Abstract
Background
Between 1940 and 1944 military gas masks with filter pads containing 20% crocidolite were assembled in a Nottingham factory.
Methods
Records supplied by the late Professor Stephen Jones were of 1154 persons, mainly women, who had worked in the factory during this period; they included many deaths from mesothelioma. A systematic effort was therefore made to establish causes of death for the whole cohort.
Results
Of 640 employees with full name and sex recorded, 567 (89%) were traced. Of these, 491 had died, including 65 from mesothelioma, though only 54 were certified as such. After exclusion of these 54, standardised mortality ratios were significantly raised for respiratory cancer (SMR 2.5) and carcinomatosis (SMR 3.2). The pattern of mortality in the remaining 514 employees without full identification was similar, but a low tracing rate (40%) did not justify their further analysis. The first death from mesothelioma was in 1963 (22 years after first exposure) and the last in 1994, whereas a further 5.0 cases would have been expected between 1996 and 2003 (p = 0.0065).
Conclusion
These findings in a cohort followed over 60 years after brief exposure to crocidolite confirm a high and specific risk of mesothelioma (28% peritoneal) and perhaps of lung cancer some 20–50 years later. The statistically significant absence of further mesothelioma cases during the past eight years suggests that crocidolite, though durable, is slowly removed.
Keywords: crocidolite, mesothelioma, biopersistence, women's work
In 1965, a 59 year old woman entered the City Hospital, Nottingham; a few months later, another woman who had worked in the same factory also attended the hospital. Both had a malignant pleural mesothelioma; by 1979, five more deaths from this tumour had come to light. All had been employed on the assembly of military gas masks, using filter pads prepared from 80% merino wool and 20% crocidolite (blue asbestos). In 1974, a man with stated occupation of musician was admitted to the Royal Victoria Hospital, Montreal, also with pleural mesothelioma. A medical student who took a detailed history discovered that he had been a scientific officer, one of a small group responsible for setting up and managing an assembly plant in Ottawa of gas masks for the Canadian army, following the British specification exactly. These events, and the early mesothelioma epidemics which followed on both sides of the Atlantic simultaneously, were described by Jones et al1,2 and McDonald and McDonald.3 Shortly before he died in December 2000, Professor Stephen Jones, who first recognised the connection, discussed with one of us (JCMcD) the possibility of investigating the mortality of all known employees in the Nottingham factory, and passed over his records so that this could be done. At that time, no systematic tracing had been made, but Jones, whose interest was widely known, had been informed by pathologists throughout the country of many further deaths from mesothelioma. Findings from a comprehensive follow up of the entire cohort are presented below.
The cohort
The records supplied by Dr Jones were of 1154 employees in the Nottingham factory, believed to have worked between 1940 and 1944 on gas mask assembly. Of these, approximately 93% were female, but both full name and sex were recorded in only 640 (Group A), and incomplete information in the other 514 (Group B). Year of birth was recorded for all but four subjects with year first employed in all but 29, and duration of employment in all but a further 64. Originally 1088 of the 1154 employees were identified from the factory wages book, although there were nine duplicate pairs, leaving 1079. Jones did not obtain a complete listing as some records had already been shredded when he rescued the wages book and, as he said, “an unknown number of names are therefore missing”.2 It is probable therefore that the cohort was increased by some of these missing former workers coming to light from pathological reports to Jones. These additions were likely to contain a disproportionate number of mesotheliomas, as was clearly the case since there were 19 mesotheliomas in the 64 for whom neither year first employed nor duration of employment were recorded; all but 10 of these were with known name, and therefore in Group A. As duration of employment was obtained from information in the wages book, we regarded the 1061 former workers with 45 mesotheliomas for whom duration of employment was recorded as an unbiased cohort, whereas the other 93 former workers with 20 mesotheliomas constituted a group made up of serendipitous additions biased by a diagnosis of mesothelioma. The analysis of mesothelioma rates is therefore based on the cohort of 1061, but the full set of 65 mesotheliomas is relevant to the total cohort albeit out of an unknown number greater than 1154 of former workers.
Cohort mortality
With help from the Office for National Statistics (ONS), 567 (89%) of Group A and 205 (40%) of Group B were traced through 2002. Of the former, 491 (87%) and of the latter, 141 (69%), had died; cause of death coded to ICD‐9 was obtained for them all. The distribution by certified cause of death is shown in table 1 for the 772 traced former workers; 54 deaths were coded to mesothelioma, and a further 11 variously coded, but from postmortem examination and other pathological evidence held by Professor Jones, more correctly attributed to the same cause. All 65 deaths due to mesothelioma, including six untraced by the ONS, were confirmed pathologically; 47 were of the pleura and 18 (28%) of the peritoneum. As these 65 cases had been ascertained by Professor Jones, with full name and sex, before the follow up was made by ONS, they qualified of necessity for Group A. Apart from mesothelioma, however, the pattern of proportional mortality in the two subcohorts was very similar, encouraging the belief that the distribution of deaths from other causes overall was probably unbiased. In table 2 the certified causes of death are given for the 640 former workers in Group A, excluding the 54 whose deaths were coded as mesothelioma.
Table 1 Deaths by certified cause with number expected for the 772 traced workers in whole cohort.
| Certified cause of death | Observed number of deaths | Expected number of deaths | SMR (95% CI) |
|---|---|---|---|
| Mesothelioma | 54 | 0.5 | 111.5 (84.5 to 146.8) |
| Respiratory cancer | 40 (2) | 19.4 | 2.1 (1.5 to 2.8) |
| Abdominal cancer | 49 | 41.2 | 1.2 (0.9 to 1.6) |
| Ovarian cancer | 10 (2) | 8.3 | 1.2 (0.6 to 2.2) |
| Carcinomatosis | 20 (2) | 6.5 | 3.1 (1.9 to 4.8) |
| Other cancer | 55 | 78.6 | 0.7 (0.5 to 0.9) |
| NMRD | 71 (1) | 69.7 | 1.0 (0.8 to 1.3) |
| Other | 333 (4) | 415.2 | 0.8 (0.7 to 0.9) |
| All deaths | 632 | 627.8 | 1.0 (0.9 to 1.1) |
*Additional deaths shown in parentheses were thought to be due to mesothelioma, though certified to other causes (see text).
NMRD, non‐malignant respiratory disease.
Table 2 Observed deaths by certified cause with number expected in selected subcohort with known name and sex, excluding 54 deaths coded as mesothelioma (n = 586).
| Certified cause of death | Observed number of deaths | Expected number of deaths | SMR (95% CI) |
|---|---|---|---|
| Respiratory cancer | 32 | 13.1 | 2.5 (1.7 to 3.5) |
| Abdominal cancer | 37 | 27.8 | 1.3 (0.9 to 1.8) |
| Ovarian cancer | 10 | 5.6 | 1.8 (0.9 to 3.3) |
| Carcinomatosis | 14 | 4.4 | 3.2 (1.8 to 5.4) |
| Other cancer | 41 | 52.8 | 0.8 (0.6 to 1.1) |
| NMRD | 58 | 47.7 | 1.2 (0.9 to 1.6) |
| Other | 245 | 282.5 | 0.9 (0.8 to 1.0) |
| All deaths (excluding mesothelioma) | 437 | 426.5 | 1.0 (0.9 to 1.1) |
NMRD, non‐malignant respiratory disease.
Although the number of mesothelioma deaths was clearly in considerable excess, interpretation of the remaining causes was less easy, without some basis for comparison. Standardised mortality ratios (SMRs) were therefore calculated, using female age specific mortality rates for England and Wales (1980) as reference in tables 1 and 2. The results in both are quite similar, though those in table 2 are probably more reliable in having been derived from a higher level of tracing. These analyses suggest that the excess of respiratory cancer deaths was about half that due to mesothelioma, and that the excess mortality from carcinomatosis, ovarian cancer, and possibly abdominal cancer may well have resulted at least in part from undiagnosed mesothelioma. Other causes of death, including other cancer sites and non‐malignant respiratory disease (NMRD), were generally less than expected.
Latency
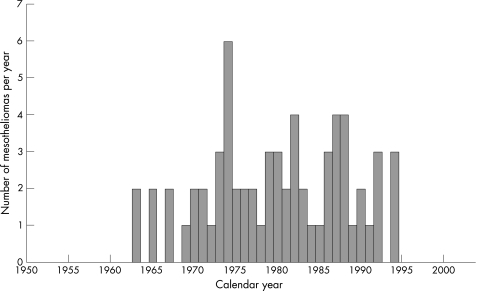
Although the first mesothelioma came to light in 1965, there had already been two deaths from this cause in 1963, and by the end of 1969 there had been seven in all. During the next three decades there had been 24, 25, and 9 deaths respectively, the most recent in 1994 (fig 1).
Figure 1 Number of mesotheliomas by calendar year in the full group of 1154 former workers.
The number of mesotheliomas in any given time period was expressed as a rate per 100 000 person‐years at risk. The number of person‐years was obtained in two steps. First, for those traced by ONS in Group A, the number of person‐years in each calendar year was known. This information was analysed in five‐year groupings of year of birth from 1880–84 to 1925–29 to give survival rates over time. These survival rates were then applied to the remainder of the cohort to give estimated person‐years which were added to the known person years in Group A. An adjustment was also applied to correct for the fact that the mesotheliomas were all put into Group A since some would have been in the remainder had not the diagnosis of mesothelioma brought their details to light.
The numbers of mesotheliomas in five‐year calendar periods and the mesothelioma rates are shown in table 3. After about the first 20 years during which there were no cases, the rate increased rapidly up to 30 years from exposure, but then remained fairly steady for the next 20 years. No mesothelioma was identified between 1996 and 2003, and if the rate had remained the same as in 1991–95, a further 5.0 cases would have been expected in 1938 person‐years; the observation of zero has a probability of 0.0065.
Table 3 Mesothelioma and calendar year.
| Calendar year (years since exposure*) | No. mesotheliomas | Total† | Cohort‡ | Person‐years‡ | Mesothelioma rate‡§ | |||
|---|---|---|---|---|---|---|---|---|
| Total† | Cohort‡ | PT | PL | PT | PL | |||
| 1956–60 (16) | 0 | 0 | 0 | 0 | 0 | 0 | 5134 | 0 |
| 1961–65 (21) | 4 | 3 | 2 | 2 | 1 | 2 | 4982 | 60 |
| 1966–70 (26) | 5 | 5 | 1 | 4 | 1 | 4 | 4741 | 105 |
| 1971–75 (31) | 14 | 10 | 4 | 10 | 3 | 7 | 4341 | 230 |
| 1976–80 (36) | 11 | 8 | 3 | 8 | 2 | 6 | 3768 | 212 |
| 1981–85 (41) | 10 | 7 | 3 | 7 | 2 | 5 | 3167 | 221 |
| 1986–90 (46) | 14 | 7 | 2 | 12 | 1 | 6 | 2582 | 271 |
| 1991–95 (51) | 7 | 5 | 3 | 4 | 2 | 3 | 1925 | 260 |
| 1996–00 (56) | 0 | 0 | 0 | 0 | 0 | 0 | 1334 | 0 |
| 2001–03 (60) | 0 | 0 | 0 | 0 | 0 | 0 | 604 | 0 |
| Total | 65 | 45 | 18 | 47 | 12 | 33 | 32578 | 138.1 |
*Average time from midpoint of production period (1940–44) to midpoint of calendar year range.
†Total number in 1154 former workers.
‡In cohort of 1061 workers with known duration of employment.
§Rate per 100 000 person‐years at risk.
PT, peritoneal; PL, pleural.
Exposure–response
In the absence of information on intensity of exposure to airborne crocidolite fibres, only duration of employment in the Nottingham plant was available as an index of risk. It was stated by Jones et al1 that although chrysotile asbestos had been employed in civilian gas masks for less than five months from December 1939, for military gas masks the use of crocidolite only began in September 1940. As there was no case of mesothelioma among 73 deaths in 147 cohort members known to have left before September, these were excluded from estimates of risk in the remaining 1007 potentially exposed to crocidolite after that date. Two analyses are shown in table 4: one of proportional mortality based on recorded data on deaths by cause, and the other of cohort mortality by estimated duration of employment. Both tabulations show that even employment of less than a year carried a substantial risk, but was highest at three years or more.
Table 4 Mesothelioma and exposure.
| Years of employment | Proportional mortality* | Mortality/cohort† | ||
|---|---|---|---|---|
| <1 | 17/260 | (6.5%) | 36/594 | (6.1%) |
| 1– | 12/115 | (10.4%) | 12/203 | (5.9%) |
| 2– | 7/75 | (9.3%) | 7/132 | (5.3%) |
| ⩾3 | 9/39 | (23.1%) | 10/78 | (12.8%) |
| Based on | 45 cases/489 all causes | 65 cases/1007 in cohort | ||
*Using only exposure data provided.
†Using above plus assumed exposure for 64 with no start date or exposure data recorded (median value of 7 months used).
Discussion
The circumstances of exposure and outcome in this small and mainly female cohort are virtually unique. Their exposure to crocidolite for a maximum of four years and average of little more than one, proved disastrous. By 2002, some 60 years later, at least 65 deaths were thought to be due to malignant mesothelioma, a high proportion of which were peritoneal (28%), plus a suspiciously high number of deaths from carcinomatosis and ovarian cancer. Deaths from respiratory cancer were also in excess, but probably accounted for only half those due to mesothelioma; there was little evidence of increased risk of death from any other malignant or non‐malignant disease. However, the SMRs for respiratory cancer in tables 1 and 2 are probably overestimates, in that they were based on the assumption that the cohort was entirely female, whereas in fact perhaps 7% were men. To correct for this, expected deaths were recalculated using male age specific mortality rates for the 7%. This adjustment reduced the SMRs for respiratory cancer in table 1 from 2.1 to 1.7 (expected deaths, 24.2 instead of 19.4), and in table 2 from 2.5 to 2.0 (expected deaths, 16.2 instead of 13.0).
These findings, including evidence of risk related to duration of exposure, might be considered little more than dramatic confirmation of the high and specific carcinogenicity of crocidolite for the mesothelium, were it not for the dearth of cases in the last decade. The generally prevailing view has been that after an initial lag period the risk of death from mesothelioma increases indefinitely with each year since first exposure, but fig 1 and table 3 suggest that the risk was stable from the mid‐1970s to the mid‐1990s, between about 35 and 55 years since exposure, and after that the absence of further cases is statistically significant. Survivors in the cohort are now relatively few and are ageing, but it seems unlikely that cases could have been missed. The first deaths with mesothelioma occurred in 1963, a little more than 20 years since first exposure. A lag period of about 20 years is in accord with other series, although a small proportion of mesothelioma deaths have occurred within 20 years from exposure—for example, in former workers at the Wittenoom crocidolite asbestos mine and mills in Western Australia, the first mesothelioma death occurred 13 years after first exposure and another 12 out of a total of 239 between 15 and 19 years from first exposure.5 The very long latency for mesothelioma has been explained by durability of amphibole asbestos fibres and their persistence in lung tissue. There is also evidence, however, that eventually even crocidolite fibres are slowly removed.4 The number of mesotheliomas occurring in former miners and millers at the Wittenoom crocidolite asbestos mines and mills during 1987–2000 has recently been shown to be similar to that predicted earlier using an elimination model.5 Hodgson and Darnton6 noted that there was evidence from cohorts with long follow up that the incidence of mesothelioma eventually falls.
Our present results provide further support for the proposition that the mesothelioma rate does not continually increase with increasing time since exposure; indeed, the absence of any cases from 1995 to 2003 is a larger reduction than would be predicted using an elimination model. It is possible that more direct evidence may be provided by the results of mineral fibre analyses made in Cardiff by Dr Allen Gibbs and Professor Fred Pooley on 71 postmortem and resected lung tissue samples from 51 cases of mesothelioma, and a smaller number of cases of lung cancer and diseases unrelated to asbestos from the cohort. In all but two of these, crocidolite fibres were identified in significant concentrations, but any detailed analysis of these results is beyond the scope of the present report and, if feasible, will be presented separately.
Acknowledgements
We wish to acknowledge the very great help given in all phases of this research by Magda Wheatley. Without the original work and records of the late Professor Stephen Jones, the whole study would not have been possible.
Footnotes
Competing interests: none
References
- 1.Jones J S P, Pooley F D, Smith P G. Factory populations exposed to crocidolite asbestos—a continuing survey. INSERM 197652117–120. [PubMed] [Google Scholar]
- 2.Jones J S P, Smith P G, Pooley F D.et al The consequences of exposure to asbestos dust in a wartime gas‐mask factory. In: Wagner JC, ed. Biological effects of mineral fibres. IARC Scientific Publications No. 30. Lyon: IARC Scientific Publications, 1980637–653. [PubMed]
- 3.McDonald A D, McDonald J C. Mesothelioma after crocidolite exposure during gas mask manufacture. Environ Res 197817340–346. [DOI] [PubMed] [Google Scholar]
- 4.Berry G. Models for mesothelioma incidence following exposure to fibers in terms of timing and duration of exposure and the biopersistence of the fibers. Inhal Toxicol 199911111–130. [DOI] [PubMed] [Google Scholar]
- 5.Berry G, De Klerk N H, Reid A.et al Malignant pleural and peritoneal mesotheliomas in former miners and millers of crocidolite at Wittenoom, Western Australia. Occup Environ Med 200461e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson J T, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg 200044565–601. [PubMed] [Google Scholar]