Abstract
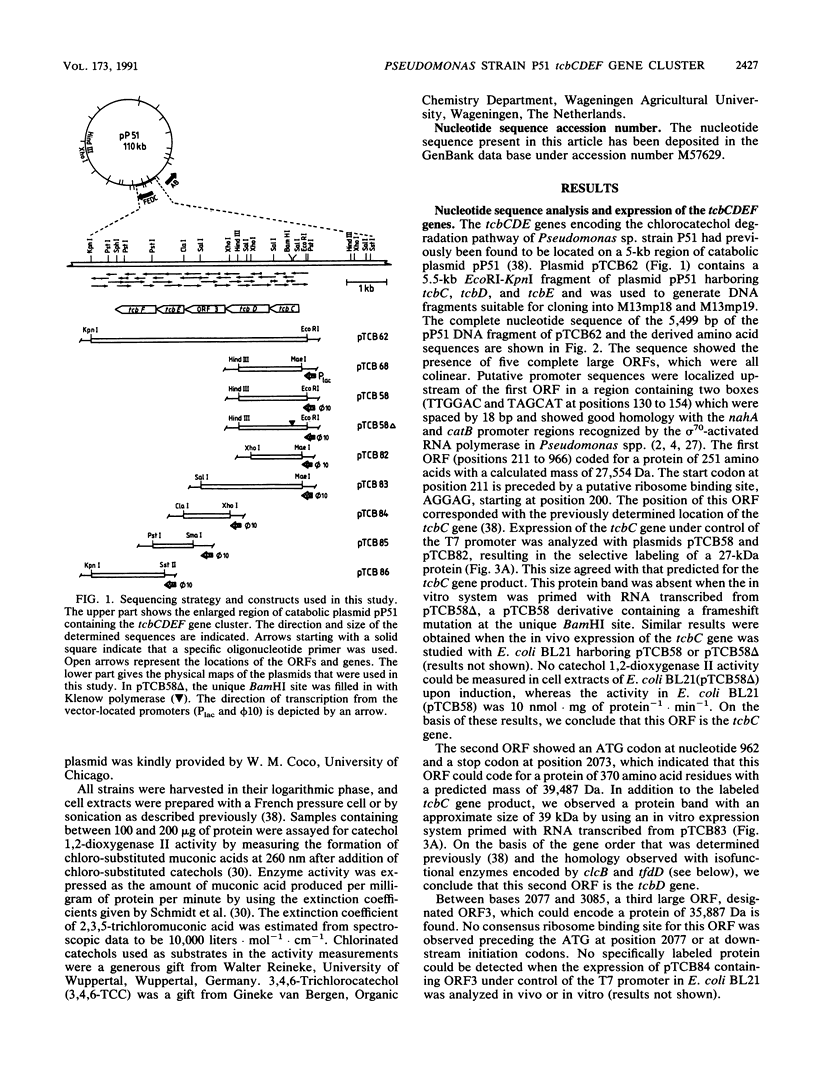
Pseudomonas sp. strain P51 contains two gene clusters located on catabolic plasmid pP51 that encode the degradation of chlorinated benzenes. The nucleotide sequence of a 5,499-bp region containing the chlorocatechol-oxidative gene cluster tcbCDEF was determined. The sequence contained five large open reading frames, which were all colinear. The functionality of these open reading frames was studied with various Escherichia coli expression systems and by analysis of enzyme activities. The first gene, tcbC, encodes a 27.5-kDa protein with chlorocatechol 1,2-dioxygenase activity. The tcbC gene is followed by tcbD, which encodes cycloisomerase II (39.5 kDa); a large open reading frame (ORF3) with an unknown function; tcbE, which encodes hydrolase II (25.8 kDa); and tcbF, which encodes a putative trans-dienelactone isomerase (37.5 kDa). The tcbCDEF gene cluster showed strong DNA homology (between 57.6 and 72.1% identity) and an organization similar to that of other known plasmid-encoded operons for chlorocatechol metabolism, e.g., clcABD of Pseudomonas putida and tfdCDEF of Alcaligenes eutrophus JMP134. The identity between amino acid sequences of functionally related enzymes of the three operons varied between 50.6 and 75.7%, with the tcbCDEF and tfdCDEF pair being the least similar of the three. Measurements of the specific activities of chlorocatechol 1,2-dioxygenases encoded by tcbC, clcA, and tfdC suggested that a specialization among type II enzymes has taken place. TcbC preferentially converts 3,4-dichlorocatechol relative to other chlorinated catechols, whereas TfdC has a higher activity toward 3,5-dichlorocatechol. ClcA takes an intermediate position, with the highest activity level for 3-chlorocatechol and the second-highest level for 3,5-dichlorocatechol.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich T. L., Frantz B., Gill J. F., Kilbane J. J., Chakrabarty A. M. Cloning and complete nucleotide sequence determination of the catB gene encoding cis,cis-muconate lactonizing enzyme. Gene. 1987;52(2-3):185–195. doi: 10.1016/0378-1119(87)90045-x. [DOI] [PubMed] [Google Scholar]
- Aldrich T. L., Rothmel R. K., Chakrabarty A. M. Identification of nucleotides critical for activity of the Pseudomonas putida catBC promoter. Mol Gen Genet. 1989 Aug;218(2):266–271. doi: 10.1007/BF00331277. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Kellogg S. T., Hamada S., Chakrabarty A. M. Plasmid specifying total degradation of 3-chlorobenzoate by a modified ortho pathway. J Bacteriol. 1981 May;146(2):639–646. doi: 10.1128/jb.146.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., Chakrabarty A. M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Ghosal D., You I. S. Nucleotide homology and organization of chlorocatechol oxidation genes of plasmids pJP4 and pAC27. Mol Gen Genet. 1988 Jan;211(1):113–120. doi: 10.1007/BF00338401. [DOI] [PubMed] [Google Scholar]
- Ghosal D., You I. S. Operon structure and nucleotide homology of the chlorocatechol oxidation genes of plasmids pJP4 and pAC27. Gene. 1989 Nov 30;83(2):225–232. doi: 10.1016/0378-1119(89)90108-x. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Nishino S. F., Spain J. C. Degradation of 1,2-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):294–301. doi: 10.1128/aem.54.2.294-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett C., Neidle E. L., Ngai K. L., Ornston L. N. DNA sequences of genes encoding Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of genes and of DNA sequences within genes during their evolutionary divergence. J Bacteriol. 1990 Feb;172(2):956–966. doi: 10.1128/jb.172.2.956-966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhm A. E., Schlömann M., Knackmuss H. J., Pieper D. H. Purification and characterization of dichloromuconate cycloisomerase from Alcaligenes eutrophus JMP 134. Biochem J. 1990 Mar 15;266(3):877–883. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakai C., Horiike K., Kuramitsu S., Kagamiyama H., Nozaki M. Three isozymes of catechol 1,2-dioxygenase (pyrocatechase), alpha alpha, alpha beta, and beta beta, from Pseudomonas arvilla C-1. J Biol Chem. 1990 Jan 15;265(2):660–665. [PubMed] [Google Scholar]
- Neidle E. L., Hartnett C., Bonitz S., Ornston L. N. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol. 1988 Oct;170(10):4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai K. L., Ornston L. N. Abundant expression of Pseudomonas genes for chlorocatechol metabolism. J Bacteriol. 1988 May;170(5):2412–2413. doi: 10.1128/jb.170.5.2412-2413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendorf D. H., Lipscomb J. D., Weber P. C. Structure and assembly of protocatechuate 3,4-dioxygenase. Nature. 1988 Nov 24;336(6197):403–405. doi: 10.1038/336403a0. [DOI] [PubMed] [Google Scholar]
- Perkins E. J., Gordon M. P., Caceres O., Lurquin P. F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990 May;172(5):2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Timmis K. N. Experimental evolution of catabolic pathways of bacteria. Microbiol Sci. 1987 Aug;4(8):228–237. [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A. Homology between nucleotide sequences of promoter regions of nah and sal operons of NAH7 plasmid of Pseudomonas putida. Proc Natl Acad Sci U S A. 1986 Jan;83(2):369–373. doi: 10.1073/pnas.83.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlömann M., Schmidt E., Knackmuss H. J. Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J Bacteriol. 1990 Sep;172(9):5112–5118. doi: 10.1128/jb.172.9.5112-5118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Remberg G., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Halogenated muconic acids as intermediates. Biochem J. 1980 Oct 15;192(1):331–337. doi: 10.1042/bj1920331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraa G., Boone M. L., Jetten M. S., van Neerven A. R., Colberg P. J., Zehnder A. J. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Appl Environ Microbiol. 1986 Dec;52(6):1374–1381. doi: 10.1128/aem.52.6.1374-1381.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Nishino S. F. Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1987 May;53(5):1010–1019. doi: 10.1128/aem.53.5.1010-1019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True A. E., Orville A. M., Pearce L. L., Lipscomb J. D., Que L., Jr An EXAFS study of the interaction of substrate with the ferric active site of protocatechuate 3,4-dioxygenase. Biochemistry. 1990 Dec 4;29(48):10847–10854. doi: 10.1021/bi00500a019. [DOI] [PubMed] [Google Scholar]
- Verver J., Goldbach R., Garcia J. A., Vos P. In vitro expression of a full-length DNA copy of cowpea mosaic virus B RNA: identification of the B RNA encoded 24-kd protein as a viral protease. EMBO J. 1987 Mar;6(3):549–554. doi: 10.1002/j.1460-2075.1987.tb04789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar M. P., Franklin F. C., Reineke W. Molecular cloning and expression of the 3-chlorobenzoate-degrading genes from Pseudomonas sp. strain B13. J Bacteriol. 1987 Jan;169(1):394–402. doi: 10.1128/jb.169.1.394-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van der Meer J. R., van Neerven A. R., de Vries E. J., de Vos W. M., Zehnder A. J. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991 Jan;173(1):6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]




