Endotoxins, part of the outer membrane of Gram negative bacteria, are a potent inducer of neutrophilic airway inflammation. A large number of studies in occupational epidemiology has shown that exposure to endotoxins increases the likelihood of organic dust toxic syndrome, chronic bronchitis, and asthma‐like syndrome. In contrast, it has been shown that exposure to endotoxins in the occupational and environmental setting protects from respiratory allergies and sensitisation to allergens.
With respect to asthma, the evidence is conflicting at first glance. While some studies indicated an increased risk of asthma after endotoxin exposure, others have shown that endotoxin exposure protects from asthma. However, these differences can be explained when different asthma phenotypes are taken into account. The risk of atopic asthma, mainly dominated by eosinophilic response, is decreased in those exposed to endotoxins. In contrast, the risk of non‐atopic asthma, characterised by neutrophilic response, is enhanced in subjects with higher endotoxin exposure. These data are in accordance with the so‐called hygiene hypothesis and have been supported by animal studies and at the cellular level.
At the workplace, measures should therefore be taken in order to reduce endotoxin exposure. The effectiveness of such measures with respect to the incidence of diseases associated with a chronic neutrophilic inflammation in the airways should be assessed in intervention studies. At the same time we need to learn whether the evidence from epidemiological studies in farming environments might help us to obtain effective intervention strategies against allergies.
What are endotoxins?
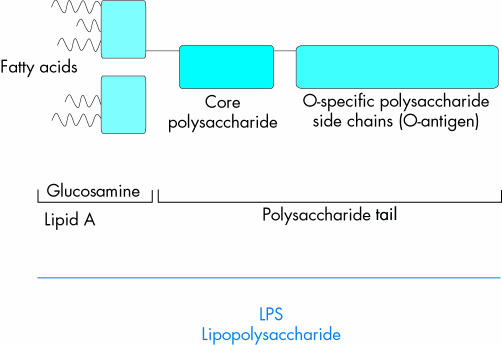
In contrast to Gram positive bacteria, Gram negative bacteria are surrounded by an inner and an outer membrane.1 Endotoxins are parts of the outer membrane. Their purified derivatives are called lipopolysaccharides (LPS).2
Endotoxins and LPS are present in the oral and nasal cavities of humans and animals. They can also be found ubiquitously in occupational and environmental settings on surfaces of animals, plants, and soil.2,3 In these environments they are quantified in highly variable concentrations.4 The immune stimulatory capacity of endotoxins can only be inactivated at high temperatures (for example, 160°C for 4 hours). Therefore, endotoxins are active for much longer than the lifetime of the bacteria themselves.
In occupational settings, endotoxins mainly occur in environments with high exposure to organic dusts. These are primarily agricultural environments (livestock, grain dust),5,6 textile production,7 and waste processing.2,8
In the domestic environment, significant concentrations occur from pets kept indoors, carpeting, as well as air conditioning.4 In the agricultural setting, endotoxins have also been shown to be transported from the animal houses into the home environment of farmers and farmers' children.9 Recent measurements on the endotoxin concentrations in house dust have been summarised by Michel.10 In these studies, the mean endotoxin content of settled bedroom floor dust varied between 7.3 and 63 EU/mg. Exposure from, for example, intensive animal production facilities increases the background exposure to endotoxins in rural areas11 compared to urban settings.12,13
Some examples for the range of exposures found in several environments are given in table 1. However, the results of these analyses largely depend on the method used (see “Exposure assessment”).
Table 1 Examples of the range of endotoxin exposure in different environments.
| Environment | Sampling | Exposure level (range), EU/m3 | Reference |
|---|---|---|---|
| Occupational setting | |||
| Pig houses | Air, personal sampling, total dust | 0.08–16720 | 5 |
| Poultry houses | Air, personal sampling, total dust | 152–13080 | 5 |
| Greenhouses | Air, personal sampling, total dust | 0.4–101 | 5 |
| Cotton workers | Area samples | 7–16970 | 64 |
| Domestic environments | |||
| Farmers | Mattress dust | 14/89 EU/mg* | 9 |
| Non‐farmers | Mattress dust | 8/63 EU/mg* | 9 |
*5th/95th centile.
Exposure assessment
Endotoxins are usually measured in samples of airborne or settled dust. One of the problems with the exposure assessment is a lack of standardisation of exposure assessment methods and environmental sampling strategies.14 The kinetic limulus amoebocyte lysate (LAL) method, an in vitro biological method, has been widely used for endotoxin measurements since the 1980s.15 The test was standardised by the US Food and Drug Administration and is usually used as a marker of Escherichia coli contamination in, for example, water, food, or infusions.16
The LAL test measures the biological activity of free cell wall dissociated LPS. The lysate used for the test is prepared from the horseshoe crab (Limulus polyphemus).17 Therefore, different test batches might give different results, making the use of an internal standard endotoxin necessary. The results of the test should be expressed as endotoxin units (EU) as the reactivity and molecular weight of different species or strains of endotoxin may vary largely in the hydrophilic and lipid A moiety.15
Due to the different protocols used, large variations in the analyses of endotoxins might also occur between laboratories. Therefore, only intra‐laboratory results should be directly compared.15 The lack of consistency between laboratories makes it difficult to propose exposure threshold values for endotoxins.14 The new European standard for endotoxin measurement at the workplace might help to overcome this problem.18
Known effects of endotoxins at the cellular level
Endotoxins are known to have strong immune stimulatory and proinflammatory properties, even in very small amounts.19 After inhalation, endotoxins enter the airways where they encounter alveolar macrophages carrying CD14, an LPS binding receptor. The binding of LPS to CD14 is mediated by the LPS binding protein (LBP). Via toll‐like receptors (TLR‐2 and TLR‐4) the alveolar macrophages are activated, leading to the production and release of proinflammatory cytokines, chemokines, adhesion molecules, and other mediators.2,20 Cytokines associated with endotoxin exposure are TNF‐α, interleukin (IL) 1‐β, IL‐6, and IL‐8, as well as metabolites of arachidonic acid.2 These cytokines recruit and activate neutrophils, resulting in local and systemic inflammation with leucocytosis and neutrophilia.2,20
Genetic variations, especially in the polymorphism of the TLR‐2 gene, are thought to be responsible for variations in the individual susceptibility to effects of endotoxins.21,22
Endotoxins and respiratory health; results from occupational epidemiology
Endotoxins can be found at higher concentrations in all occupational environments with exposure to organic dust (for example, farming, cotton production, waste processing). Of these, farmers are the professional group that has been most extensively studied throughout the world. The results of these studies may well serve as a model for the association between endotoxin exposure and airway inflammation.
As early as 1555, Olaus Magnus indicated that the respiratory tract of farmers is at increased risk for occupational diseases.23 This has been confirmed in a large number of studies (for reviews, see Schenker15 and Radon and Nowak24). Respiratory diseases among farmers can be compensated as occupational disorders in many industrialised countries, and the number of claims for compensation is high in these countries. In this context, the most important diseases affecting the airways of farmers are:
Extrinsic allergic alveolitis or farmer's lung
Organic dust toxic syndrome
Chronic bronchitis
Respiratory allergies, asthma, and asthma‐like syndrome.
Of these, organic dust toxic syndrome, chronic bronchitis, and asthma have been considered to be associated with endotoxin exposure at the workplace.
Figure 1 Structure of lipopolysaccharides (LPS).
Organic dust toxic syndrome
Organic dust toxic syndrome (ODTS) is a systemic inflammatory reaction with flu‐like symptoms. These include fever, myalgias, chest tightness, chills, and dyspnoea 4–8 hours after exposure.4,24 The symptoms resemble the acute form of extrinsic allergic alveolitis, a type III and type IV sensitisation that is much less common than ODTS with a longer duration.25 In the airways, obstruction might occur together with increased airway hyperreactivity and a reduced alveolar diffusion capacity.4 The affected worker usually recovers within 36 hours without need for treatment. Often the disease is characterised by a clustering of cases in one occupational setting after, for example, cleaning of animal houses.
Endotoxins are considered to be of uppermost importance in the development of ODTS.6 However, if repeatedly exposed, some degree of tolerance seems to develop. The mechanism leading to this tolerance is still poorly understood, but one study indicated that it is associated with interference with transcription factor pathways and a reduced gene expression.26
Epidemiological studies have shown that ODTS is very common in farmers. In the European Farmers' Study, a cross‐sectional study including 7000 farmers across Europe, the lifetime prevalence ranged from 15% in crop producers27 to 23% in pig farmers.28 In one study from Sweden the annual incidence was 1%.29
In the past, ODTS has been considered a self‐limiting, harmless syndrome.25 However, newer studies indicate that ODTS is associated with an increased risk of chronic bronchitis in farmers.30,31,32 Therefore, the acute neutrophilic inflammation of the lung seems to cause a chronic neutrophilic inflammation associated with chronic symptoms in repeatedly exposed workers.
Chronic bronchitis
There is a large body of literature showing that exposure to farming environments increases the risk of chronic bronchitis. The prevalence among farmers ranges from 5%33 to 39%.34 In the European Farmers' Study, farmers in the age group 20–44 years were already at increased risk for chronic bronchitis.28 In a recent meta‐analysis, the summary odds ratio for chronic bronchitis among animal farmers compared to unexposed reference populations was 2.0 (95% CI 1.7 to 2.4).35
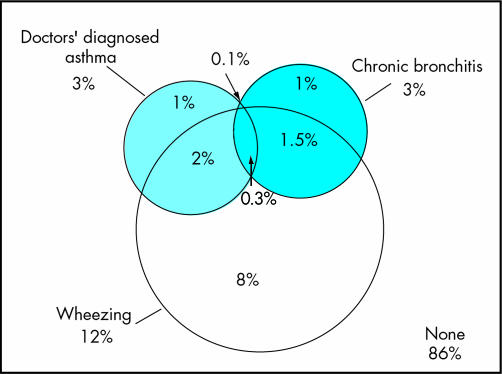
The endotoxin and β‐glucan concentrations in organic dust are thought to be major risk factors for the chronic neutrophilic inflammation of the airways among farmers.6 However, several studies have indicated that among farmers the symptoms of chronic bronchitis do not correlate with an airway obstruction.15 Beside the fact that many of the epidemiological studies were done on a cross‐sectional base, potentially resulting in a healthy worker survivor effect,36,37 it is difficult to clearly distinguish between the different chronic respiratory diseases in questionnaire based studies. As shown in fig 2, wheezing might indicate early symptoms of chronic bronchitis as well as asthma. In addition, without allergic sensitisation, physicians might fail to diagnose asthma in a patient with asthma‐like symptoms.11 This problem is discussed in the next section.
Figure 2 Venn diagram of doctors' diagnosed asthma, wheezing without a cold, and chronic bronchitis among 18–44 year old adults living in rural areas of Lower Saxony, Germany (n = 6820).
Allergic sensitisation, asthma, and asthma‐like syndrome
In the past, cattle farming was shown to be a risk factor for allergic sensitisation against cattle epithelium.15 At the same time, sensitisation against storage mites and moulds has been shown, especially among farmers (see Schenker15 for a review). In the cotton industry, asthma symptoms mainly occurring after days off work (“Monday asthma” or byssinosis) were associated with endotoxin exposure.38 In a prospective cohort study in the Chinese cotton industry, the cumulative incidence over 15 years was 24% in cotton workers compared to 0% in silk workers.38 At the same time, it is well known that endotoxins can exacerbate airflow obstruction and inflammation in patients with atopic asthma. Likewise, subjects with atopic asthma have an enhanced respiratory response to endotoxin.39
During recent years more studies have included unexposed reference groups. In this context, the evidence for the association between farming environments and asthma and allergies became conflicting. For example, the European Respiratory Health Survey, a general population survey among adults throughout Europe, indicated an increased odds ratio of 2.6 (95% CI 1.3 to 5.4) for asthma among farmers.40 In contrast, in the context of the European Farmers' Study, allergic asthma and respiratory allergies were only more frequent among crop farmers, especially among farmers involved in flower production, with a prevalence of 5%.27 In this study, the prevalence of asthma was 1% among animal farmers aged 20–44 years compared to 3% in the general population.28 Of 100 pig farmers with work related respiratory symptoms, 16% were sensitised to common allergens compared to 18% in the general population, even though the exposure to, for example, house dust mites, was 50‐fold increased.41
Atopic and non‐atopic asthma
One potential explanation for these contradictory results is that not all asthma is associated with allergic sensitisation. For farmers with symptoms of asthma but without allergic sensitisation, the term asthma‐like syndrome was coined.15 Symptoms associated with asthma‐like syndrome are chest tightness, wheeze, or dyspnoea, and a cross‐shift decline in FEV1 of usually less than 10%. In contrast to atopic asthma the syndrome is associated with a neutrophilic inflammation of the airways.15 As described by Schenker 1998,15 several studies have indicated that endotoxins might be the agent responsible, causing the airway inflammation responsible for the asthma‐like syndrome.
Overall, asthma‐like syndrome mainly describes what now is known as non‐atopic asthma.42 It has been estimated that about 50% of all asthma cases are attributable to this non‐atopic asthma based on neutrophilic airway inflammation; in the occupational environment it might even be more.42 This is supported by our recent finding that only about half of young adults living in a rural area of Lower Saxony, Germany, who reported wheezing were also sensitised against common allergens or reported symptoms of allergic rhinitis. Endotoxins are one of the relevant exposures associated with non‐atopic asthma.15,25,30,42,43
Eduard and colleagues44 have shown that animal farmers are at increased risk of non‐atopic asthma with increasing endotoxin concentration at the workplace, while allergic sensitisation and atopic asthma was inversely related to endotoxin levels in airborne dust. A similar association has recently be shown by our group.11 Therefore, it seems to be important that the different phenotypes of asthma are taken into account when the prevalence of asthma in different populations is compared.
It has been argued that genetic differences might explain the reduced risk of atopic asthma and allergic sensitisation in animal farmers.45,46 However, while genetics might play some role in the observed differences (healthy worker effect), environmental studies as well as findings from in vivo and in vitro studies contradict this explanation.47
Range of exposure in different areas of agriculture
Beside the different phenotypes of asthma, the type of farming has to be taken into account when the effect of farming on respiratory health is under consideration. The term “farming” covers a large variety of tasks, techniques, and products. Type, duration, and level of exposure to allergens and irritants vary from, for example, grain production to greenhouse farming and from dairy farming to poultry production.5 Exposure also depends on climate.48 Therefore, the European Farmers' Study has shown large differences in the prevalence of atopic asthma and allergies between animal farmers and greenhouse farmers.27,28 These environments differ considerably in the amount of endotoxins, ranging from a median of 3 EU/m3 in greenhouses to 610 EU/m3 in swine confinement houses.5
Current evidence therefore suggests that endotoxins in the occupational environment are associated with a decreased risk of atopic diseases. In contrast, the risk for non‐atopic asthma increases with increasing endotoxin exposure. These hypotheses are supported by data from the general environment.
Endotoxins and respiratory health; results from environmental epidemiology
Several epidemiological studies have been conducted on an international base in order to determine the prevalence and risk factors for asthma and allergies across the world.49 These studies have indicated that the prevalence of respiratory allergies and asthma is considerably higher in high income countries compared to developing countries. In addition, while the prevalence of respiratory allergies and asthma was significantly lower in Eastern Germany compared to the Western part of the country at the time of the German reunification, these differences have been levelling off within less than 10 years due to a rise of atopic diseases in Eastern Germany. One explanation for the described differences is covered by the “hygiene hypothesis”, stating that contact to microbial components can protect from the development of hay fever and possibly other allergic diseases.50 This has been supported by several factors shown to be associated with a decreased risk of respiratory allergies in epidemiological studies:47
Increasing number of older siblings
Day care attendance during the first years of life
Gastrointestinal microbes
Early exposure to farms.
The latter has been confirmed by a large number of studies from several countries across the world, showing a protective effect of being raised on a farm (for reviews, see Braun‐Fahrlander47 and Kabesch and Lauener51) and exposure to animal confinement houses during childhood on the prevalence of asthma and nasal allergies. As in the occupational studies discussed above, the protective effect of early contact to farms seems to be confined to those with contact to livestock farming as opposed to children with contact to farms with mainly crop production.52
Although it is assumed that it is the early‐life farm contact that confers a protective effect against allergic diseases, new data show that regular farm animal contact starting later in life is also associated with a decreased prevalence of sensitisation among adults.53 Two small longitudinal studies among young adults54 and schoolchildren55 suggest that farm animal contact starting at a later age not only reduces the incidence, but is also associated with a more frequent loss of sensitisation. The study among 42 Austrian agricultural students54 showed a remission of allergic disease among those with new contact to farm animals; the study, however, was hampered by a high loss to follow up. The other study55 was a three year follow up of 844 Austrian schoolchildren (mean age at baseline 8 years). Farm children (15%) lost sensitisation significantly more often than non‐farm children (odds ratio 8, 95% CI 2 to 32). To our knowledge, no other study of the influence of late starting farm contacts is available.
Possible causal agents explaining the protective effect of farm contact were sought in the exposure to endotoxin, bacterial DNA, muramic acid, pathogens like Toxoplasma gondii and Helicobacter pylori, and possibly mould components.56 The reaction of the immune system on stimulation of Toll‐like receptors (TLRs) may determine the protective effect against allergic disease seen with the contact to microbial components.57 Thus, endotoxins seem to be one of the important exposures occurring in the animal farming environments that might protect from respiratory allergies. Therefore, the distribution of endotoxins in the home environment of children living in rural areas has been studied. A greater exposure to endotoxin in the mattresses of schoolchildren in Switzerland, Austria, and Germany was associated with a lower risk of asthma and allergies.58 In the context of this study it was shown for farmers' and non‐farmers' children that the indoor home endotoxin levels were associated with the child's activity on a farm. Additionally, pet keeping was a predictor of the endotoxin level at home.9 This association is consistent with findings from other studies (see Liu59 for a review). The level of indoor endotoxin exposure was inversely related to sensitisation or respiratory allergies in some of these studies, but not for all subpopulations.
Overall, the prevalence of allergic symptoms and symptoms of chronic bronchitis depend on the dose of endotoxins and allergens in the environment (table 2). Higher concentrations of endotoxins in the occupational environment lead to an increased risk of chronic bronchitis and non‐atopic asthma. This association was recently confirmed for subjects living in areas with environmental exposure to endotoxins due to intensive livestock production.11 In contrast, increased endotoxin concentrations in the occupational and general environment might protect from sensitisation to allergens.
Table 2 Prevalence of respiratory diseases depends on concentration of endotoxins and allergens in the occupational and home environment.
| Level of exposure to | Allergens | Low | Low | High | High |
|---|---|---|---|---|---|
| Level of exposure to | Endotoxins | Low | High | Low | High |
| Prevalence of | Allergic rhinitis/atopic asthma | Low | Low | High | Low |
| Prevalence of | Asthma‐like syndrome/chronic bronchitis | Low | High | Low | High |
There is still some controversy as to whether endotoxin exposure is actually the cause for the inverse association between farm contact and the lower rate of respiratory allergies.60 However, as the inverse association between endotoxin exposure and respiratory allergies is also consistently seen among non‐farmers, most other factors do not seem to be crucial (for example, genetics, level of air pollution, nutrition).60
Potential mechanisms
The classical hygiene hypothesis assumes that the increase in Th1 cell production associated with infections skews the immune system away from Th2 cell production. Therefore, the prevalence of Th2 dominated diseases like atopic diseases might be decreased in subjects exposed to infectious agents.50
The observation that parasites associated with a Th2 dominated immune response like, for example, hookworms, also resulted in a reduced prevalence of atopic diseases contradicted the classical hygiene hypothesis.50,61 Therefore, it has recently been hypothesised that the immune response might be modified by IL‐10 produced by Th3 cells. With respect to cat allergen exposure it has been suggested that a combination of IL‐10 with IL‐4 may skew the immune response in favour of IgG4 production instead of IgE production.62 The result is that a non‐pathogenic Th2 immune response might be induced.63
The underlying mechanisms of this bidirectional response have been shown in animal studies and in vitro experiments. The results of these studies have been excellently reviewed by Renz and Herz.64
Nevertheless, it is still unknown whether endotoxins are the causal component for the protection from respiratory allergies. Cohort and intervention studies are on the way to giving further evidence for the prevention of atopic diseases in the long run. Currently it has been recommended that bacterial extracts should not yet been used clinically.65 Our current knowledge on the adverse health effects of endotoxins with respect to chronic bronchitis and non‐atopic asthma has to be used for effective prevention strategies at workplaces with high exposures to organic dust.
Acknowledgements
I am grateful to Rudolf Schierl and Anja Schulze for critical review of the paper.
QUESTIONS (SEE ANSWERS ON P 16)
-
Endotoxins are:
Part of the outer membrane of Gram negative bacteria
Part of the inner membrane of Gram negative bacteria
Part of the outer membrane of Gram positive bacteria
-
One of the occupations mostly affected by endotoxin exposure is:
Bakers
Greenhouse workers
Hairdressers
Animal farmers
-
Exposure to endotoxins is highest in mattresses of:
Urban citizens
Rural citizens with regular contact to animal farms
Rural citizens without contact to animal farms
-
Exposure to endotoxins decreases the risk of:
Chronic bronchitis
Asthma
Atopy
Extrinsic allergic alveolitis (farmer's lung)
Organic dust toxic syndrome (ODTS)
-
Organic dust toxic syndrome (ODTS):
Is characterised by clustering of cases
Has long term effects
Is a type IV sensitisation
Is associated with restrictive lung function changes
Is associated with Th2 cell production
Footnotes
Competing interests: none declared
References
- 1.Bos M P, Tommassen J. Biogenesis of the Gram‐negative bacterial outer membrane. Curr Opin Microbiol 20047610–616. [DOI] [PubMed] [Google Scholar]
- 2.Michel O. Systemic and local airways inflammatory response to endotoxin. Toxicology 200015225–30.This paper gives a very concise overview of the effects of endotoxin. The main focus is on human challenge studies. [DOI] [PubMed] [Google Scholar]
- 3.Liu A H, Redmon A H., Jr Endotoxin: friend or foe? Allergy Asthma Proc 200122337–340. [PubMed] [Google Scholar]
- 4.Singh J, Schwartz D A. Endotoxin and the lung: insight into the host‐environment interaction. J Allergy Clin Immunol 2005115330–333.Most recent review on the pathophysiology and mechanisms of asthma and chronic obstructive lung disease, taking into account genetic aspects. The role of endotoxin in asthma and COPD is critically discussed and new areas of research are revealed. [DOI] [PubMed] [Google Scholar]
- 5.Radon K, Danuser B, Iversen M.et al Air contaminants in different European farming environments. Ann Agric Environ Med 2002941–48.This paper summarises results of exposure assessment in different European farming environments. [PubMed] [Google Scholar]
- 6.Von Essen S. The role of endotoxin in grain dust exposure and airway obstruction. Curr Opin Pulm Med 19973198–202. [DOI] [PubMed] [Google Scholar]
- 7.Lane S R, Nicholls P J, Sewell R D. The measurement and health impact of endotoxin contamination in organic dusts from multiple sources: focus on the cotton industry. Inhal Toxicol 200416217–229. [DOI] [PubMed] [Google Scholar]
- 8.Douwes J, Thorne P, Pearce N.et al Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg 200347187–200. [DOI] [PubMed] [Google Scholar]
- 9.Waser M, Schierl R, von Mutius E.et al Determinants of endotoxin levels in living environments of farmers' children and their peers from rural areas. Clin Exp Allergy 200434389–397.This publication describes the factors associated with indoor exposure to endotoxins in farming and non‐farming environments. [DOI] [PubMed] [Google Scholar]
- 10.Michel O. Role of lipopolysaccharide (LPS) in asthma and other pulmonary conditions. J Endotoxin Res 20039293–300. [DOI] [PubMed] [Google Scholar]
- 11.Radon K, Schulze A, van Strien R.et alRespiratory health of young adults living in rural areas of Lower Saxony [final report]. Ludwig‐Maximilians‐University, 2004. http://aumento.web.med.uni‐muenchen.de/nils/index.html
- 12.Carty C L, Gehring U, Cyrys J.et al Seasonal variability of endotoxin in ambient fine particulate matter. J Environ Monit 20035953–958. [DOI] [PubMed] [Google Scholar]
- 13.Mueller‐Anneling L, Avol E, Peters J M.et al Ambient endotoxin concentrations in PM10 from Southern California. Environ Health Perspect 2004112583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorne P S, Duchaine C, Douwes J.et al Working Group report 4: exposure assessment for biological agents. Am J Ind Med 200446419–422. [DOI] [PubMed] [Google Scholar]
- 15.Schenker M. Respiratory health hazards in agriculture. Am J Respir Crit Care Med 1998158S1–76.Very detailed review paper published by the American Thoracic Society on respiratory health effects of farming. [DOI] [PubMed] [Google Scholar]
- 16.Poole F.Class II special controls guidance document: endotoxin assay. Rockville, MD: US Department of Health and Human Services Food and Drug Administration, 2003
- 17.Williams K L, Roberts K, Nnalue N A.et alEndotoxins: pyrogens, Lal testing, and depyrogenation, 2nd edn. New York: Marcel Dekker, 2001
- 18. DIN EN 14031. Workplace atmospheres—determination of airborne endotoxins. 2003
- 19.Douwes J, Pearce N, Heederik D. Does environmental endotoxin exposure prevent asthma? Thorax 20025786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed C E, Milton D K. Endotoxin‐stimulated innate immunity: a contributing factor for asthma. J Allergy Clin Immunol 2001108157–166. [DOI] [PubMed] [Google Scholar]
- 21.Vercelli D. Genetics, epigenetics, and the environment: switching, buffering, releasing. J Allergy Clin Immunol 2004113381–386.In this very interesting review, endotoxin exposure and asthma serves as a model to explain the complex issues of genetics and epigenetics. [DOI] [PubMed] [Google Scholar]
- 22.Eder W, Klimecki W, Yu L.et al Toll‐like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol 2004113482–488. [DOI] [PubMed] [Google Scholar]
- 23.Ramazzini B.De morbis artificium diatriba. Chicago, IL: The University of Chicago Press, 1940
- 24.Radon K, Nowak D. Farming. In: Hendrick DJ, Burge PS, Beckett WS, Churg A, eds. Occupational disorders of the lung: recognition, management, and prevention. London: WB Saunders, 2002427–437.
- 25.Linaker C, Smedley J. Respiratory illness in agricultural workers. Occup Med 20028451–459. [DOI] [PubMed] [Google Scholar]
- 26.Medvedev A E, Kopydlowski K M, Vogel S N. Inhibition of lipopolysaccharide‐induced signal transduction in endotoxin‐tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll‐like receptors 2 and 4 gene expression. J Immunol 20001645564–5574. [DOI] [PubMed] [Google Scholar]
- 27.Monso E, Magarolas R, Radon K.et al Respiratory symptoms of obstructive lung disease in European crop farmers. Am J Respir Crit Care Med 2000162(4 pt 1)1246–1250.This paper is one of the few describing respiratory health effects of crop farming. [DOI] [PubMed] [Google Scholar]
- 28.Radon K, Danuser B, Iversen M.et al Respiratory symptoms in European animal farmers. Eur Respir J 200117747–754.Paper presenting the results of a large scale study on respiratory health effects of animal farming throughout Europe. The prevalence of respiratory diseases among farmers is compared to the general population. [DOI] [PubMed] [Google Scholar]
- 29.Malmberg P, Rask‐Andersen A, Hoglund S.et al Incidence of organic dust toxic syndrome and allergic alveolitis in Swedish farmers. Int Arch Allergy Appl Immunol 19888747–54. [DOI] [PubMed] [Google Scholar]
- 30.Radon K, Monso E, Weber C.et al Prevalence and risk factors of airway diseases in farmers—summary of results of the European farmers' project. Ann Agric Environ Med 20029207–213. [PubMed] [Google Scholar]
- 31.Monso E, Schenker M, Radon K.et al Region‐related risk factors for respiratory symptoms in European and Californian farmers. Eur Respir J 200321323–331. [DOI] [PubMed] [Google Scholar]
- 32.Von Essen S, Fryzek J, Nowakowski B.et al Respiratory symptoms and farming practices in farmers associated with an acute febrile illness after organic dust exposure. Chest 19991161452–1458. [DOI] [PubMed] [Google Scholar]
- 33.Brouwer R, Biersteker K, Bongers P.et al Respiratory symptoms, lung function, and IgG4 levels against pig antigens in a sample of Dutch pig farmers. Am J Ind Med 198610283–285. [DOI] [PubMed] [Google Scholar]
- 34.Morris P D, Lenhart S W, Service W S. Respiratory symptoms and pulmonary function in chicken catchers in poultry confinement units. Am J Ind Med 199119195–204. [DOI] [PubMed] [Google Scholar]
- 35.Goy S. Chronic bronchitis in farmers—a meta‐analysis. Medical doctorate, Ludwig‐Maximilians‐University, in preparation
- 36.Thelin A, Hoglund S. Change of occupation and retirement among Swedish farmers and farm workers in relation to those in other occupations. A study of “elimination” from farming during the period 1970–1988. Soc Sci Med 199438147–151. [DOI] [PubMed] [Google Scholar]
- 37.Radon K, Goldberg M, Becklake M. Healthy worker effect in cohort studies on chronic bronchitis. Scand J Work Environ Health 200228328–332. [DOI] [PubMed] [Google Scholar]
- 38.Wang X R, Eisen E A, Zhang H X.et al Respiratory symptoms and cotton dust exposure; results of a 15 year follow up observation. Occup Environ Med 200360935–941.Carefully conducted cohort study in the Chinese cotton industry, nicely illustrating the adverse effects of endotoxins on the respiratory tract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz D A. Does inhalation of endotoxin cause asthma? Am J Respir Crit Care Med 2001163305–306. [DOI] [PubMed] [Google Scholar]
- 40.Kogevinas M, Anto J M, Sunyer J.et al Occupational asthma in Europe and other industrialised areas: a population‐based study. European Community Respiratory Health Survey Study Group. Lancet 19993531750–1754. [DOI] [PubMed] [Google Scholar]
- 41.Radon K, Schottky A, Garz S.et al Distribution of dust‐mite allergens (Lep d 2, Der p 1, Der f 1, Der 2) in pig‐farming environments and sensitization of the respective farmers. Allergy 200055219–225.This paper illustrates the low rate of sensitisation among farmers irrespective of a very high exposure to workplace and ubiquitous allergens. [DOI] [PubMed] [Google Scholar]
- 42.Douwes J, Gibson P, Pekkanen J.et al Non‐eosinophilic asthma: importance and possible mechanisms. Thorax 200257643–648.This paper summarises the current knowledge about eosinophilic and non‐eosinophilic asthma. It is shown that at least 40% of adult asthma are not associated with eosinophilic mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Essen S. The role of farm exposures in occupational asthma and allergy. Opin Allergy Clin Immunol 20011151–156. [DOI] [PubMed] [Google Scholar]
- 44.Eduard W, Douwes J, Omenaas E.et al Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax 200459381–386.Large study on Norwegian farmers illustrating a lower prevalence of atopic asthma with increasing endotoxin exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Schayck C P, Knottnerus J A. Can the ‘hygiene hypothesis' be explained by confounding by behavior? J Clin Epidemiol 200457435–437. [DOI] [PubMed] [Google Scholar]
- 46.Vogelzang P F, van der Gulden J W, Tielen M J.et al Health‐based selection for asthma, but not for chronic bronchitis, in pig farmers: an evidence‐based hypothesis. Eur Respir J 199913187–189. [DOI] [PubMed] [Google Scholar]
- 47.Braun‐Fahrlander C. Environmental exposure to endotoxin and other microbial products and the decreased risk of childhood atopy: evaluating developments since April 2002. Curr Opin Allergy Clin Immunol 20033325–329.Recent summary on the epidemiological evidence for the effects of environmental endotoxin exposure on atopy in childhood. [DOI] [PubMed] [Google Scholar]
- 48.Omland O. Exposure and respiratory health in farming in temperate zones—a review of the literature. Ann Agric Environ Med 20029119–136. [PubMed] [Google Scholar]
- 49.Pearce N, Sunyer J, Cheng S.et al Comparison of asthma prevalence in the ISAAC and the ECRHS. ISAAC Steering Committee and the European Community Respiratory Health Survey. International Study of Asthma and Allergies in Childhood. Eur Respir J 200016420–426.Excellent presentation of two recent population based studies on the prevalence and risk factors of asthma and allergies around the world. [DOI] [PubMed] [Google Scholar]
- 50.Strachan D P. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 200055(suppl 1)S2–10.This review explains the idea of the hygiene hypothesis as well as the pros and cons of the hygiene hypothesis from a current point of view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabesch M, Lauener R P. Why Old McDonald had a farm but no allergies: genes, environments, and the hygiene hypothesis. J Leukoc Biol 200475383–387.Excellent review on the gene‐environment interactions of farming and allergies. [DOI] [PubMed] [Google Scholar]
- 52.Downs S H, Marks G B, Mitakakis T Z.et al Having lived on a farm and protection against allergic diseases in Australia. Clin Exp Allergy 200131570–575. [DOI] [PubMed] [Google Scholar]
- 53.Radon K, Ehrenstein V, Praml G.et al Childhood visits to animal buildings and atopic diseases in adulthood: an age‐dependent relationship. Am J Ind Med 200446349–356. [DOI] [PubMed] [Google Scholar]
- 54.Prior C, Falk M, Frank A. Longitudinal changes of sensitization to farming‐related antigens among young farmers. Respiration 20016846–50. [DOI] [PubMed] [Google Scholar]
- 55.Horak F, Jr, Studnicka M, Gartner C.et al Parental farming protects children against atopy: longitudinal evidence involving skin prick tests. Clin Exp Allergy 2002321155–1159. [DOI] [PubMed] [Google Scholar]
- 56.Eder W, von Mutius E. Hygiene hypothesis and endotoxin: what is the evidence? Curr Opin Allergy Clin Immunol 20044113–117. [DOI] [PubMed] [Google Scholar]
- 57.Lauener R P, Birchler T, Adamski J.et al Expression of CD14 and Toll‐like receptor 2 in farmers' and non‐farmers' children. Lancet 2002360465–466. [DOI] [PubMed] [Google Scholar]
- 58.Braun‐Fahrlander C, Riedler J, Herz U.et al Environmental exposure to endotoxin and its relation to asthma in school‐age children. N Engl J Med 2002347869–877.Results of a study on endotoxin exposure and atopic diseases in children living in rural areas of South Germany, Austria and Switzerland. This study includes careful exposure assessment as well as objective makers of disease. [DOI] [PubMed] [Google Scholar]
- 59.Liu A H. Endotoxin exposure in allergy and asthma: reconciling a paradox. J Allergy Clin Immunol 2002109379–392. [DOI] [PubMed] [Google Scholar]
- 60.Wjst M. Another explanation for the low allergy rate in the rural Alpine foothills. Clin Mol Allergy 200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radon K, Dressel H, Windstetter D.et al Toxoplasma gondii infection, atopy and autoimmune disease. Eur J Med Res 20038147–153. [PubMed] [Google Scholar]
- 62.Platts‐Mills T A, Vaughan J W, Blumenthal K.et al Serum IgG and IgG4 antibodies to Fel d 1 among children exposed to 20 µg Fel d 1 at home: relevance of a nonallergic modified Th2 response. Int Arch Allergy Immunol 2001124126–129.Interesting study on the U‐shaped association between cat allergen exposure in early life and allergic sensitisation in children. This study gives some insights into the hygiene hypothesis with respect to the Th1 and Th2 response. [DOI] [PubMed] [Google Scholar]
- 63.Liu A H, Murphy J R. Hygiene hypothesis: fact or fiction? J Allergy Clin Immunol 2003111471–478.Critical review of the hygiene hypothesis. In this paper the hypothesis is put into context with respect to autoimmune disease. In addition, implications for prevention studies are carefully discussed. [DOI] [PubMed] [Google Scholar]
- 64.Renz H, Herz U. The bidirectional capacity of bacterial antigens to modulate allergy and asthma. Eur Respir J 200219158–171.This is a most useful paper summarising the results of in‐vivo and in‐vitro studies on endotoxin exposure and allergies. [DOI] [PubMed] [Google Scholar]
- 65.Matricardi P M, Björksten B, Bonini S.et al Microbial products in allergy prevention and therapy. Allergy 200358461–471. [DOI] [PubMed] [Google Scholar]