical data, results were compared between PSP, controls, LBD and AD. Where possible, data were correlated with clinical measures. To ensure a normal distribution, results were logged before analysis and the means were antilogged. Results are presented as geometric means and SD. Statistical tests included one‐way analysis of variance between disease groups, with post hoc analysis using Tukey's pairwise comparisons where indicated. Regression analysis was used to explore potential correlations between clinical variables, demographics and neurochemical data.
Results
Progressive supranuclear palsy
M2 receptor binding
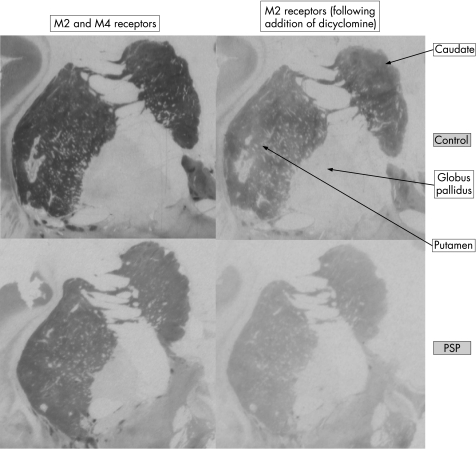
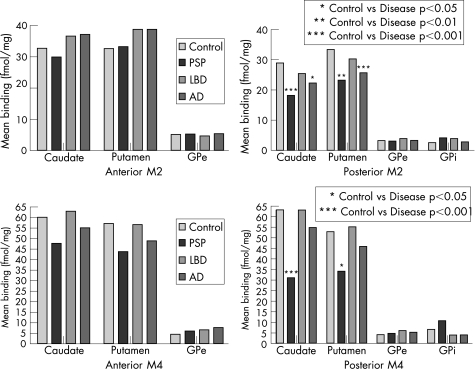
Table 3 and figs 1 and 2 show the results for M2 receptor binding. M2 binding in controls was highest in the caudate and putamen, and lowest in the pallidum. A striatal rostrocaudal reduction was seen in M2 binding. This gradient was more marked in the group with PSP. The density of M2 receptor binding was significantly reduced in the posterior caudate (–37%, p<0.001) and putamen (–31%, p<0.01) in PSP compared with controls. In the anterior striatum, there was no significant change in M2 binding. There was no significant difference in M2 binding in either the internal or external globus pallidus. Reduced M2 binding was seen in the posterior insular cortex compared with controls (–27%, p<0.05).
Table 3 M2 receptor binding in the anterior and posterior striatum.
| M2 anterior M2 posterior | Control | PSP | LBD | AD |
|---|---|---|---|---|
| Caudate | 32.5 (0.1) | 29.7 (0.1) | 36.4 (0.1) | 36.9 (0.2) |
| 28.7 (0.1) | 18.0 (0.2)‡ | 25.2 (0.1)§ | 22.1 (0.2)* | |
| Putamen | 32.4 (0.1) | 33.0 (0.1) | 38.6 (0.1) | 38.6 (0.2) |
| 33.1 (0.1) | 23.0 (0.1)† | 30.0 (0.1) | 25.4 (0.2)‡ | |
| GPe | 4.9 (0.3) | 5.0 (0.3) | 4.4 (0.3) | 5.2 (0.3) |
| 2.9 (0.4) | 2.8 (0.1) | 3.6 (0.2) | 3.0 (0.5) | |
| GPi | 2.3 (0.4) | 3.9 (0.2) | 3.7 (0.2) | 2.6 (0.5) |
| Insular cortex | 14.0 (0.1) | 17.1 (0.2) | 19.7 (0.2)† | 18.8 (0.2)* |
| 15.6 (0.1) | 11.4 (0.2)* | 15.7 (0.1)§ | 13.5 (0.2) |
AD, Alzheimer's disease; GPe, external globus pallidus; GPi, internal globus pallidus; LBD, Lewy body dementia; PSP, progressive supranuclear palsy.
Values in bold indicate M2 receptor binding in the anterior striatum.
Values expressed as geometric means (SD) in fmol/mg.
*Disease vs control, p<0.05.
†Disease vs control, p<0.01.
‡Disease vs control, p<0.001.
§PSP vs LBD, p<0.05.
Figure 1 Autoradiography photographs showing total M2/M4 binding using 3H AFDX and M2 receptors only, using dicyclomine to block M4 receptors.
Figure 2 M2 and M4 receptors.
M4 receptor binding
Table 4 and figs 1 and 2 show the results for M4 receptor binding. Highest binding in controls was again in the striatum with low binding in the pallidum. There was a rostrocaudal reduction in M4 receptors in PSP, which was not evident in the other groups. The posterior striatum showed significantly lower M4 binding than controls (caudate reduced by 51%, p<0.001, and putamen by 36%, p<0.05). M4 binding in the caudate was also significantly lower than in groups with LBD and AD and the M4 binding in the putamen was lower than in groups with LBD. GPi M4 binding was increased in those with PSP relative to all groups, but this did not reach statistical significance. M4 receptor binding in the insular cortex was lower in cases of PSP than controls (–33%, p<0.05) and LBD (–44%, p<0.001).
Table 4 M4 receptor binding in the anterior and posterior striatum.
| M4 anterior M4 posterior | Control | PSP | LBD | AD |
|---|---|---|---|---|
| Caudate | 59.7 (0.1) | 47.3 (0.3) | 62.6 (0.2) | 54.7 (0.2) |
| 62.8 (0.1) | 30.7 (0.4)†§¶ | 62.7 (0.2) | 54.4 (0.2) | |
| Putamen | 56.8 (0.1) | 43.4 (0.4) | 56.3 (0.2) | 48.5 (0.2) |
| 52.5 (0.2) | 33.8 (0.3)*‡ | 54.8 (0.2) | 45.5 (0.2) | |
| GPe | 4.1 (0.3) | 5.7 (0.4) | 6.3 (0.3) | 7.4 (0.2) |
| 3.7 (0.3) | 4.4 (0.2) | 5.7 (0.2) | 5.0 (0.3) | |
| GPi | 6.3 (0.2) | 10.4 (0.1) | 3.6 (0.6) | 3.6 (0.7) |
| Insular cortex | 28.1 (0.1) | 23.6 (0.4) | 31.0 (0.4) | 24.9 (0.2) |
| 26.8 (0.2) | 17.9 (0.3)*§ | 32.0 (0.1) | 23.9 (0.2) |
AD, Alzheimer's disease; GPe, external globus pallidus; GPi, internal globus pallidus; LBD, Lewy body dementia; PSP, progressive supranuclear palsy.
Values expressed as geometric means (standard deviation) in fmol/mg.
Values in bold indicate M4 receptor binding in anterior striatum.
*Disease vs control p<0.05.
†Disease vs control p<0.001.
‡PSP vs LBD, p<0.05.
§PSP vs LBD, p<0.001.
¶PSP vs AD, p<0.01.
Lewy body dementias
M2 receptor binding in the striatum and pallidum was normal. In the anterior insular cortex, M2 binding was increased compared with controls (+40%, p<0.01), and in the posterior insular cortex M2 binding was increased compared with PSP (+38%, p<0.05). There was a trend towards higher M4 binding in the GPe in LBD compared with controls although this did not reach statistical significance.
Alzheimer's disease
There was a decrease in M2 binding in the posterior striatum of 23%, caudate (p<0.05) and putamen (p<0.001). There was a significant increase in M2 binding in the anterior insular cortex (+34%, p<0.05). There was a trend towards reduced M4 binding in the striatum but this was not significant.
Effect of demographic variables
In the control group, there was no correlation between age or PM delay and receptor binding in any brain area. In cases of PSP, there was no correlation between M2 or M4 receptor binding and age, disease duration or PM delay. There was no difference in binding of M2 or M4 receptors between those patients noted to have frontal executive dysfunction or dementia and those without, although numbers where data were available in each group were small. In cases of LBD and AD, there were no correlations between M2 or M4 receptors and age, PM delay or disease duration in the striatum or pallidum.
Discussion
We have shown a reduction in M2 and M4 receptors in the posterior striatum in PSP.
Although subtypes of muscarinic receptor have not been previously measured in PSP, two studies using [3H]N‐methyl‐scopolamine which binds to all muscarinic receptors, found a 18–30% reduction in the striatum.7,18 By contrast, the total number of striatal muscarinic receptors measured by [3H] quinuclidynyl benzilate ([3H]QNB) was similar between controls and patients with PSP.19
The reduced striatal M2 receptor density is likely to reflect loss of posterior cholinergic interneurones, previously shown in vitro. Choline acetyltransferase is a marker of cholinergic neurone integrity and this was reduced by approximately 50% in the caudate and by 40% in the putamen in nine patients with PSP compared with controls.19 Another study showed a 50% reduction in striatal ACh vesicular transporter expression and choline acetyltransferase activity in 11 cases with PSP.20 This reduction was more marked in the posterior striatum, as we found in this study. Interestingly, nerve growth factor receptors are localised on intrinsic striatal cholinergic neurones21 and were reduced by 30% in three cases with PSP compared with controls and patients with PD.21 The percentage reduction in cholinergic interneurones found by us is less than some previous reports, probably reflecting the presence of M2 receptors on corticostriatal and thalamic afferents, which are not known to be affected in PSP.
The posterior striatum, particularly the putamen, is involved predominantly in motor function and the anterior striatum and most of the caudate in cognitive and associative functions.22 Posterior striatal neuronal loss suggests cholinergic dysfunction may be involved in motor as well as cognitive impairment, although cholinergic loss in the basal forebrain system may be more relevant to the cognitive deficits in PSP.
M4 receptors are predominantly located postsynaptically, on medium spiny neurones of the direct pathway and colocalise with D1 receptors. They may also be present presynaptically on striatal cholinergic interneurones,23 and to a lesser extent on spiny neurones of the indirect pathway.24 The marked reduction in striatal M4 receptors in our study could indicate loss of medium spiny projection neurones of the direct pathway, although D1 receptors were reported to be within the normal range in one case of PSP.25 Therefore, M4 receptor loss may be selective or, more likely, represent the loss of cholinergic interneurones. It is possible that the M4 receptors are down regulated, although this is unlikely as the reduction in ACh should, if anything, cause upregulation in functioning neurones.
Gamma‐aminobutyric acid is the predominant neurotransmitter in the GP and cholinergic inputs to this nucleus are modest, as reflected by low overall binding in controls. The normal, or even increased, M4 receptor binding is supported by a previous study which measured total muscarinic receptors in the globus pallidus and found a marked increase in GPi in patients with PSP.7 Pathologically the GPe and GPi both show evidence of atrophy and typical PSP pathology.26,27 M4 receptors may, therefore, be upregulated in the GP in response to neuronal loss as a compensatory mechanism.
Reduced M2 receptor binding in the insular cortex suggests decreased cholinergic input. The insular cortex has been implicated in many functions including somatosensory,28,29 gustatory,29,30 vestibular‐like functions and cardiovascular disturbances,29 but none of these symptoms are prominent in PSP.
Failure to detect any difference in receptor binding between those cases with PSP who are with and without cognitive impairment may be due to the fact that this information was available only for a small number of cases.
The normal M2 and M4 receptor binding in the striatum in LBD suggests cholinergic interneurones and medium spiny projection neurones of the direct pathway are intact. This is supported by previous work showing normal striatal M2/M431 and D1 receptors in DLB.32 Medium spiny neurones of the indirect pathway may be reduced, however, reflected by decreased numbers of striatal M1 and D2 receptors.31
The increase in M2 binding in the insular cortex of cases with LBD and AD was not expected as cholinergic cortical projections are reduced in these dementing illnesses.33,34 However, an increase in presynaptic receptors has been shown previously in AD35 and may be due to axonal sprouting of the remaining cholinergic neurones.
The reduction in posterior striatal M2 in AD may represent a reduction in cortical afferents rather than a reduction in intrinsic cholinergic neurones. Normal striatal M4 receptors in AD suggest medium spiny projection neurones are preserved. This is supported by a previous study, although muscarinic subtypes were not measured.31
In conclusion, this study highlights differences in cholinergic dysfunction between the three disease groups, and is compatible with marked striatal cholinergic pathology in PSP. Potential therapeutic approaches suggested by our findings include specific targeting of the remaining M2 receptors with an M2 antagonist, or use of an M1 agonist to stimulate intact postsynaptic receptors. The striatum, however, is only part of the basal ganglia–thalamo–cortico loop and dysfunction of other sites and neurotransmitter systems are likely to be clinically relevant.
Acknowledgements
We thank Mary Johnson for her help with the tissue preparation at the Newcastle Brain Bank, and also all at the Sara Koe PSP Research Centre, London, in particular, Linda Parsons and Susan Stoneham for their help with the tissue samples and data collection. We also thank those patients and controls who donated their brains for this research.
Abbreviations
ACh - acetylcholine
AD - Alzheimer's disease
DLB - dementia with Lewy bodies
GPe - external globus pallidus
GPi - internal globus pallidus
LBD - Lewy body dementia
PD - Parkinson's disease
PDD - Parkinson's disease with dementia
PM - post mortem
PSP - progressive supranuclear palsy
Footnotes
Funding: NMW was sponsored by the PSP (Europe) Association.
Competing interests: None declared.
References
- 1.Steele J C, Richardson J C, Olszewski J. Progressive supranuclear palsy. A heterogenous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 196410333–359. [DOI] [PubMed] [Google Scholar]
- 2.Goetz C G, Leurgans S, Lang A E.et al Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 200360917–922. [DOI] [PubMed] [Google Scholar]
- 3.Maher E R, Lees A J. The clinical features and natural history of the Steele‐Richardson‐Olszewski syndrome (progressive supranuclear palsy). Neurology 1986361005–1008. [DOI] [PubMed] [Google Scholar]
- 4.Birdi S, Rajput A H, Fenton M.et al Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord 2002171255–1264. [DOI] [PubMed] [Google Scholar]
- 5.Nath U B ‐ S Y, Thomson R G, Lees A J.et al Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology 200325910–916. [DOI] [PubMed] [Google Scholar]
- 6.Albers D S, Augood S J. New insights into progressive supranuclear palsy. Trends Neurosci 200124347–353. [DOI] [PubMed] [Google Scholar]
- 7.Landwehrmeyer B, Palacios J. Alterations of neurotransmitter receptors and neurotransmitter transporters in progressive supranuclear palsy. J Neural Transm 199442(Suppl)229–246. [DOI] [PubMed] [Google Scholar]
- 8.Levy R, Ruberg M, Herrero M T.et al Alterations of GABAergic neurons in the basal ganglia of patients with progressive supranuclear palsy: an in situ hybridization study of GAD67 messenger RNA. Neurology 199545127–134. [DOI] [PubMed] [Google Scholar]
- 9.Foster N L, Aldrich Ms, Bluemlein L.et al Failure of cholinergic agonist RS‐86 to improve cognition and movement in PSP despite effects on sleep. Neurology 198939257–261. [DOI] [PubMed] [Google Scholar]
- 10.Litvan I, Phipps M, Pharr V L.et al Randomized placebo‐controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology 200157467–473. [DOI] [PubMed] [Google Scholar]
- 11.Fabbrini G, Barbanti P, Bonifati V.et al Donepezil in the treatment of progressive supranuclear palsy. Acta Neurol Scand 2001103123–125. [DOI] [PubMed] [Google Scholar]
- 12.Warren N M, Piggott M A, Greally E.et al Basal ganglia cholinergic and dopaminergic function in progressive supranuclear palsy. In press. [DOI] [PubMed]
- 13.McKeith I, Mintzer J, Aarsland D.et al Dementia with Lewy bodies. Lancet Neurol 2004319–28. [DOI] [PubMed] [Google Scholar]
- 14.Emre M, Aarsland D, Albanese A.et al Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 20043512509–2518. [DOI] [PubMed] [Google Scholar]
- 15.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: current status and new perspectives. Lancet Neurol 20042539–547. [DOI] [PubMed] [Google Scholar]
- 16.Litvan I, Phipps M, Pharr V.et al Randomized placebo‐controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology 200157467–473. [DOI] [PubMed] [Google Scholar]
- 17.Perry R H. Coronal map of Brodmann areas in the human brain. Roberts GLP, ed. Neuropsychiatric disorders. London: Wolfe, 19931–10.
- 18.Pascual J, Figols J, Grijalba B.et al Changes in aminergic receptors in a PSP postmortem brain: correlation with pathological findings. J Neural Transm 199442(Suppl)247–260. [DOI] [PubMed] [Google Scholar]
- 19.Ruberg M, Javoy‐Agid F, Hirsch E.et al Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol 198518523–529. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Desmond T J, Albin R L.et al Cholinergic vesicular transporters in progressive supranuclear palsy. Neurology 2002581013–1018. [DOI] [PubMed] [Google Scholar]
- 21.Villares J, Strada O, Faucheux B.et al Loss of striatal high affinity NGF binding sites in progressive supranuclear palsy but not in Parkinson's disease. Neurosci Lett 199418259–62. [DOI] [PubMed] [Google Scholar]
- 22.Parent A, Hazrati L N. Functional anatomy of the basal ganglia. I. The cortico‐basal ganglia‐thalamo‐cortical loop. Brain Res Brain Res Rev 19952091–127. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Basile A S, Gomeza J.et al Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock‐out mice. J Neurosci 2002221709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Z, Flores‐Hernandez J, Surmeier D J. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 20011031017–1024. [DOI] [PubMed] [Google Scholar]
- 25.Pascual J, Berciano J, Grijalba B.et al Dopamine D1 and D2 receptors in progressive supranuclear palsy: an autoradiographic study. Ann Neurol 199232703–707. [DOI] [PubMed] [Google Scholar]
- 26.Hardman C D, Halliday G M. The internal globus pallidus is affected in progressive supranuclear palsy and Parkinson's disease. Exp Neurol 1999158135–142. [DOI] [PubMed] [Google Scholar]
- 27.Hardman C D, Halliday G M. The external globus pallidus in patients with Parkinson's disease and progressive supranuclear palsy. Mov Disord 199914626–633. [DOI] [PubMed] [Google Scholar]
- 28.Hanamori T. Chemical stimulation of the thalamic reticular nucleus inhibits the neuronal activity of the posterior insular cortex in rats. Chem Senses 200328717–728. [DOI] [PubMed] [Google Scholar]
- 29.Cereda C, Ghika J, Maeder P.et al Strokes restricted to the insular cortex. Neurology 2002591950–1955. [DOI] [PubMed] [Google Scholar]
- 30.Sakai N, Imada S. Bilateral lesions of the insular cortex or of the prefrontal cortex block the association between taste and odor in the rat. Neurobiol Learn Mem 20038024–31. [DOI] [PubMed] [Google Scholar]
- 31.Piggott M A, Owens J, O'Brien J.et al Muscarinic receptors in basal ganglia in dementia with Lewy bodies, Parkinson's disease and Alzheimer's disease. J Chem Neuroanat 200325161–173. [DOI] [PubMed] [Google Scholar]
- 32.Piggott M A, Marshall E F, Thomas N.et al Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer's and Parkinson's diseases: rostrocaudal distribution. Brain 19991221449–1468. [DOI] [PubMed] [Google Scholar]
- 33.Tiraboschi P, Hansen L A, Alford M.et al Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Arch Gen Psychiatr 200259946–951. [DOI] [PubMed] [Google Scholar]
- 34.Shinotoh H, Namba H, Fukushi K.et al Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer's disease: a positron emission tomography study. Ann Neurol 200048194–200. [PubMed] [Google Scholar]
- 35.Vogt B A, Crino P B, Vogt L J. Reorganization of cingulate cortex in Alzheimer's disease: neuron loss, neuritic plaques, and muscarinic receptor binding. Cereb Cortex 19922526–535. [DOI] [PubMed] [Google Scholar]