Abstract
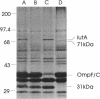
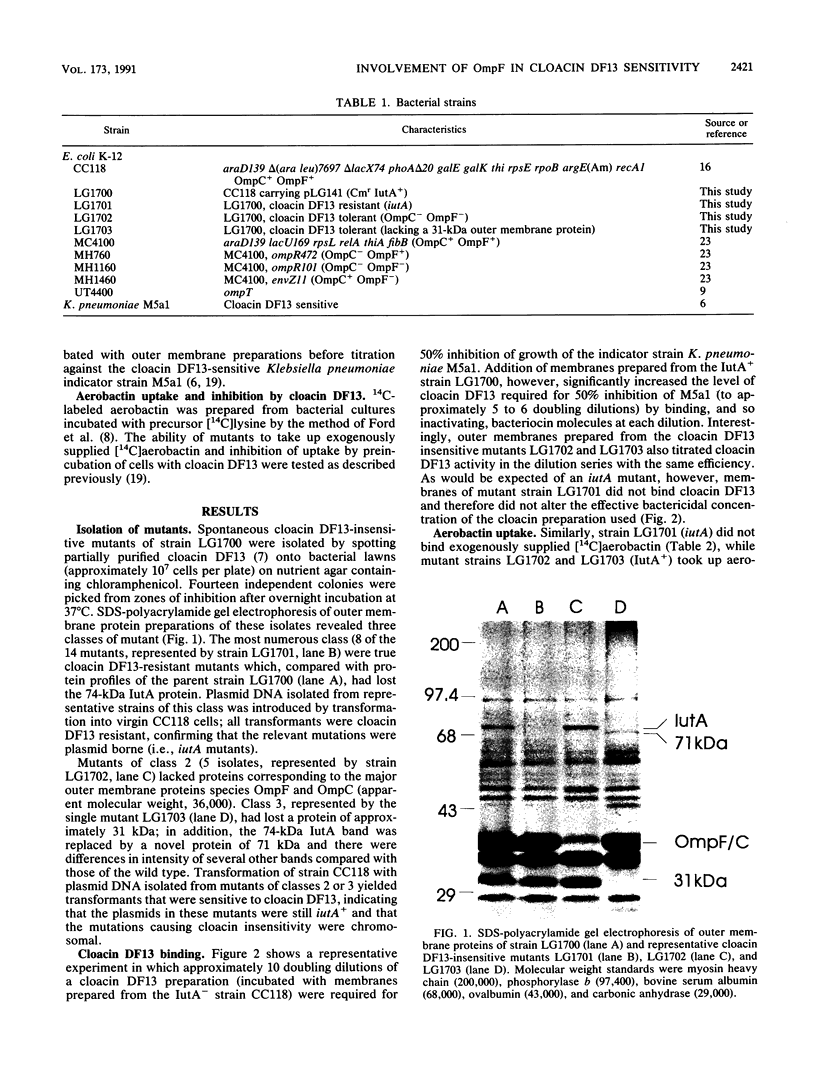
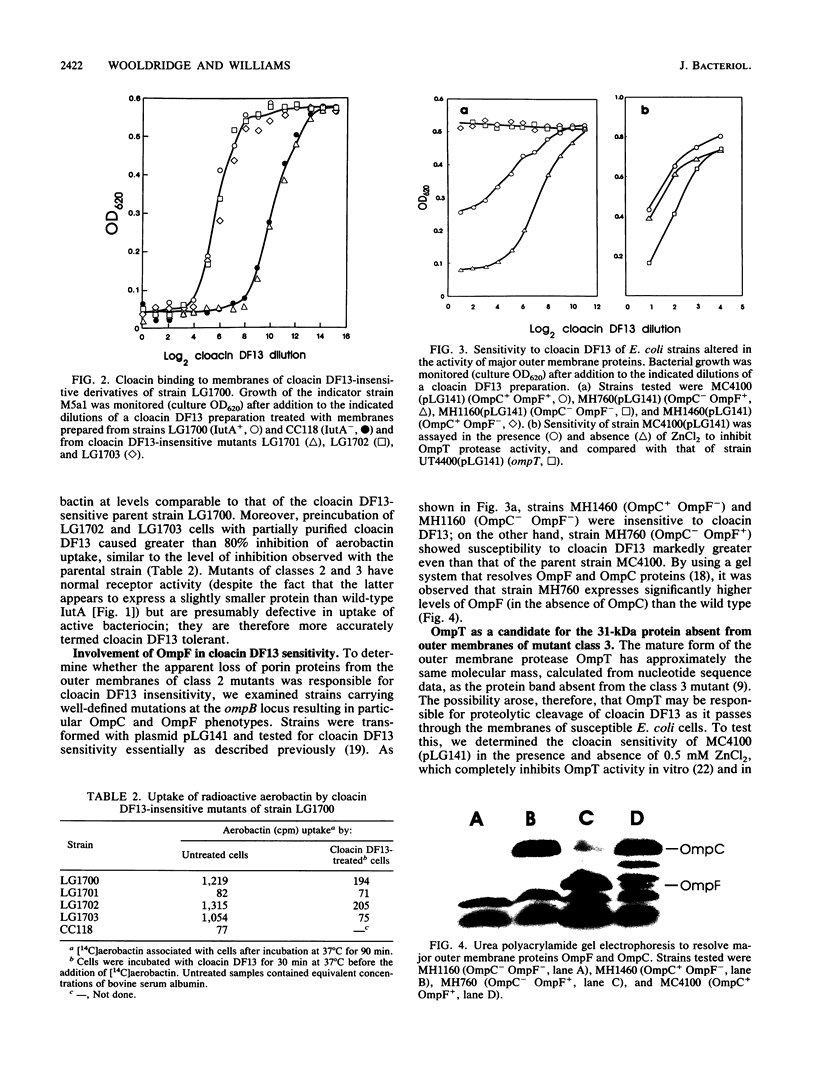
Fourteen spontaneous cloacin DF13-insensitive mutants of an Escherichia coli strain expressing the aerobactin-cloacin DF13 receptor protein IutA were isolated. The mutants fell into three classes on the basis of outer membrane profiles analyzed by electrophoresis in denaturing polyacrylamide gels. The most frequent class lacked the IutA protein and was unable to bind cloacin DF13 or aerobactin. A second class of mutants had lost protein species corresponding in size to the porin proteins OmpF and OmpC. To determine which porin was required for the bactericidal activity of cloacin DF13, defined strains with mutations at the ompB (ompR envZ) locus were transformed with a recombinant plasmid carrying the iutA gene and screened for cloacin DF13 sensitivity. OmpF- strains, whether OmpC+ or OmpC-, were insensitive to cloacin DF13, indicating involvement of the OmpF protein in cloacin DF13 killing. An OmpC- OmpF+ strain, on the other hand, was more sensitive than the wild-type parent strain, probably because of compensatory overexpression of OmpF. The third class of cloacin DF13-insensitive mutant had lost an outer membrane protein of approximately 31 kDa. The nature and function of this protein are not yet known, but it is not the protease OmpT. Mutants of classes 2 and 3 bound cloacin DF13 and aerobactin as effectively as the cloacin DF13-sensitive parental strain, indicating that they remained IutA+. We propose that these mutants (more accurately described as cloacin DF13 tolerant) are defective in translocation of the active portion of cloacin DF13 across the bacterial membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx F., Georgiou G. In vivo degradation of secreted fusion proteins by the Escherichia coli outer membrane protease OmpT. J Bacteriol. 1990 Jan;172(1):491–494. doi: 10.1128/jb.172.1.491-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Aerobactin genes in clinical isolates of Escherichia coli. J Bacteriol. 1985 Feb;161(2):727–735. doi: 10.1128/jb.161.2.727-735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. C., James R. Three immunity types of klebicins which use the cloacin DF13 receptor of Klebsiella pneumoniae. J Gen Microbiol. 1985 Sep;131(9):2313–2318. doi: 10.1099/00221287-131-9-2313. [DOI] [PubMed] [Google Scholar]
- Grodberg J., Lundrigan M. D., Toledo D. L., Mangel W. F., Dunn J. J. Complete nucleotide sequence and deduced amino acid sequence of the ompT gene of Escherichia coli K-12. Nucleic Acids Res. 1988 Feb 11;16(3):1209–1209. doi: 10.1093/nar/16.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., Koningstein G., de Graaf F. K., Oudega B. Plasmid-determined cloacin DF13-susceptibility in Enterobacter cloacae and Klebsiella edwardsii; identification of the cloacin DF13/aerobactin outer membrane receptor proteins. Antonie Van Leeuwenhoek. 1985;51(2):203–218. doi: 10.1007/BF02310013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. Escherichia coli K12 strains for use in the identification and characterization of colicins. J Gen Microbiol. 1985 Feb;131(2):369–376. doi: 10.1099/00221287-131-2-369. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Wooldridge K. G., Gavine H., Kuswandi S. I., Williams P. H. Inhibition of biological activities of the aerobactin receptor protein in rough strains of Escherichia coli by polyclonal antiserum raised against native protein. J Gen Microbiol. 1989 Sep;135(9):2387–2398. doi: 10.1099/00221287-135-9-2387. [DOI] [PubMed] [Google Scholar]
- Schramm E., Mende J., Braun V., Kamp R. M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987 Jul;169(7):3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Tieze G. A. Bacteriocin production by members of the genus Klebsiella. Antonie Van Leeuwenhoek. 1966;32(2):171–182. doi: 10.1007/BF02097457. [DOI] [PubMed] [Google Scholar]
- Sugimura K., Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Silhavy T. J. Isolation and characterization of mutations altering expression of the major outer membrane porin proteins using the local anaesthetic procaine. J Mol Biol. 1983 May 25;166(3):273–282. doi: 10.1016/s0022-2836(83)80085-0. [DOI] [PubMed] [Google Scholar]
- Van Tiel-Menkveld G. J., Mentjox-Vervuurt J. M., Oudega B., de Graaf F. K. Siderophore production by Enterobacter cloacae and a common receptor protein for the uptake of aerobactin and cloacin DF13. J Bacteriol. 1982 May;150(2):490–497. doi: 10.1128/jb.150.2.490-497.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Spanjaerdt Speckman E. A., Stouthamer A. H. Mode of action of a bacteriocin produced by Enterobacter cloacae DF13. Antonie Van Leeuwenhoek. 1969;35(3):287–306. doi: 10.1007/BF02219150. [DOI] [PubMed] [Google Scholar]