Abstract
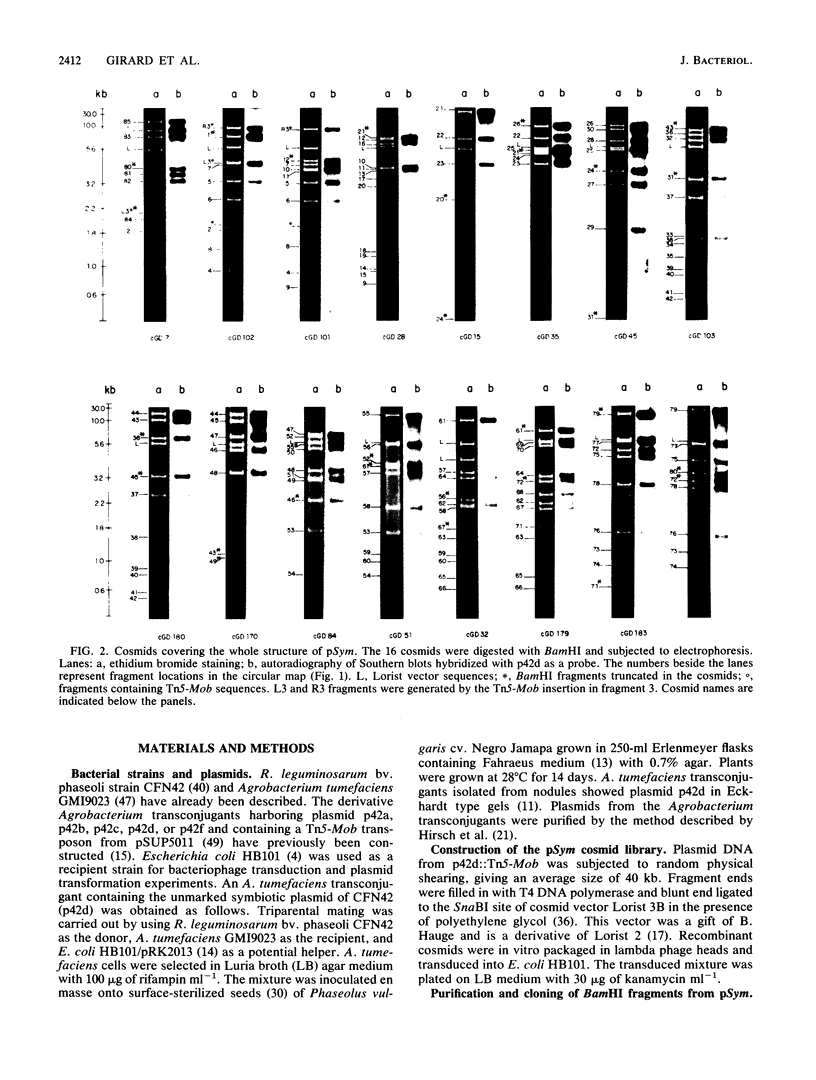
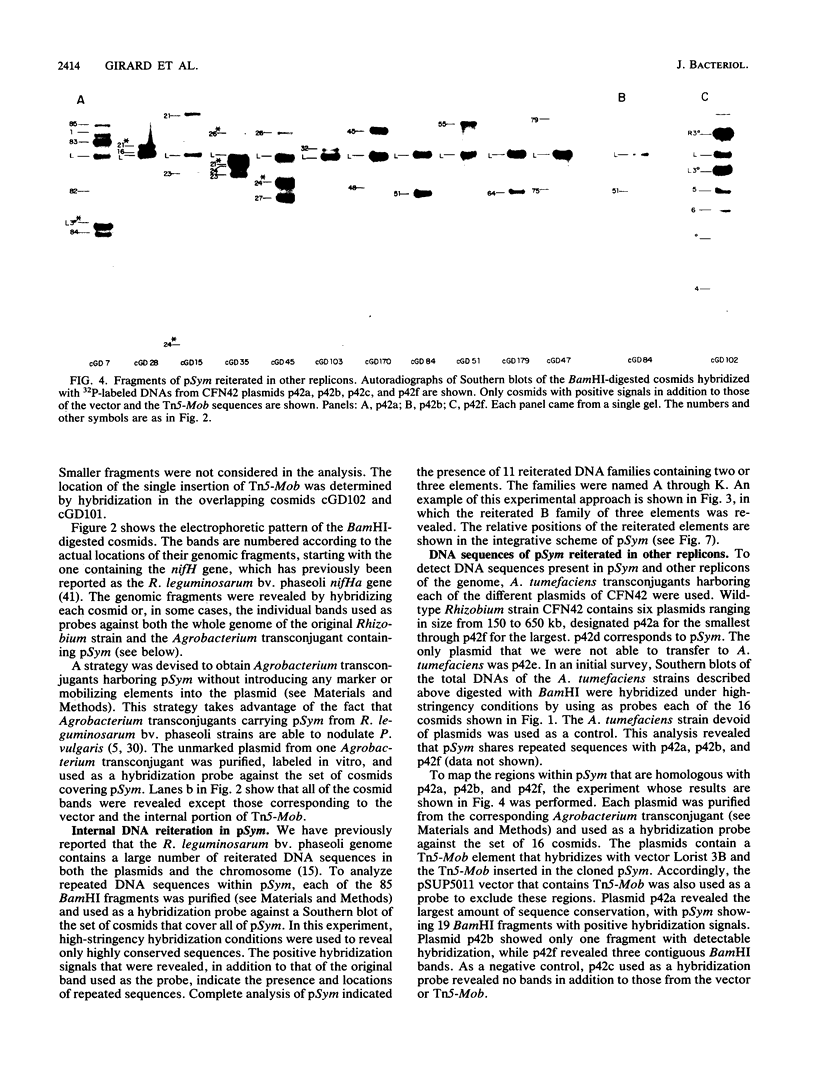
The complete physical map of the symbiotic plasmid of Rhizobium leguminosarum bv. phaseoli strain CFN42 was established. The data support the concept that Rhizobium symbiotic genes are part of a complex genomic structure which contains a large amount of reiterated DNA sequences. This plasmid is a circular structure of 390 kb with approximately 10 families of internally reiterated DNA sequences of two to three elements each. One family includes two directly oriented nitrogenase operons situated 120 kb apart. We also found several stretches of pSym that are reiterated in other replicons of the cell. Localization of symbiotic gene sequences by heterologous hybridization revealed that nodABC sequences are separated in two regions, each of which contains a nod boxlike element, and it also suggested the presence of two copies of the nifA and nodD gene sequences. We propose that the complex structure of the symbiotic plasmid allows interactions between repeated DNA sequences which, in turn, might result in frequent rearrangements.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A. 1981 May;78(5):3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum E. R., Thompson D. V., Idler K., Chartrain N. Rhizobium japonicum USDA 191 has two nodD genes that differ in primary structure and function. J Bacteriol. 1988 Jan;170(1):12–20. doi: 10.1128/jb.170.1.12-20.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brom S., Martinez E., Dávila G., Palacios R. Narrow- and Broad-Host-Range Symbiotic Plasmids of Rhizobium spp. Strains That Nodulate Phaseolus vulgaris. Appl Environ Microbiol. 1988 May;54(5):1280–1283. doi: 10.1128/aem.54.5.1280-1283.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Cevallos M. A., Vázquez M., Dávalos A., Espín G., Sepúlveda J., Quinto C. Characterization of Rhizobium phaseoli Sym plasmid regions involved in nodule morphogenesis and host-range specificity. Mol Microbiol. 1989 Jul;3(7):879–889. doi: 10.1111/j.1365-2958.1989.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Charles T. C., Finan T. M. Genetic map of Rhizobium meliloti megaplasmid pRmeSU47b. J Bacteriol. 1990 May;172(5):2469–2476. doi: 10.1128/jb.172.5.2469-2476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Hadley R. G. IS2-IS2 and IS3-IS3 relative recombination frequencies in F integration. Plasmid. 1980 Jan;3(1):48–64. doi: 10.1016/s0147-619x(80)90033-5. [DOI] [PubMed] [Google Scholar]
- Dusha I., Kovalenko S., Banfalvi Z., Kondorosi A. Rhizobium meliloti insertion element ISRm2 and its use for identification of the fixX gene. J Bacteriol. 1987 Apr;169(4):1403–1409. doi: 10.1128/jb.169.4.1403-1409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Long S. R. Rhizobium meliloti nodulation genes: identification of nodDABC gene products, purification of nodA protein, and expression of nodA in Rhizobium meliloti. J Bacteriol. 1985 Nov;164(2):591–599. doi: 10.1128/jb.164.2.591-599.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Brom S., Martínez E., Piñero D., Romero D., Dávila G., Palacios R. Reiterated DNA sequences in Rhizobium and Agrobacterium spp. J Bacteriol. 1987 Dec;169(12):5782–5788. doi: 10.1128/jb.169.12.5782-5788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. J., Coulson A. R., Sulston J. E., Little P. F. Lorist2, a cosmid with transcriptional terminators insulating vector genes from interference by promoters within the insert: effect on DNA yield and cloned insert frequency. Gene. 1987;53(2-3):275–281. doi: 10.1016/0378-1119(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Inversions between ribosomal RNA genes of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7069–7072. doi: 10.1073/pnas.78.11.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M. A., Ausubel F. M. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8558–8562. doi: 10.1073/pnas.84.23.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza K., Hahn M., Hennecke H. Repeated sequences similar to insertion elements clustered around the nif region of the Rhizobium japonicum genome. J Bacteriol. 1985 May;162(2):535–542. doi: 10.1128/jb.162.2.535-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. J., Capage M., Hill C. W. A repetitive DNA sequence, rhs, responsible for duplications within the Escherichia coli K-12 chromosome. J Mol Biol. 1984 Jul 25;177(1):1–18. doi: 10.1016/0022-2836(84)90054-8. [DOI] [PubMed] [Google Scholar]
- Martínez E., Palacios R., Sánchez F. Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J Bacteriol. 1987 Jun;169(6):2828–2834. doi: 10.1128/jb.169.6.2828-2834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson R. V., Atherly A. G. The presence of repeated DNA sequences and a partial restriction map of the pSym of Rhizobium fredii USDA193. Plasmid. 1986 Jul;16(1):37–44. doi: 10.1016/0147-619x(86)90077-6. [DOI] [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Recombination sites in plasmid drug resistance gene amplification. J Bacteriol. 1985 Dec;164(3):1359–1361. doi: 10.1128/jb.164.3.1359-1361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pheiffer B. H., Zimmerman S. B. Polymer-stimulated ligation: enhanced blunt- or cohesive-end ligation of DNA or deoxyribooligonucleotides by T4 DNA ligase in polymer solutions. Nucleic Acids Res. 1983 Nov 25;11(22):7853–7871. doi: 10.1093/nar/11.22.7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. K., Atherly A. G. Reiteration of genes involved in symbiotic nitrogen fixation by fast-growing Rhizobium japonicum. J Bacteriol. 1984 Nov;160(2):785–787. doi: 10.1128/jb.160.2.785-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. K., van Veen R. J., Schilperoort R. A. Restriction endonuclease mapping of a Rhizobium leguminosarum Sym plasmid. Plasmid. 1982 May;7(3):271–280. doi: 10.1016/0147-619x(82)90008-7. [DOI] [PubMed] [Google Scholar]
- Priefer U. B., Burkardt H. J., Klipp W., Pühler A. ISR1: an insertion element isolated from the soil bacterium Rhizobium lupini. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):87–91. doi: 10.1101/sqb.1981.045.01.016. [DOI] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan N., Prakash R. K., Shantharam S., Duteau N. M., Atherly A. G. Molecular cloning and expression of Rhizobium fredii USDA 193 nodulation genes: extension of host range for nodulation. J Bacteriol. 1986 Dec;168(3):1087–1095. doi: 10.1128/jb.168.3.1087-1095.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renalier M. H., Batut J., Ghai J., Terzaghi B., Gherardi M., David M., Garnerone A. M., Vasse J., Truchet G., Huguet T. A new symbiotic cluster on the pSym megaplasmid of Rhizobium meliloti 2011 carries a functional fix gene repeat and a nod locus. J Bacteriol. 1987 May;169(5):2231–2238. doi: 10.1128/jb.169.5.2231-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Romero D., Brom S., Martínez-Salazar J., Girard M. L., Palacios R., Dávila G. Amplification and deletion of a nod-nif region in the symbiotic plasmid of Rhizobium phaseoli. J Bacteriol. 1991 Apr;173(8):2435–2441. doi: 10.1128/jb.173.8.2435-2441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero David, Singleton Paul W., Segovia Lorenzo, Morett Enrique, Bohlool B. Ben, Palacios Rafael, Dávila Guillermo. Effect of Naturally Occurring nif Reiterations on Symbiotic Effectiveness in Rhizobium phaseoli. Appl Environ Microbiol. 1988 Mar;54(3):848–850. doi: 10.1128/aem.54.3.848-850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Rossen L., Johnston A. W., Downie J. A. DNA sequence of the Rhizobium leguminosarum nodulation genes nodAB and C required for root hair curling. Nucleic Acids Res. 1984 Dec 21;12(24):9497–9508. doi: 10.1093/nar/12.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Török I., Kondorosi E., Stepkowski T., Pósfai J., Kondorosi A. Nucleotide sequence of Rhizobium meliloti nodulation genes. Nucleic Acids Res. 1984 Dec 21;12(24):9509–9524. doi: 10.1093/nar/12.24.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez M., Dávalos A., de las Peñas A., Sánchez F., Quinto C. Novel organization of the common nodulation genes in Rhizobium leguminosarum bv. phaseoli strains. J Bacteriol. 1991 Feb;173(3):1250–1258. doi: 10.1128/jb.173.3.1250-1258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]