Abstract
Objectives
The chronic effects of urban air pollution are not well known. The authors' aim was to investigate the association between the prevalence and new onset of chronic bronchitis and urban air pollution.
Methods
Subjects from the general population randomly selected for the European Community Respiratory Health Survey (ECRHS I) during 1991–93 in 21 centres in 10 countries were followed up from the years 2000 to 2002 (n = 3232 males and 3592 females; average response rate = 65.3%). PM2.5 and elements, with the same equipment at centre level, and home outdoor NO2 in 1634 individuals were measured. Hierarchical models were used.
Results
The prevalence and new onset of chronic phlegm during follow up were 6.9% and 4.5%, respectively, 5.3% in males and 3.5% in females. Smoking, rhinitis, poor education, and low social class were associated with (prevalence and new onset of) chronic phlegm in both genders, and occupational exposures in males and traffic intensity (adjusted odds ratio for constant traffic, OR = 1.86; 95% CI 1.24 to 2.77) as well as home outdoor NO2 (OR > 50 μg/m3v < 20μg3 = 2.71; 95% CI 1.03 to 7.16) among females. PM2.5 and S content at centre level did not show any association with prevalence or new onset of chronic phlegm. Similar results were obtained with chronic productive cough.
Conclusion
Individual markers of traffic at household level such as reported intensity and outdoor NO2 were risk factors for chronic bronchitis among females.
Keywords: bronchitis, air pollution, ECRHS, NO2 , PM2.5
The majority of deaths occurring during the London fog episode in 1952 were considered to be due to bronchitis.1 In the 1960s, researchers in the UK had shown that air pollution was not only the cause of sudden exacerbations in patients suffering from a chronic airways obstructive disease but also that prevalence of chronic bronchitis appeared to be greater in areas with higher pollution,2,3 which was also observed in the United States4,5,6 and Poland.7 However, further small studies in areas with lower levels of air pollution created doubts about the chronic role of air pollution,8,9 until the recent appearance of studies comparing urban air pollution10,11 and the intensity of transport related air pollutants12,13 with the prevalence of symptoms of bronchitis in children. In adults, recent cross sectional studies on prevalence of symptoms comparing different areas are rare (only one study in a particular population in California14 and another in eight areas of Switzerland15). In addition, there are a few studies using national interview surveys in the United States16,17 and Germany.18 All studies consistently found a higher prevalence of symptoms of chronic bronchitis in areas with higher particulate air pollution. However, there is a need for prospective studies in a larger number of geographical areas to confirm that current urban air pollution is associated with incidence of chronic bronchitis.19 Our aim is to assess the association between the prevalence and new onset of chronic bronchitis and urban air pollution in the European Community Respiratory Health Study‐II (ECRHS‐II), an international follow up study.
Methods
Subjects from the general population randomly selected for ECRHS I, carried out in 1991–93,20 who belong to the 21 centres in 10 countries measuring air pollution were included. Follow up took place 8.9 years after among 6924 persons (response rate, 65.3%). Responders were slightly older and were more likely to be from a higher social class (p<0.05), but there were no marked differences in chronic bronchitis at baseline (p = 0.60). The response rate per centre was correlated negatively with the average levels of fine particle mass (r = −0.55) and positively with the incidence of chronic phlegm (r = 0.49) and with the average levels of home nitrogen dioxide (NO2) (r = 0.44). Ethical approval was obtained for each centre from the appropriate institutional or regional ethics committee, and written consent was obtained from each participant.
Two definitions for symptoms of chronic bronchitis were employed: firstly, productive chronic cough for chronic cough and chronic phlegm (more than three months each year), and; secondly, chronic phlegm alone. The two definitions yielded similar results and given the higher frequency only the latter is shown in tables. Risk factors for chronic bronchitis such as smoking, age at end of education, occupational groups, occupational exposures, respiratory infections during childhood, rhinitis, asthma, in addition to traffic intensity at household level (cars, trucks or buses, or both) were extracted from questionnaires at follow up.21
The same central monitoring site equipment was placed in each area in a single background monitoring station during the period June 2000 to December 2001, running every second day over a two week period during each month, and the annual mean mass concentration of fine particles with a median size of 2.5 μm aerodynamic diameter (PM2.5) and its sulfur content (S) measured and analysed centrally.22 PM2.5 mass and S are spatially probably rather homogenous. However, personal exposure to tail pipe emissions is poorly characterised at a central site. A home based measurement of NO2 as a marker for local tail pipe emissions was implemented. At this individual level, outdoor (at the kitchen window) and kitchen indoor NO2 concentrations were collected during a 14 day period in 16 centres involving 1634 households of subjects who did not move house during the follow up. After about six months this procedure was repeated in 659 households (45%) who volunteered to repeat the measurement. The passive samplers (Palmes tubes) were analysed centrally.22
The outcomes of interest were prevalence at follow up and new onset (prevalence at follow up among the subjects without the symptoms of chronic bronchitis at baseline). Results were almost identical with the two outcomes (and if any stronger with the latter). Hierarchical models23 including the effect of risk factors at individual level and PM2.5 mass and S at the centre level, as well as other centre level variables such as the proportion of smokers or poorly educated subjects, were fitted using MLwiN 1.1.24 Confounding variables were retained at individual level if p<0.10 or the coefficient of the air pollution variable was modified by 10% or more. The association was measured with the odds ratios for individual level variables and with the interval odds ratio (that is, covering 80% of the odds ratios) for centre level variables.25 Hierarchical models were not applied for NO2 due to its measurement at individual level and the small number of individuals in some centres. General additive models (GAM) and logistic regression were used to fit NO2 using Stata8 (Statacorp, Seattle, WA, USA). GAM modeling depicted the association with NO2 without any parameterisation while logistic model provided the odds ratio measuring the adjusted association.
Results
New onset of chronic phlegm during the follow up was 4.0% (4.5% in males and 3.5% in females; for chronic productive cough it was 1.2% and 1.1%, respectively). However, the prevalence at the end of follow up was similar, or if anything slightly lower, to that at baseline in most of the centres (table 1) (the prevalence at the end of follow up being 6.9% in males and 5.3% in females) indicating a similar proportion of individuals with new symptoms or having ceased experiencing symptoms. The proportion of individuals with new and remitting symptoms was very homogeneous between centres (p for heterogeneity >0.50), even after stratifying by smoking and sex. Centres showed considerable variations in NO2 and particulate levels, with Nordic centres having lower values, while the relative variation in symptoms was lower (table 1).
Table 1 Frequency of chronic bronchitis symptoms and average of air pollutants (outdoor home NO2 and city average PM2.5 and S) by centre.
| Centre* | n | Prevalence at ECRHS‐I | Prevalence at ECRHS‐II | New onset† at ECRHS‐II | n | NO2 (μg/m3) | Range | PM2.5 (μg/m3) | S (ng/m3) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic phlegm | Productive chronic cough | Chronic phlegm | Productive chronic cough | Chronic phlegm | Productive chronic cough | |||||||
| Erfurt (C) | 287 | 2.75 | 1.94 | 3.48 | 3.07 | 1.89 | 1.99 | 0 | – | 16.3 | 1144.3 | |
| Turin (S) | 123 | 7.79 | 2.47 | 3.25 | 0.85 | 3.57 | 0.86 | 72 | 70.81 | (35–136) | 44.9 | 1827.4 |
| Antwerp City (C) | 299 | 9.61 | 6.07 | 7.82 | 4.25 | 4.28 | 3.25 | 52 | 47.79 | (28–78) | 24.1 | 1464.7 |
| Basel (C) | 459 | 3.43 | 2.03 | 3.28 | 2.06 | 2.48 | 1.42 | 89 | 34.62 | (18–69) | 17.4 | 1038.9 |
| Norwich (C) | 257 | 3.85 | 1.51 | 5.49 | 2.55 | 3.51 | 2.17 | 118 | 25.69 | (9.2–74) | 16.2 | 977.4 |
| Verona (S) | 205 | 2.92 | 1.17 | 2.94 | 0.53 | 2.55 | 0.53 | 121 | 52.52 | (9.4–110) | 41.5 | 2015.2 |
| Tartu (N) | 259 | 5.61 | 3.07 | 4.63 | 0.00 | 2.76 | 0.00 | 37 | 22.90 | (14–37) | 14.8 | 892.0 |
| South Antwerp (C) | 340 | 4.32 | 1.98 | 3.56 | 1.27 | 2.29 | 1.29 | 86 | 35.27 | (23–57) | 20.8 | 1453.5 |
| Pavia (S) | 192 | 8.80 | 4.96 | 4.17 | 3.85 | 1.92 | 1.91 | 87 | 43.12 | (18–86) | 35.3 | 1782.6 |
| Paris (S) | 433 | 6.00 | 3.71 | 6.26 | 3.46 | 3.53 | 2.54 | 0 | – | 17.8 | 1080.5 | |
| Oviedo (S) | 242 | 8.73 | 4.51 | 10.74 | 5.21 | 6.51 | 1.99 | 135 | 45.61 | (12–143) | 15.9 | 1181.2 |
| Ipswich (C) | 297 | 5.19 | 3.22 | 6.42 | 3.32 | 4.78 | 2.07 | 82 | 30.09 | (0.34–69) | 16.5 | 998.6 |
| Barcelona (S) | 272 | 5.61 | 2.3 | 7.12 | 1.24 | 6.58 | 1.26 | 125 | 76.86 | (41–146) | 22.2 | 1387.9 |
| Albacete (S) | 308 | 14.02 | 7.59 | 9.09 | 4.64 | 4.69 | 3.09 | 142 | 36.88 | (7.1–125) | 13.1 | 1008.5 |
| Goteborg (N) | 505 | 9.38 | 3.81 | 7.54 | 3.82 | 4.13 | 2.75 | 0 | – | 12.6 | 903.4 | |
| Galdakao (S) | 360 | 6.79 | 2.06 | 8.06 | 3.65 | 6.71 | 3.1 | 176 | 40.55 | (5.8–102) | 16.3 | 1584.5 |
| Huelva (S) | 204 | 14.76 | 5.54 | 11.76 | 8.52 | 5.42 | 5.95 | 59 | 35.34 | (13–98) | 17.3 | 1557.8 |
| Umea (N) | 421 | 8.17 | 4.17 | 6.7 | 2.08 | 4.59 | 1.07 | 171 | 6.03 | (0.34–20) | 5.6 | 414.8 |
| Grenoble (S) | 384 | 5.51 | 2.12 | 5.21 | 3.03 | 3.5 | 1.97 | 0 | – | 19.0 | 887.7 | |
| Reykjavik (N) | 460 | 5.21 | 2.33 | 5.9 | 1.71 | 4.51 | 1 | 82 | 12.82 | (0.34–29) | 3.7 | 155.3 |
| Uppsala (N) | 517 | 6.47 | 2.81 | 4.66 | 1.47 | 3.27 | 1.31 | 0 | – | 10.4 | 752.1 | |
| Total | 6824 | 6.53 | 3.20 | 6.07 | 2.80 | 3.96 | 1.93 | 1634 | 38.22 | (0.34–146) | 19.12 | 1167.07 |
*Ordered by participation rate in the follow up.
†New onset among those without these symptoms at baseline.
Geographical area: N, North; C, Centre; S, South.
Smoking, childhood respiratory infections, rhinitis, occupational exposure, social class (based on occupational groups), education, as well as traffic intensity and NO2 levels showed large variations between centres both in men and women (table 2). Women reported higher traffic intensity but had average NO2 levels similar to males, and both males and females showed a similar association between reported constant traffic intensity and NO2 (odds ratio for reporting constant traffic versus none = 2.90 (95% CI 2.01–4.21) for males and 2.54 (95% CI 1.82–3.55) for females per each increase of 30 μg/m3. After stratifying by centre, this association occurred in all centres except one and with a similar strength for cars and for buses and trucks).
Table 2 Frequency (%) of variables of interest at follow up and variation per centres in 3232 males and 3592 females.
| Males | Females | Centres with min and max frequencies | ||||
|---|---|---|---|---|---|---|
| Mean or % | Range by centres | Mean or % | Range by centres | |||
| Mean age (years) | 42.62 | (38.12–45.62) | 42.57 | (39.92–45.69) | Tartu | Grenoble |
| Smoking | ||||||
| Never, no passive smoking | 29.02 | (8.25–51.22) | 34.45 | (15.13–50.33) | Huelva | Umea |
| With passive smoking | 9.19 | (2.05–17.36) | 11.46 | (1.87–23.86) | Uppsala | Pavia |
| Former | 30.61 | (7.62–41.09) | 26.13 | (5.96–35.98) | Tartu | Grenoble |
| ⩽10/day | 13.40 | (4.46–37.14) | 14.45 | (6.82–31.79) | Uppsala | Tartu |
| 10–20/day | 11.34 | (2.44–25.22) | 10.69 | (3.54–21.01) | Uppsala | Oviedo |
| >20/day | 6.43 | (0.00–18.56) | 2.82 | (0.00–10.92) | Norwich, Umea | Huelva |
| Respiratory infections before 5 years | 9.04 | (2.78–13.64) | 10.24 | (2.91–24.00) | Huelva | Verona |
| Rhinitis | 25.87 | (10.11–34.92) | 29.84 | (11.38–43.40) | Galdakao | Paris |
| Any occupational exposure to biological or mineral dust, gas and fumes | ||||||
| None | 47.03 | (24.85–69.95) | 62.00 | (45.33–80.60) | Umea | Paris |
| Low | 27.53 | (18.00–33.70) | 33.18 | (19.40–47.20) | Turin | Uppsala |
| High | 25.44 | (7.25–46.15) | 4.82 | (0.00–8.89) | Paris | Galdakao |
| Social class (based on occupational group) | ||||||
| Professional and managerial (ref) | 31.03 | (10.71–52.02) | 24.25 | (8.57–44.26) | Huelva | Paris |
| Other non‐manual | 32.83 | (19.63–53.57) | 50.92 | (37.14–70.80) | Galdakao | Erfurt |
| Skilled manual | 17.95 | (3.54–28.99) | 2.81 | (0.00–7.24) | Paris | Albacete |
| Semi/unskilled manual | 12.93 | (5.05–22.47) | 7.32 | (2.13–18.40) | Paris | Galdakao |
| Unclassified | 5.26 | (0.00–17.36) | 14.70 | (1.25–37.08) | Norwich | Antwerp city |
| Educational level (years) | ||||||
| ⩽16 | 20.50 | (1.82–66.14) | 22.06 | (3.70–58.24) | Erfurt | Ipswich |
| 17–20 | 37.14 | (20.39–59.73) | 34.71 | (19.83–69.63) | Erfurt | Albacete |
| >20 | 42.36 | (12.60–59.36) | 43.23 | (12.94–70.61) | Ipswich | Uppsala |
| Reported traffic at home front door | ||||||
| Never | 45.64 | (17.36–68.93) | 39.33 | (15.25–64.04) | Antwerp–city | Pavia |
| Seldom | 19.14 | (8.74–29.75) | 20.67 | (12.10–37.93) | Pavia | South–Antwerp |
| Frequent | 15.78 | (9.96–31.31) | 17.29 | (10.11–28.85) | Goteborg | Huelva |
| Constant | 19.45 | (9.71–34.34) | 22.71 | (11.24–38.42) | Pavia | Antwerp city |
| Mean NO2 (μg/m3) | 38.00 | (5.86–78.37) | 38.44 | (6.18–76.16) | Umea | Barcelona |
Smoking, rhinitis, poor education, and low social class showed a crude association with chronic phlegm in both males and females (table 3). In addition, occupational exposures showed an association among males, and traffic intensity as well as home outdoor NO2 and season of symptoms report among females (table 3), the association with NO2 being only borderline significant (p = 0.052). The same results were observed for chronic productive cough.
Table 3 Individual unadjusted association between chronic phlegm (prevalence in %, 95% CI) at follow up and variables of interest by gender in 3232 males and 3592 females.
| Males | p Value trend | Females | p Value trend | |||||
|---|---|---|---|---|---|---|---|---|
| n | Chronic phlegm | (95% CI) | n | Chronic phlegm | (95% CI) | |||
| Age at II (years) | ||||||||
| <35 | 604 | 7.62 | (5.63–10.03) | 668 | 5.09 | (3.55‐7.04) | ||
| 35–45 | 1296 | 7.33 | (5.97–8.89) | 1466 | 5.32 | (4.23–6.60) | ||
| >45 | 1310 | 6.18 | (4.94 –7.63) | 0.387 | 1439 | 5.42 | (4.31–6.72) | 0.952 |
| Smoking | ||||||||
| Non‐smoker | 912 | 4.06 | (2.87–5.55) | 1216 | 3.54 | (2.57–4.73) | ||
| With passive smoking | 288 | 3.82 | (1.92–6.73) | 405 | 3.46 | (1.90–5.73) | ||
| Former smoker | 962 | 6.13 | (4.70–7.84) | 920 | 4.13 | (2.94–5.63) | ||
| Smoker: <10/day | 420 | 7.62 | (5.27–10.59) | 508 | 6.50 | (4.51–9.00) | ||
| Smoker: 10–20/day | 354 | 12.71 | (9.43–16.64) | 377 | 11.14 | (8.15–14.76) | ||
| Smoker: >20/day | 201 | 16.42 | (11.58–22.28) | <0.001 | 100 | 19.00 | (11.84–28.07) | <0.001 |
| Respiratory infections <5 years | ||||||||
| No | 2666 | 6.41 | (5.51–7.41) | 2954 | 5.18 | (4.41–6.04) | ||
| Yes | 264 | 9.47 | (6.22–13.66) | 0.058 | 337 | 6.23 | (3.90–9.37) | 0.414 |
| Rhinitis | ||||||||
| No | 2371 | 6.11 | (5.18–7.16) | 2501 | 4.36 | (3.59–5.23) | <0.001 | |
| Yes | 825 | 9.21 | (7.33–11.39) | 0.003 | 1059 | 7.65 | (6.12–9.42) | |
| Occupational exposure | ||||||||
| None | 1451 | 5.31 | (4.21–6.59) | 2098 | 5.24 | (4.33–6.29) | ||
| Low | 850 | 7.41 | (5.74–9.38) | 1124 | 5.43 | (4.18–6.92) | ||
| High | 783 | 9.71 | (7.72–12.00) | <0.001 | 161 | 4.35 | (1.77–8.75) | 0.847 |
| Educational level | ||||||||
| Low | 641 | 9.36 | (7.22–11.88) | 771 | 8.04 | (6.22–10.19) | ||
| Medium | 1171 | 7.69 | (6.23–9.36) | 1209 | 5.13 | (3.95–6.53) | ||
| High | 1335 | 5.17 | (4.04–6.50) | 0.001 | 1512 | 4.03 | (3.10–5.15) | <0.001 |
| Social class | ||||||||
| Professional and managerial (ref) | 1000 | 4.20 | (3.04–5.64) | 865 | 3.35 | (2.26–4.78) | ||
| Other non‐manual | 1052 | 6.56 | (5.14–8.23) | 1821 | 5.49 | (4.49–6.64) | ||
| Skilled manual | 576 | 10.24 | (7.89–13.01) | 99 | 4.04 | (1.11–10.02) | ||
| Semi/unskilled manual | 416 | 10.10 | (7.37–13.40) | 262 | 8.40 | (5.34–12.44) | ||
| Unclassified | 166 | 6.02 | (2.93–10.80) | <0.001 | 526 | 6.65 | (4.68–9.13) | 0.008 |
| Reported traffic intensity | ||||||||
| Never | 1463 | 6.63 | (5.41–8.03) | 1399 | 4.15 | (3.16–5.33) | ||
| Seldom | 613 | 7.34 | (5.40–9.70) | 734 | 4.36 | (3.00–6.10) | ||
| Frequent | 503 | 8.35 | (6.08–11.12) | 618 | 5.83 | (4.11–7.97) | ||
| Constant | 620 | 6.13 | (4.37–8.32) | 0.466 | 806 | 7.69 | (5.95–9.75) | 0.002 |
| Geographical area | ||||||||
| North | 1241 | 7.30 | (5.77–9.08) | 1451 | 4.79 | (3.62–6.21) | ||
| Centre | 660 | 3.94 | (2.59–5.72) | 716 | 4.75 | (3.31–6.57) | ||
| South | 1309 | 7.72 | (6.33–9.30) | 0.006 | 1406 | 6.40 | (5.18–7.81) | 0.110 |
| NO2 (μg/m3) | ||||||||
| <20 | 166 | 6.02 | (2.93–10.80) | 191 | 3.14 | (1.16–6.71) | ||
| 20– 35 | 186 | 5.91 | (2.99–10.34) | 244 | 4.51 | (2.27–7.92) | ||
| 35– 50 | 208 | 6.73 | (3.73–11.04) | 235 | 4.26 | (2.06–7.69) | ||
| >50 | 180 | 6.67 | (3.49–11.36) | 0.982 | 219 | 8.68 | (5.30–13.22) | 0.052 |
| Season of symptoms report | ||||||||
| Spring | 997 | 6.32 | (4.89–8.01) | 1146 | 4.45 | (3.33–5.81) | ||
| Summer | 566 | 7.07 | (5.10–9.50) | 636 | 4.87 | (3.34–6.85) | ||
| Autumn | 859 | 6.17 | (4.66–7.99) | 914 | 4.70 | (3.43–6.29) | ||
| Winter | 788 | 8.38 | (6.54–10.53) | 0.27 | 877 | 7.41 | (5.77–9.35) | 0.016 |
At the individual level, in the hierarchical model following adjustment for confounding variables, constant traffic retained a significant association with chronic phlegm at follow up among females (table 4). The association with traffic did not vary after stratifying by smoking or after stratifying by type of traffic (car or trucks and buses). These associations were homogeneous among centres (p for heterogeneity >0.52). The association was stronger when the outcome was chronic productive cough (odds ratio for constant traffic = 2.70; 95% CI 1.07 to 7.12). When the models were adjusted for education instead of social class very similar results were obtained. Exclusion of individuals with asthma or rhinitis did not modify the association of traffic intensity among females. No effect for traffic was observed among males.
Table 4 Multilevel model on chronic phlegm prevalence* at follow up (odds ratio, 95% confidence interval) by gender.
| Males | Females | |
|---|---|---|
| Individual level | ||
| Age in years | 0.97 (0.95–1.00) | 1.00 (0.98–1.03) |
| Smoking status | ||
| Non‐smokers (ref) | 1 | 1 |
| With passive smoking | 1.11 (0.52‐2.36) | 0.79 (0.41–1.54) |
| Ex‐smokers | 1.81 (1.13–2.91) | 1.08 (0.68–1.71) |
| ⩽ 10/day | 2.21 (1.27–3.87) | 1.81 (1.10–2.96) |
| 10–20/day | 2.56 (1.48–4.42) | 3.14 (1.95–5.04) |
| > 20/day | 4.86 (2.68–8.82) | 4.81 (2.49–9.31) |
| Respiratory infections before age 5 (yes) | 1.68 (1.05–2.71) | 1.12 (0.76–1.66) |
| Rhinitis (yes) | 1.78 (1.27–2.51) | 2.00 (1.45–2.78) |
| Social class | ||
| Professional and managerial (ref) | 1 | 1 |
| Other non‐manual | 1.58 (1.02–2.46) | 1.46 (0.93–2.30) |
| Skilled manual | 1.94 (1.20–3.14) | 0.92 (0.30–2.86) |
| Semi/unskilled manual | 2.52 (1.51–4.19) | 2.13 (1.14–3.96) |
| Unclassified | 1.47 (0.64–3.36) | 1.79 (1.03–3.08) |
| Traffic | ||
| None (ref) | 1 | 1 |
| Seldom | 1.25 (0.82–1.93) | 1.23 (0.77–1.96) |
| Frequent | 1.26 (0.82–1.95) | 1.46 (0.92–2.31) |
| Constant | 0.88 (0.56–1.38) | 1.86 (1.24–2.77) |
| Centre level† | ||
| PM2.5 in μg/m3 | 0.97 (0.70–1.35) | 0.99 (0.85–1.17) |
| Sulfur content in μg/m3 | 1.00 (0.70–1.44) | 1.00 (0.85–1.17) |
| Odds ratio between % chronic phlegm v response rate | 0.88 (0.52–1.50) | 1.46 (1.07–1.99) |
| Average educational level in years | 1.01 (0.59–1.75) | 0.98 (0.83–1.15) |
| % Smokers | 0.36 (0.22–0.60) | 4.44 (0.89–22.1) |
*Each column is a multivariate model.
†The measure of association is the interval odds ratio; PM2.5 was introduced in an alternative model instead of S content, but coefficients for individual level variables remain stable.
None of the variables included at the individual level showed a random variation between centres, suggesting a lack of different effects of these variables among the centres. In addition the variance of the random intercept was small and not significant indicating minimal heterogeneity for the individual level adjusted frequency of chronic phlegm between centres. Among females, the variability between centres was reduced by around 6% after including the individual variables for traffic intensity in the model.
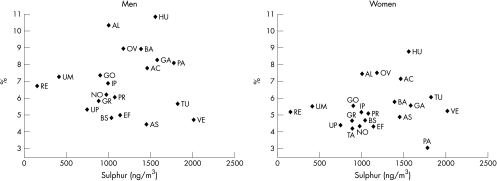
Table 4 also shows a lack of association for centre variables because the interval odds ratio for any of the pollutants in males and females include the value 1, indicating large variability of the PM2.5 and S centre variables in comparison with the unexplained variation between centres. On the other hand, the variation in chronic phlegm between centres was not reduced after including the area of residence ranked by PM2.5 (or S) in the model. This lack of heterogeneity between centres could alternatively be assessed by looking at the plot of the association between the adjusted chronic phlegm prevalence in each centre, obtained from the model in table 4, against the average S values (fig 1) (figure with PM2.5 was identical). Similarly, no association was observed with new onset of chronic phlegm or chronic productive cough or after excluding the Italian centres with outlier values on air pollution. Adjustment for other centre variables such as response rate, educational level, or percentage of smokers did not modify this association with particles at centre level. Prevalence of smoking showed an opposite association in males than females.
Figure 1 Adjusted prevalences of chronic phlegm at follow up versus average of sulphur concentrations by sex. Adjusted for age, smoking, respiratory infections before age 5, rinhitis, and social class. AS, South Antwerp; AC, Antwerp City; EF, Efurt; BA, Barcelona; GA, Galdakao; AL, Albacete; OV, Oviedo; HU, Huelva; GR, Grenoble; PR, Paris; PA, Pavia; TU, Turin; VE, Verona; IP, Ipswich; NO, Norwich; RE, Reyjavik; GO, Goteborg; UM, Umea; UP, Uppsala; BS, Basel; TA, Tartu.
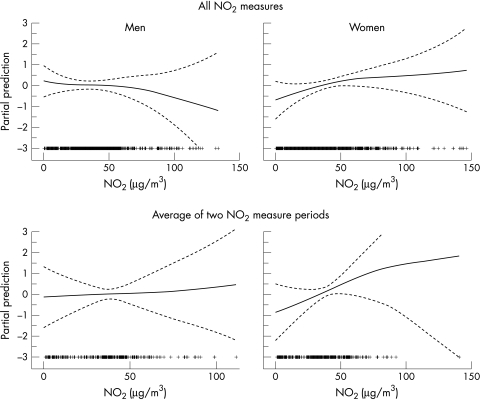
Figure 2 shows the dose‐response relationship with NO2 using the GAM modeling after adjusting for variables in table 3. The dose‐response with NO2 was linear in females but not in males (p for gain of non‐linearity 0.15 and 0.03, respectively). The association with NO2 was significant among females but not among males (when NO2 was treated as a discrete variable—that is, the interquartile change) (table 5). Adjustment for other risk factors of chronic bronchitis increased the association, particularly in males; although the association in males was not statistically significant. The association in females remained similar after inclusion of only never smokers, or excluding areas with low participation rates (p for heterogeneity between areas = 0.7), or restricting the analysis to individuals with at least two NO2 periods measured; and was stronger after exclusion of housewife or people with manual social class or among women with higher education. Adjustment for the risk factors in the stratified analysis yielded to slightly stronger associations. Adjustment for indoor NO2 did not affect these associations. Among females, the association remained when the outcome was chronic productive cough instead of chronic phlegm (the OR for a change of 30 μg/m3 being 1.87 (95% CI 0.99 to 3.42) and 2.93 (95% CI 1.14 to 7.49) for interquartile change).
Figure 2 Non‐parametric smoothed association (and 95% confidence bands) between chronic phlegm and average of home outdoor NO2 by sex. All NO2 measurements included one or two 2‐week measurement periods. Adjusted for season of NO2 measurement (top) or day of year of the first day of measurement and interval (bottom), smoking, and centre.
Table 5 Sensitivity analysis of the association (odds ratio, 95% confidence interval) between NO2 and prevalence of chronic phlegm.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| n | Per each increase in 30 μg/m3* | <20 μg/m3v >50 μg/m3† | n | Per each increase in 30 μg/m3* | <20 μg/m3v >50 μg/m3† | |
| All | 734 | 0.85 (0.56–1.31) | 0.99 (0.40–2.46) | 886 | 1.38 (0.97–1.95) | 2.71 (1.03–7.16) |
| Adjusted‡ | 639 | 0.86 (0.42–1.77) | 3.37 (0.56–20.0) | 752 | 1.76 (1.04–2.98) | 3.53 (0.72–17.2) |
| Never smokers | 266 | 1.00 (0.46–2.17) | 1.20 (0.29–5.04) | 428 | 1.49 (0.77–2.91) | 3.13 (0.75–13.1) |
| Non‐manual social class | 455 | 0.95 (0.49–1.85) | 1.29 (0.27–6.17) | 633 | 1.98 (1.26–3.11)§ | 3.98 (1.04–15.3) |
| Years education >16 | 538 | 0.82 (0.47–1.44) | 0.98 (0.32–2.97) | 604 | 1.90 (1.23–2.93)§ | 5.81 (1.22–27.7) |
| Centres with participation rate above the median | 483 | 0.84 (0.54–1.30) | 1.03 (0.38–2.85) | 537 | 1.34 (0.89–2.02) | 2.29 (0.72–7.28) |
| Average of two NO2 periods† | 302 | 0.95 (0.44–2.03) | 1.02 (0.18–5.71) | 356 | 1.22 (0.56–2.66) | 3.35 (0.59–19.1) |
*Adjusted for season.
†Interquartile change. Adjusted for day of week and interval between the two measures.
‡Adjusted for age, smoking, social class, rhinitis, early respiratory infections, and centre. Data from Anstwerp and Tartu dropout from the model due to small numbers.
§p for interaction with social class or education <0.05.
Discussion
Prevalence and new onset of chronic phlegm and chronic productive cough among the centres of the ECRHS in both males and females vary by a factor of three, but these variations were not explained by the average levels of PM2.5. However, at the individual level, self‐reported traffic intensity and home outdoor levels of NO2 (a surrogate of traffic exposure) were associated with frequency of chronic phlegm and chronic productive cough in females homogeneously throughout the centres. Fitting either prevalence or new onset of chronic phlegm at the end of follow up yielded similar findings. The persistence of the effect of traffic intensity in non‐smokers reinforces the validity of this finding, as well as the fact that NO2 was measured in subjects who had not moved residence since baseline and also that the findings were homogenous across the geographical areas.
Measurement of individual exposure is the greatest challenge of population studies on air pollution. A central monitoring measurement does not reflect the individual variability within a community for primary tail pipe emissions,26 particularly in large cities as many of those participating in ECRHS. This probably explains the apparent inconsistency of a lack of effects using centre‐level measurements and a presence of effects when using individual measurements. An exception could be the S content, which is expected to be homogeneously distributed in a city because it reflects long range transport pollution. However, we also did not find an association with S content. In addition to the potential variation in the S content within individuals from the same city because of different time‐activity patterns, social and cultural heterogeneity in Europe is probably too large to be adjusted for in the hierarchical models, in contrast to studies carried out in more homogeneous areas such as the SAPALDIA study.15 Moreover, non‐response might have heterogeneously biased the prevalence as centres with higher pollution showed higher non‐response. Overall, a true ecological association could have been underestimated.
The assessment of the cumulative exposure at home outdoors is a major challenge when the time between exposure and effect assessment is long. A measure of a single period does not capture the seasonal variations while the average of at least two periods of measurement notably reduces the error in relation to the yearly average. The deployment of home NO2 samplers across seasons happened randomly with subject selections based neither on health nor local air quality characteristics. Thus, we expect that the use of a single or a few NO2 sampling periods as an estimate of the annual means introduced random rather than systematic errors with a bias most likely towards null findings. The lack of comparable monitoring data for the time up to ECRHS II is an inherent weakness of the study. As a consequence, we are unable to investigate the effect of changes in air quality between ECRHS I and II and its effects on the development of respiratory diseases. However, the relevant period of reported symptoms, namely the 12 months before the follow up assessment and the ECRHS II air quality assessment, are very well matched and valid to investigate the hypothesis.
At high concentrations in animals and humans, NO2 damages the epithelial cells by oxidant injury, reduces the clearance of infecting organisms, depresses alveolar macrophages, and releases pro‐inflammatory mediators.27 The toxicological evidence suggests that NO2 at the low concentrations found in everyday life may play a role in lung inflammation.28,29 Both a recent Dutch birth cohort30 and a German birth cohort31 showed an association of respiratory symptoms in infants with outdoor NO2 measurements, something which has also been observed in studies of school age children.32,33 In contrast, cohort studies measuring indoor NO2 did not find an association.34,35 Furthermore, in a Swiss study, the duration of lower respiratory symptoms in children less than 5 years of age was related to an individual outdoor measurement, but not with indoor levels.36 Similarly, the effect of home outdoor NO2 was associated with an increase of wheeze in a German study in 317 children, which was not found with personal NO2 measurements.37 These studies suggest that outdoor NO2 per se is probably only a surrogate of the pollutant mixture responsible for chronic respiratory effects, while substances such as polycyclic aromatic hydrocarbons or diesel particles may be the most important aetiological components. It has been shown that the concentration of fine particulate matter varies with nearby traffic roads and with NO2.38 The role of traffic exposure, mostly fine particulates, in chronic bronchitis is consistent with experimental findings that generated free radicals are capable of causing cell oxidative stress and lung inflammation.39 An inflammatory pathway correlates with the persistence of the observed effects seen in this study after excluding individuals with asthma, suggesting that traffic effects a respiratory phenotype, defined by reporting of chronic respiratory symptoms—even in non‐smokers.
In our study, a simple measure of self‐reported traffic intensity, whether cars or trucks and buses (we did not find differences between the two items, data not shown), was associated with chronic phlegm and chronic productive cough as was the individual measure of NO2. In fact, we found a strong correlation between reported frequent or constant traffic and the home‐outdoor NO2 levels (an increase of 40% in the NO2 levels in comparison with those reporting no traffic). However, evidence of the poor value of reported traffic40 and the impossibility of assessing the potential role of reverse causation between symptoms and reporting of traffic means the findings on traffic intensity must be viewed with caution.
A major finding is the gender differences in traffic effects (when measured either as a report of traffic intensity or as an individual level measurement of NO2) consistent with results from a small study in Sweden which found an association between NO2 and chronic cough only among women.41 One explanation is that home NO2 and traffic reflects the personal exposure among women but not among men given the potential for women to spend longer periods at home. On average women spend more time at home than men, according to the EXPOLIS study in several cities in Europe (N Kunzli, personal communication). Another explanation, though less likely, is that females are more susceptible to air pollution. Differences in susceptibility factors (hormones), risk factors (smoking), perception of the disease, and access to health services have been related to sex differences in asthma prevalence and chronic obstructive pulmonary disease (COPD).42 In a cohort of individuals with COPD, females had a higher risk of dying in relation to particle levels than males.43 A recent study has shown different mechanisms between males and females in the development of asthma, females being more affected by non‐atopic mechanisms.44 A third possible explanation could be residual confounding by smoking but analysis among never smokers did not show any association in males, while the association among females of both traffic and NO2 did not change. A final explanation refers to the question of whether women have better/different perceptions of their environment and their symptoms. The fact that we observed the same association between NO2 and reporting of intensity of traffic in women and men, homogeneously across all centres, seems to suggest a similar perception of traffic intensity. In addition, the consistency of the findings—regardless of whether chronic bronchitis was defined by chronic phlegm or chronic productive cough (or chronic cough, data not shown)—reduces the potential for a differential diagnostic bias in women compared with men. Finally, the finding of a stronger association with NO2 among the more educated women and the non‐manual social class possibly was due to a more valid perception and reporting of symptoms of chronic bronchitis, given that the variations in NO2 levels in our study were not perceivable.
One strength of the present study, in addition to the individual measurement of air pollution, is the prospective nature of the design. That allowed consideration of two potentially different symptomatic groups, namely those with symptoms at baseline, and those with symptoms only at follow up (“new onset”). The repeated survey showed a high fluctuation in chronic bronchitis symptoms, something which has also been shown with other respiratory symptoms.45 In light of temporal changes in occurrence and reporting of symptoms, the questionnaire captures in particular symptomatic episodes during the past year or months before the two surveys (ECRHS I and II) rather than a “chronic condition”. Accordingly, “new onset” may not necessarily reflect the incidence of a chronic condition not present at ECRHS I, but the period prevalence of recent symptomatic episodes among those who did not report symptomatic episodes 7–10 years before. The group with symptoms reported in both surveys may more likely consist of subjects with chronic conditions. The findings of an association of exposure during ECRHS II with symptom prevalence in the total population, as well as in those who did not report symptoms in ECHRS I, suggests that air pollution may contribute to symptomatic episodes not only in those with underlying chronic respiratory diseases. The high turnover of incident and remitting cases in our study suggests that the predictive nature of having chronic bronchitis symptoms at middle age is uncertain. The present results may refer to the acute and subchronic, rather than chronic, effects of air pollution. To investigate the contribution of ambient pollution on the chronic development of symptomatic respiratory diseases one may need longitudinal studies with several annual repeated surveys, coupled with continuous monitoring across the entire follow up.
A limitation of the present study in the assessment of the individual exposures (that is, traffic, NO2) was the non‐response rate, however the consistency of the coefficients after adjusting for the odds ratio between non‐response and symptoms and the geographical homogeneity of the findings are reassuring in this respect. Non‐response is notable for the home NO2 measurement, however participants had the same prevalence of chronic productive cough (p = 0.7) and chronic phlegm (p = 0.5) than non‐participants, which reduces the probability that association with NO2 was due to a selection bias. In addition, it seems unlikely that NO2 effects were driven by a selective participation of diseased females, because there was no association between participation and symptoms among females (p>0.4).
We performed a hierarchical analysis in order to incorporate the geographical structure of the data and to incorporate contextual (that is, aggregated) variables, related with air pollution, and with chronic bronchitis, such as the smoking patterns by area. Thus, we adjusted for social conditions and occupation not only at the individual level, but also at the aggregated level in order to avoid an imperfect control of the confounding. We found that inclusion of aggregated variables did not change our estimation of the effect of traffic among females, and also that geographical variations in chronic bronchitis were only moderately explained by known risk factors, as already found in the cross sectional analysis of ECRHS I.46
Overall, chronic bronchitis symptoms increased among females in relation to indicators of exposures to traffic pollution at the home level, reinforcing public health concerns about the health effects of urban air pollution emissions, and provoking unsolved questions about gender differences in bronchitis and susceptibility to air pollutants.
Acknowledgements
We acknowledge Gemma Perello for her help in the editing of the manuscript and all the participants of the study for their generosity.
Abbreviations
COPD - chronic obstructive pulmonary disease
ECRHS - European Community Respiratory Health Survey
Footnotes
Funding: “Instituto de Salud Carlos III” Red de Grupos INMA (G03/176) and “Instituto de Salud Carlos III, Red de Centros RCESP (C03/09).
Competing interests: none.
References
- 1.Logan W P D. Mortality in the London fog incident. Lancet 19531336–338. [DOI] [PubMed] [Google Scholar]
- 2.Lambert P M, Reid D D. Smoking, air pollution and bronchitis in Britain. Lancet 19701853–857. [DOI] [PubMed] [Google Scholar]
- 3.Holand W N, Reid D D. The urban factor in chronic bronchitis. Lancet 19651445–448. [DOI] [PubMed] [Google Scholar]
- 4.Ferris B G, Jr, Higgins I T, Higgins M W.et al Chronic no‐specific respiratory disease, Berlin NH. 1961–1967. A cross‐sectional study. Am Rev Resp Dis 1971104232–244. [DOI] [PubMed] [Google Scholar]
- 5.Love G J, Lan S, Shy C M.et al The incidence and severely of acute respiratory illness in families exposed to different levels of air pollution, New York Metropolitan Area, 1971–1972. Arch Environ Health 19813666–74. [DOI] [PubMed] [Google Scholar]
- 6.Burrows B, Kellogg A L L, Buskey S. Relationship of symptoms of chronic bronchitis and emphysema to matter and air pollution. Arch Environ Health 196816406–413. [DOI] [PubMed] [Google Scholar]
- 7.Sawiki F. Chronic non‐specific respiratory diseases in the city of Cracow. Statistical analyses of air pollution by suspended particulate matter and sulphur dioxide. Epidemiol Rev 196923221–231. [Google Scholar]
- 8.Aubry F, Gibbs G W, Beckake M R. Air pollution and health in three urban communities. Arch Environ Health 197934360–367. [DOI] [PubMed] [Google Scholar]
- 9.Bouhuys A, Beck G J, Schoenberg J B. Do present levels of air pollution outdoors affect respiratory health? Nature 1978276466–471. [DOI] [PubMed] [Google Scholar]
- 10.Braun‐Fahrlander C, Vuille J C, Sennhauser F H.et al Respiratory health and long‐term exposure to air pollutants in Swiss schoolchildren. SCARPOL Team. Am J Respir Crit Care Med 19971551042–1049. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich J, Hoelscher B, Wichman H E. Decline of ambient air pollution and respiratory symptoms in children. Am J Respir Crit Care Med 20001611930–1936. [DOI] [PubMed] [Google Scholar]
- 12.Kim J J, Smorodinsky S, Lipsett M.et al Traffic‐related air pollution near busy roads: the East Bay Children's Health Study. Am J Respir Crit Care Med 2004170520–526. [DOI] [PubMed] [Google Scholar]
- 13.Ryan P H, LeMasters G, Biagini J.et al Is it traffic type, volume or distance. Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol 2005116279–284. [DOI] [PubMed] [Google Scholar]
- 14.Euler G, Abbey D E, Magie A R.et al Chronic obstructive pulmonary disease symptom effects of long‐term cumulative exposure to ambient levels of total suspended particulates and sulfur dioxide in California Seventh‐day Adventist residents. Arch Environ Health 198742213–222. [PubMed] [Google Scholar]
- 15.Zemp E, Elsasser S, Schindler C.et al Long‐term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). Am J Respir Crit Care Med 19991591257–1266. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz J. Particulate air pollution and chronic respiratory disease. Environ Res 1993627–13. [DOI] [PubMed] [Google Scholar]
- 17.Portney P R, Mullahy J. Urban air quality and chronic respiratory disease. Reg Sci Urban Econ 199020407–418. [Google Scholar]
- 18.Heinrich J, Topp R, Gehring U.et al Traffic at residential address, respiratory health, and atopu in adults: the National German health survey 1988. Environ Res 200598240–249. [DOI] [PubMed] [Google Scholar]
- 19.Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J 2001171024–1033. [DOI] [PubMed] [Google Scholar]
- 20. Burney PG, Laitinen LA, Perdrizet S, et al Validity and repeatability of the IUALTD (1984) bronchial symptoms questionnaire: an international comparison. Eur Respir J 19892940–945. [PubMed] [Google Scholar]
- 21.The European Community Respiratory Health Survey II Steering Committee The European Community Respiratory Health Survey II. Eur Respir J 2002201071–1079. [DOI] [PubMed] [Google Scholar]
- 22.Hazenkamp‐von Arx M E, Götschi T, Acklerman‐Liebrich N.et al PM2.5 and NO2 assesment in 21 European study centres of ECRHS II annual means and seasonal differences. Atmos Environ 2004381943–1953. [Google Scholar]
- 23.Hox J J.Applied multilevel analysis. Amsterdam: TT‐Publications, 1995
- 24. MlwiN version 1.1. Multilevel models project. London: Institute of Education, University of London, Hedeker D, Gibbons RD. MIXOR: A computer program for mixed‐effects ordinal regression analysis. Comput Methods Programs Biomed 199649157–176. [DOI] [PubMed] [Google Scholar]
- 25.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 200516181–88. [DOI] [PubMed] [Google Scholar]
- 26.Nerriere E, Zmirou‐Navier D, Blanchard O.et al Can we use fixed ambient air monitors to estimate population long‐term exposure to air pollutants? The case of spatial variability in the Genotox ER study. Environ Res 20059732–42. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society Committee of the environmental and occupational health assembly. Health effects of outdoor pollution. Am J Respir Crit Care Med 1996153477–484. [DOI] [PubMed] [Google Scholar]
- 28.Blomberg A, Krishna M T, Helleday R.et al Persistent airway inflammation but accommodated antioxidant and lung function responses after repeated daily exposure to nitrogen dioxide. Am J Respir Crit Care Med 1999159536–543. [DOI] [PubMed] [Google Scholar]
- 29.Wegmann M, Fehrenbach A, Heimann S.et al NO2.induced airway inflammation is associated with progressive airflow limitation and development of emphysema‐like lesions in C57bl6 mice. Exp Toxicol Pathol. 2005;56;341–50. [DOI] [PubMed]
- 30.Brauer M, Hoek G, Van Vliet P.et al Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 20021661092–1098. [DOI] [PubMed] [Google Scholar]
- 31.Gehring U, Cyrys J, Sedlmeir G.et al Traffic‐related pollution and respiratory health during the first 2 years of life. Eur Respir J 200219690–698. [DOI] [PubMed] [Google Scholar]
- 32.Van Vliet P, Knape M, de Hartog J.et al Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ Res 199774122–132. [DOI] [PubMed] [Google Scholar]
- 33.Shima M, Adachi M. Effect of outdoor and indoor nitrogen dioxide on respiratory symptoms in schoolchildren. Int J Epidemiol 200029862–870. [DOI] [PubMed] [Google Scholar]
- 34.Samet J M, Lambert W E, Skipper B J.et al Nitrogen dioxide and respiratory illnesses in infants. Am Rev Respir Dis 19931481258–1265. [DOI] [PubMed] [Google Scholar]
- 35.Sunyer J, Basagaña X. Particles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary disease. Int J Epidemiol 2001301138–1140. [DOI] [PubMed] [Google Scholar]
- 36.Braun‐Fahrlander C, Ackerman‐Lievrich U, Schwartz J.et al Air pollution and respiratory symptoms in preschool children. Am Rev Respir Dis 199214542–47. [DOI] [PubMed] [Google Scholar]
- 37.Brauer M, Hoek G, Van Vliet P.et al Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 20021661902–1908. [DOI] [PubMed] [Google Scholar]
- 38.Brauer M, Hoek G, van Vliet P.et al Estimating long‐term average particulate air pollution concentrations; application of traffic indicators and geographic information systems. Epidemiology 200314228–239. [DOI] [PubMed] [Google Scholar]
- 39.MacNee W, Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur Respir J Suppl 20034047s–51s. [DOI] [PubMed] [Google Scholar]
- 40.Heinrich J, Gerhing U, Cyris J.et al Exposure to traffic related air pollutants: self‐reported traffic intensity versus GIS modelled exposure. Occup Environ Med 200562517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsberg B, Sjternberg N, Wall S. Prevalence of respiratory symptoms and hypereactivity symptoms in relation to levels of criteria air pollutants in Sweeden. Eur J Public Health 19977291–296. [Google Scholar]
- 42.Kauffmann F, Becklale M R. Maladies respiratoires obstructives. Un paradigme de la complexité des differences en santé entre femmes et hommes. In: Saurel‐Cubizolles MJ, Blondel B (eds), La santé des femmes. Paris: Medecine‐Sciences Flammarion 1996
- 43.Sunyer J, Schwartz J, Tobías A.et al Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: A case‐crossover analysis. Am J Epidemiol 200015150–56. [DOI] [PubMed] [Google Scholar]
- 44.Mandhane P J, Greene J M, Cowan J O.et al Sex differences in factors associated with childhood and adolescent‐onset wheeze. Am J Respir Crit Care Med 200517245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinn S, Jarvis D, Burney P.et al Increase in diagnosed asthma but not in symptoms in the European Community Respiratory Health Survey. Thorax 200459646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerveri I, Accordini S, Verlato G.et al Variations in the prevalence across countries of chronic bronchitis and smoking habits in young adults. Eur Respir J 20011885–92. [DOI] [PubMed] [Google Scholar]