Abstract
Objectives
Ambient particulate air pollution has been associated with increased risk of cardiovascular morbidity and mortality. Pathways by which particles may act involve autonomic nervous system dysfunction or inflammation, which can affect cardiac rate and rhythm. The importance of these pathways may vary by particle component or source. In an eastern US location with significant regional pollution, the authors examined the association of air pollution and odds of cardiac arrhythmia in older adults.
Methods
Thirty two non‐smoking older adults were evaluated on a weekly basis for 24 weeks during the summer and autumn of 2000 with a standardised 30 minute protocol that included continuous electrocardiogram measurements. A central ambient monitoring station provided daily concentrations of fine particles (PM2.5, sulfate, elemental carbon) and gases. Sulfate was used as a marker of regional pollution. The authors used logistic mixed effects regression to examine the odds of having any supraventricular ectopy (SVE) or ventricular ectopy (VE) in association with increases in air pollution for moving average pollutant concentrations up to 10 days before the health assessment.
Results
Participant specific mean counts of arrhythmia over the protocol varied between 0.1–363 for SVE and 0–350 for VE. The authors observed odds ratios for having SVE over the length of the protocol of 1.42 (95% CI 0.99 to 2.04), 1.70 (95% CI 1.12 to 2.57), and 1.78 (95% CI 0.95 to 3.35) for 10.0 μg/m3, 4.2 μg/m3, and 14.9 ppb increases in five day moving average PM2.5, sulfate, and ozone concentrations respectively. The other pollutants, including elemental carbon, showed no effect on arrhythmia. Participants reporting cardiovascular conditions (for example, previous myocardial infarction or hypertension) were the most susceptible to pollution induced SVE. The authors found no association of pollution with VE.
Conclusion
Increased levels of ambient sulfate and ozone may increase the risk of supraventricular arrhythmia in the elderly.
Keywords: air pollution, PM2.5, epidemiology, cardiovascular disease, arrhythmia
Several recent studies provide evidence of the relation between ambient particle concentrations and cardiovascular morbidity1,2,3,4 and mortality.5,6,7 Autonomic nervous system dysfunction, as well as inflammation, may be pathways by which particles affect cardiac rate and rhythm.
Findings from recent panel studies suggest that particulate air pollution can affect the risk of ectopy,8,9,10,11 defined as extra cardiac depolarisations within either the atria (that is, supraventricular ectopy (SVE)) or the ventricles (that is, ventricular ectopy (VE)). For example, a study examining chronic obstructive pulmonary disease (COPD) patients in Vancouver found a 22% increased rate of SVE for each 5.8 μg/m3 increase in fine particles (PM2.5).11 Riediker and colleagues (2004) found 23.0% and 19.1% increased rates of SVE and VE, respectively, for 10 μg/m3 increases in PM2.5 in a study of North Carolina highway patrolmen.10 In contrast to these two studies, where the primary sources of air pollution were traffic, this paper reports the effects of particle pollution on the risk of ectopy in a population of older adults in the small town of Steubenville, OH. Steubenville is located in an industrial area of the Ohio River Valley, characterised by coal fired power plants and steel mills.
Methods
Study design and recruitment
We recruited 32 non‐smoking older adults to participate in a cardiovascular health and air pollution exposure study in Steubenville, OH. Most participants lived in one of three centrally located apartment buildings. Sampling was conducted over two 12 week sampling sessions during the summer (June 4–August 18) and fall (September 25–December 15) of 2000. Of the 32 participants, 28 participated in both seasons. Before the study, we held screening appointments to obtain information on the health status of each participant and baseline resting electrocardiograms (ECG) (12‐lead MAC6, Marquette Medical Systems Inc., Milwaukee, WI, USA). As heart rate variability (HRV) was an endpoint of interest, exclusion criteria for this study included having a pacemaker, a recent acute coronary syndrome, atrial flutter, or atrial fibrillation. The research protocol was approved by Institutional Review Boards of the Harvard School of Public Health and the Brigham and Women's Hospital in Boston, MA. Informed consent was obtained from all individuals prior to their participation in the study.
Health measurements
Participants visited one of two clinic rooms, set up in rooms of two main apartment buildings where participants resided, on a weekly basis following a regular schedule (Monday–Friday, 8am–4pm). During each visit, we administered a questionnaire regarding symptoms, doctor and hospital visits, changes in medication, and participant medication use that morning. Holter monitoring (SEER MC, GE Medical Systems, Milwaukee, WI, USA) with electrodes in a modified V5 and AVF position provided continuous ECG data throughout a 30 minute protocol, comprised of: (1) five minutes of rest in a supine position; (2) three supine blood pressure (BP) measurements (NIBP Vital Signs Monitor, Welch Allyn, Skaneateles Falls, NY, USA); (3) five minutes of standing with three standing BP measurements taken after two minutes; (4) five minutes of exercise (walking) outdoors (weather and health permitting); (5) five minutes of rest in a supine position; and (6) two minutes and 20 seconds of paced breathing.
Research technicians downloaded the ECG recordings onto a Sparq station in the field. Cardiology technicians at the Brigham & Women's Hospital analysed the ECG files using a MARS Marquette Workstation. For each clinic visit, we obtained data on heart rate and counts of SVE and VE, as compiled over the whole protocol and for each separate interval.
Exposure measurements
We measured 24 hour integrated fine particulate (PM2.5, sulfate (SO42−), elemental carbon (EC)) and gaseous (ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2)) pollutant concentrations at a central ambient site at the Franciscan University of Steubenville, located within one mile of participants' residences, beginning at 9am each day (except Saturday) using a Harvard multi‐pollutant monitor (HMPM). CONSOL Energy Inc Research & Development analysed the SO42−, O3, NO2, and SO2 filters using ion chromatography and EC filters using thermal optical transmission. A complete description of the HMPM and its performance in the current study has been described previously.12,13 Due to the high number of HMPM SO2 samples below the limit of detection,13 these data were not used in the current analysis.
The HMPM data were supplemented with continuous measurements of PM2.5 (R&P, TEOM 1400A (50°C)), SO2 (API, Model 100UV Fluorescent), carbon monoxide (CO) (API, Model 300 GFC), and meteorological variables (Met‐1, 10 meter station) collected at the same University of Steubenville site by CONSOL Energy Inc Research & Development as part of a broader monitoring effort in Steubenville.14,15 The PM2.5 data were used in sensitivity analyses only, to fill in missing Saturday values in the HMPM PM2.5 data series. The SO2 and CO data supplemented the pollutants measured with the HMPM sampler. Using the meteorological variables, we calculated apparent temperature as: −2.653+(0.994×Ta)+(0.0153×Td2), where Ta is the air temperature and Td is the dew point.
Data analysis
We excluded one participant who dropped out of the study after four weeks due to the limited number of samples, and another participant who had atrial fibrillation. Intervals or sessions with less than 50% valid ECG data were invalidated.
We used logistic regression to examine the odds of having at least one arrhythmia (that is, during the 30 minute protocol or for specific protocol intervals) associated with increases in air pollution. In these analyses, we excluded participants who experienced no change in their binomial (0/1) outcome, as they would have no variation in the outcome. Excluded from primary analyses were participants who experienced (a) ectopic beats during all clinic visits, or (b) no ectopic beats during any clinic visit. Sensitivity analyses were conducted that included all participants regardless of their binomial outcomes.
We additionally examined the relative rate of arrhythmia (for example, number of beats/30 minute protocol) associated with increases in air pollution by fitting overdispersed Poisson regression models, otherwise known as negative binomial regression, to the arrhythmia counts. These analyses included the entire cohort.
We conducted all analyses using PROC NLMIXED (SAS Release 8.02; SAS Institute, Cary, NC, USA), controlling for a random subject effect and the fixed effects of apparent temperature, season (summer v autumn), day of week, hour of day, age, and body mass index (BMI). We chose “current hour of the clinic visit”, a priori, as the most appropriate exposure window for apparent temperature since participants went outside during the ECG protocol. Apparent temperature was modeled as linear, quadratic, or as a linear spline with one knot. The final models included a linear term for apparent temperature, based on Akaike's Information Criterion comparisons. A sensitivity analysis, conducted by including a categorical “building of residence” variable, did not affect model results.
We created separate models to examine the relative effects of particles (PM2.5), particle components (SO42− and EC), and gases (O3, NO2, SO2, and CO) on cardiac arrhythmia. Based on autonomic nervous system dysfunction (which may be acute) and inflammation (which may take several days to develop) as potential mechanisms by which particles could influence cardiac arrhythmia, we examined the relative effects of one day to five day moving average concentrations of each pollutant before the health assessment. Moving averages up to 10 days before the health assessment were examined in sensitivity analyses. Effect estimates are presented for interquartile range (IQR) increases in pollutant concentrations at each exposure period evaluated.
Finally, we explored the potential for heterogeneity in response across the cohort by including participant characteristics, medications use (recorded at each clinic visit) and participant‐specific mean ectopy levels as potential effect modifiers of the PM2.5 associations. These factors were included as main effects and interaction terms with PM2.5 in the models.
Results
Participant characteristics and arrhythmia occurrence
Participants (mean age 71.2 years, range 53.5–90.3 years) were predominantly female (n = 28) and over the age of 60 (n = 27) (table 1). The majority of participants (n = 29) reported at least one of the listed cardiovascular or respiratory illnesses. Twenty three participants reported multiple diagnoses. Of the six participants that reported angina, for example, four also reported having at least one of the respiratory diseases. Similarly, of the nine participants that reported having asthma, six also reported having COPD. Participants with cardiovascular conditions (for example, previous myocardial infarction (MI), heart failure, and/or hypertension) reported taking cardiovascular medications more frequently than those without the condition. For example, of the 22 participants who reported ever having had hypertension, 18 (82%) were on beta‐blockers, Ca2+ channel blockers, and/or ACE inhibitors compared with only one (13%) of eight participants without hypertension. Similarly, of the 14 participants with respiratory illness (bronchitis, COPD, and/or asthma), 10 (71%) reported taking respiratory medications (bronchodilators, beta‐agonists, and/or steroids) compared with none of the 16 participants without respiratory illness.
Table 1 Participant characteristics, disease diagnoses, and medications use.
| Whole cohort | SVE models | VE models | ||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Obs | n (%) | Obs | n (%) | Obs | |||
| Overall | 30 (100) | 630 | 23 (100) | 482 | 18 (100) | 374 | ||
| Arrhythmia | SVE (beats/hr) | Mean (SD) | 50.0 (162.8) | 8.9 (47.1) | – | |||
| Min, Med, Max | 0, 1.9, 1389.3 | 0, 0, 642.9 | – | |||||
| VE (beats/hr) | Mean (SD) | 52.6 (173.5) | – | 7.4 (22.9) | ||||
| Min, Med, Max | 0, 0, 1225.6 | – | 0, 0, 239.9 | |||||
| General | Age <65 | 10 (33) | 202 | 9 (39) | 178 | 6 (33) | 116 | |
| 65–74 | 9 (30) | 196 | 6 (26) | 138 | 4 (22) | 92 | ||
| ⩾75 | 11 (37) | 232 | 8 (35) | 166 | 8 (44) | 166 | ||
| Male | 2 (7) | 33 | 1 (4) | 22 | 0 (0) | 0 | ||
| Black (v white) | 9 (30) | 184 | 7 (30) | 137 | 6 (33) | 121 | ||
| Ever smoker (v never) | 14 (47) | 289 | 9 (39) | 186 | 8 (44) | 167 | ||
| Diagnoses* | ||||||||
| Cardiovascular | Angina pectoris | 6 (20) | 116 | 5 (22) | 93 | 4 (22) | 73 | |
| Previous MI | 6 (20) | 118 | 5 (22) | 97 | 6 (33) | 118 | ||
| Heart failure | 5 (17) | 103 | 5 (22) | 103 | 3 (17) | 65 | ||
| Hypertension | 22 (73) | 464 | 15 (65) | 316 | 14 (78) | 294 | ||
| Diabetes | 7 (23) | 149 | 7 (30) | 149 | 6 (33) | 125 | ||
| Obesity | 20 (67) | 388 | 17 (74) | 342 | 11 (61) | 210 | ||
| Respiratory | Bronchitis | 9 (30) | 173 | 5 (22) | 96 | 6 (33) | 120 | |
| COPD | 9 (30) | 177 | 5 (22) | 97 | 6 (33) | 118 | ||
| Asthma | 9 (30) | 170 | 6 (26) | 114 | 5 (28) | 97 | ||
| Medications† | ||||||||
| Cardiovascular | Beta‐blockers | 12 (40) | 184 | 8 (35) | 108 | 8 (44) | 136 | |
| Ca2+ channel blockers | 11 (37) | 204 | 8 (35) | 161 | 6 (33) | 106 | ||
| ACE inhibitors | 11 (37) | 176 | 8 (35) | 120 | 7 (39) | 96 | ||
| Digoxin | 3 (10) | 56 | 3 (13) | 56 | 3 (17) | 56 | ||
| Statins | 8 (27) | 129 | 7 (30) | 109 | 7 (39) | 105 | ||
| Anticoagulants | 5 (17) | 82 | 3 (13) | 49 | 3 (17) | 50 | ||
| Respiratory | Bronchodilator‡ | 8 (27) | 123 | 5 (22) | 64 | 5 (28) | 64 | |
| Asthma steroids | 8 (27) | 96 | 5 (22) | 48 | 4 (22) | 46 | ||
*Diagnoses are self‐reported doctor's diagnoses except for: asthma is self‐reported; diabetes determined via diabetic medications use, obesity defined as BMI >30, bronchitis defined as chronic cough or phlegm on most days for three consecutive months or more during the year.
†Medications use includes participants who reported taking the medication at least once throughout the study on the day of their clinic visit.
‡Bronchodilator grouping includes beta‐agonist and atrovent medications.
Over the 24 week study period, 623 clinic visits provided valid ECG data. The mean (standard deviation) duration of the structured ECG protocol session was 33 (3) minutes and mean counts of arrhythmia for the whole cohort were 27.2 (87.8) beats/protocol session (or 50.0 (162.8) beats/hour) for SVE and 28.0 (93.3) beats/protocol session (or 52.6 (173.5) beats/hour) for VE. There was considerable variability in participant‐specific SVE and VE counts, as participant‐specific mean counts (over the whole protocol) varied between 0.1–363 for SVE and 0–350 for VE. The correlation between the two arrhythmia measures was low, with counts of SVE and VE over the whole protocol showing a Spearman correlation coefficient (r) of 0.03.
Air quality data
Tables 2 and 3 present descriptive statistics and correlations for air pollutants, respectively. During the study, 24 hour PM2.5 concentrations averaged approximately 19 μg/m3 and ranged from 3.6 to 48.4 μg/m3. Ambient PM2.5 and SO42− were highly correlated (r = 0.89 for 24 hour averages), with lower correlations between PM2.5 and the other pollutants (range of r = 0.20–0.51). Correlations were moderately strong between apparent temperature and SO42− and O3 concentrations (r = 0.55 and 0.67, respectively), likely due to the direct influence of meteorology on the formation of these secondary pollutants. In contrast, correlations were weaker between apparent temperature and EC, NO2, SO2, and CO, which are all primary pollutants (r = 0.22, −0.10, −0.04, and −0.30, respectively). The correlations between pollutants showed similar patterns whether examining 24 hour (that is, one day) or five day moving averages. SO42− comprised approximately 50% of the ambient PM2.5 mass, similar to other locations in the eastern US; this suggests that the regional influence on PM2.5 in Steubenville was relatively high. EC comprised approximately 6% of the ambient PM2.5 mass, which also compares well to other areas of the country.
Table 2 Summary of 24 hour (one day) and five day moving average air pollution concentrations.
| Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Pollutant | n | Mean (SD) | Min | 25th | 50th | 75th | Max | IQR* |
| 1 day | PM2.5 (μg/m3) | 113 | 19.6 (10.4) | 3.6 | 11.7 | 17.7 | 25.0 | 48.4 | 13.3 |
| SO42− (μg/m3) | 109 | 6.8 (4.7) | 0.0 | 3.3 | 5.7 | 8.5 | 25.0 | 5.2 | |
| EC (μg/m3) | 103 | 1.1 (0.6) | 0.3 | 0.7 | 1.0 | 1.3 | 3.6 | 0.6 | |
| O3 (ppb) | 111 | 21.8 (12.6) | −0.8 | 12.0 | 20.2 | 28.5 | 74.8 | 16.5 | |
| NO2 (ppb) | 111 | 10.7 (6.7) | −0.9 | 6.3 | 9.6 | 14.0 | 37.9 | 7.7 | |
| SO2 (ppb) | 106 | 10.4 (8.3) | 1.8 | 5.2 | 8.1 | 12.4 | 58.3 | 7.2 | |
| CO (ppm) | 110 | 0.2 (0.3) | −0.1 | 0.0 | 0.1 | 0.3 | 1.5 | 0.3 | |
| 5 day | PM2.5 (μg/m3) | 107 | 19.8 (7.5 | 4.9 | 13.9 | 19.0 | 23.9 | 38.7 | 10.0 |
| SO42− (μg/m3) | 97 | 6.7 (3.1) | 1.5 | 4.2 | 6.1 | 8.4 | 14.8 | 4.2 | |
| EC (μg/m3) | 80 | 1.0 (0.4) | 0.4 | 0.7 | 0.9 | 1.2 | 2.2 | 0.5 | |
| O3 (ppb) | 101 | 22.2 (9.1) | 6.5 | 14.2 | 20.7 | 29.1 | 44.0 | 14.9 | |
| NO2 (ppb) | 98 | 10.5 (4.2) | 4.5 | 7.4 | 9.2 | 12.9 | 20.4 | 5.5 | |
| SO2 (ppb) | 103 | 10.7 (5.5) | 2.4 | 7.4 | 9.1 | 12.8 | 31.3 | 5.4 | |
| CO (ppm) | 106 | 0.2 (0.2) | 0.0 | 0.1 | 0.2 | 0.3 | 1.3 | 0.2 | |
*IQR, interquartile range (75th–25th percentile).
Table 3 Spearman correlations between 24‐hour air quality parameters.
| PM2.5 | SO42− | EC | O3 | NO2 | SO2 | CO | |
|---|---|---|---|---|---|---|---|
| App temp‡ | 0.46† | 0.55† | 0.22* | 0.67† | −0.10 | −0.04 | −0.30* |
| PM2.5 | 0.89† | 0.51† | 0.20* | 0.34* | 0.47† | 0.45† | |
| SO42− | 0.42† | 0.31* | 0.23* | 0.44† | 0.30* | ||
| EC | −0.06 | 0.65† | 0.44† | 0.60† | |||
| O3 | −0.34* | −0.21* | −0.37† | ||||
| NO2 | 0.54† | 0.66† | |||||
| SO2 | 0.62† |
*p<0.05; †p⩽0.0001; ‡apparent temperature.
Associations between air pollution and cardiac arrhythmia
Results of the logistic regression models showed that air pollution increased the odds of having at least one SVE, but not VE, over the entire protocol (table 4). The results for SVE were greatest for PM2.5, SO42−, and O3, while EC, NO2, SO2, and CO demonstrated weaker effects that were consistent with the null. The impacts of air pollution on the odds of SVE and VE did not differ when examining the individual protocol intervals (that is, resting, standing, exercise, etc) in separate models.
Table 4 Odds ratios for SVE and VE for IQR increases in five day moving average pollutant concentrations*.
| Pollutant | 5 day IQR | n | SVE | p Value | n | VE | p Value |
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | ||||||
| Particles | |||||||
| PM2.5 | 10.0 μg/m3 | 400 | 1.42 (0.99–2.04) | 0.07 | 314 | 1.02 (0.63–1.65) | 0.93 |
| SO42− | 4.2 μg/m3 | 356 | 1.70 (1.12–2.57) | 0.02 | 286 | 1.08 (0.65–1.80) | 0.78 |
| EC | 0.5 μg/m3 | 310 | 1.15 (0.73–1.81) | 0.57 | 243 | 1.00 (0.57–1.75) | 0.99 |
| Gases | |||||||
| O3 | 14.9 ppb | 387 | 1.78 (0.95–3.35) | 0.09 | 304 | 1.43 (0.63–3.27) | 0.41 |
| NO2 | 5.5 ppb | 379 | 0.89 (0.61–1.28) | 0.53 | 296 | 0.61 (0.36–1.03) | 0.08 |
| SO2 | 5.4 ppb | 397 | 1.04 (0.78–1.39) | 0.78 | 306 | 1.28 (0.85–1.92) | 0.25 |
| CO | 0.2 ppm | 410 | 0.99 (0.76–1.29) | 0.93 | 317 | 1.05 (0.75–1.46) | 0.80 |
*Models control for apparent temperature at hour of clinic visit, season, day of week, hour of day, age, and BMI.
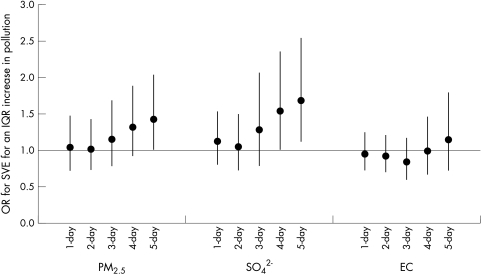
Over the entire protocol, the odds ratio (OR) for having at least one air pollution related SVE was greatest at longer moving average concentrations prior to the health assessment (fig 1). For example, the OR for SVE was 1.42 (95% CI 0.99 to 2.04) for a 10 μg/m3 increase in five day PM2.5 as compared with 1.04 (95% CI 0.73 to 1.47) for a 13.3 μg/m3 increase in one day PM2.5. The observed particle effects remained elevated or increased at moving average concentrations longer than five days. In these analyses, the effects of PM2.5 on the odds of SVE were not different when the HMPM PM2.5 time series was filled in for missing Saturday data using regressions with 24 hour averaged TEOM data.
Figure 1 Odds ratios for SVE for IQR increases in pollutant concentrations at 1–5 day moving average concentrations (n = 310–421). Models control for apparent temperature at hour of clinic visit, season, day of week, hour of day, age, and BMI. Error bars indicate 95% confidence intervals.
Including heart rate in the models resulted in more precise estimation of effects, with the five day moving average PM2.5 effect on the odds of SVE (OR = 1.48, 95% CI 1.02 to 2.15) becoming significant. Inclusion of five day O3 in models together with either five day PM2.5 or SO42− reduced the effects of both pollutants slightly. For example, with five day SO42− in the model, the five day O3 effect fell to 1.57 (95% CI 0.74 to 3.35) and the SO42− effect fell to 1.54 (95% CI 1.00 to 2.36).
In sensitivity analyses, the logistic model results were similar when all 30 participants were included in the analyses. In order to use the information provided by the counts of SVE and VE in our dataset, we additionally modeled the outcome as a count variable using negative binomial regression. Estimated associations between pollution levels and the rate of arrhythmias per 30 minute interval from this model were not significantly different from zero (results not shown).
Examination of effect modification
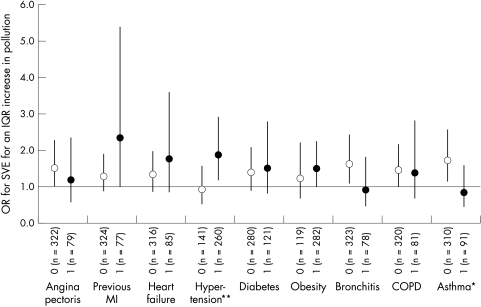
Figure 2 presents the results of interactions between five day moving average PM2.5 concentrations and participant characteristics on the odds of SVE. Pollution effects were greatest for participants with cardiovascular conditions. Participants who reported a previous MI, for example, had an OR for SVE of 2.35 (95% CI 1.03 to 5.39) as compared to an OR of 1.31 (95% CI 0.89 to 1.91) in those without a previous MI, each for a 10.0 μg/m3 increase in five day PM2.5 (test of significant difference, p = 0.20). In addition, participants who reported ever having hypertension showed a significantly higher effect (OR = 1.88, 95% CI 1.21 to 2.92) compared to those without hypertension (OR = 0.94, 95% CI 0.56 to 1.59) (test of significant difference, p = 0.04). Medications use showed collinear effects with diagnosis. For example, participants on medications for cardiac conditions, such as beta blockers or Ca2+ channel blockers, experienced greater air pollution effects than those not on cardiac medications (corresponding to those without diagnosed cardiac disease). In contrast to those with cardiovascular disease, participants with respiratory conditions (for example, bronchitis, asthma) did not influence the effect of PM2.5 on the odds of SVE. The null effect of PM2.5 on participants with angina is likely influenced by the fact that a majority of these participants (4 of 6) also had respiratory disease.
Figure 2 Participant characteristics as effect modifiers of the five day moving average PM2.5‐SVE association. 0 = participants without the characteristic, 1 = participants with the characteristic. Difference in pollution effect is significant at **p<0.05, *p<0.10. Models control for apparent temperature at hour of clinic visit, season, day of week, hour of day, age, and BMI. Error bars indicate 95% confidence intervals.
The temporal association between PM2.5 and SVE noted above was similar when considering only those participants with cardiac diagnoses. For example, subsetting the analyses only on those participants with hypertension, the OR for having at least one air pollution related SVE was greater at five day moving average concentrations (2.07 (95% CI 1.29 to 3.32) for a 10 μg/m3 increase in five day PM2.5) before the health assessment as compared to one day moving average concentrations (1.28 (95% CI 0.82 to 1.99) for a 13.3 μg/m3 increase in one day PM2.5).
We also examined whether pollution effects were greater in participants with higher levels of ectopy. We observed that five day PM2.5 effects on the odds of SVE were significantly higher for participants with mean SVE rates greater than the population median rate of 1.5 beats/hour (OR = 1.99, 95% CI 1.20 to 3.29) as compared to participants with mean rates <1.5 beats/hour (OR = 1.03, 95% CI 0.65 to 1.64) (test of significant difference, p = 0.05).
Discussion
In a community with significant industrial sources for air pollution, our study demonstrates an association of particle pollution with increased odds of supraventricular arrhythmia in a cohort of older adults, with findings of 42%, 70%, and 78% increases in odds of SVE associated with IQR increases in five day moving average PM2.5, SO42−, and O3, respectively. Air pollution effects were greatest for participants with a history of clinically significant cardiac disease. Since two‐pollutant models demonstrated stability in the effects of both particles and O3, collectively our results may provide evidence of the combined effect of the secondary pollutant mix in Steubenville on cardiac arrhythmia. Specifically, the strong effects found with SO42− are interesting as Steubenville is located in an industrial area of the Ohio River Valley, with little traffic but with a number of coal fired power plants, which are the major source of SO2, a SO42− precursor. It is important to note that ambient SO42− concentrations were measured with higher overall precision, and further, that ambient SO42− concentrations were better proxies of corresponding personal exposures as compared to EC.13 Both factors may have resulted in sufficient power to detect associations between arrhythmia and ambient concentrations of SO42−. A previous study conducted in Boston, reporting on patients with implantable cardioverter defibrillators (ICD), found that traffic related pollutants, particularly NO2, showed the greatest odds of arrhythmia.16 Our data suggest that pollution in an industrial location may also contribute to the risk of arrhythmia and they indicate the potential for varying impacts of air pollution by geographical location and source contributions.
Recent air pollution health effects studies have suggested mechanisms to support the association between ambient particle levels and SVE occurrence.8,9,10,11 A leading hypothesis regarding the biological mechanism of air pollution health effects centres on the relation between increased air pollution levels and autonomic nervous system imbalance,17,18,19,20 which is known to aggravate SVE.21 Our observation that long moving average pollutant concentrations exerted the greatest impact on the odds of SVE is consistent with the previous ICD patient studies,16,22 and the results may suggest the presence of an inflammatory mechanism as well.23 Stone and Godleski suggest that increased circulating cytokine levels as part of the systemic response can act to impair cardiac myocyte and electrophysiological function,24,25 which could cause cardiac irritability, thus increasing SVE occurrence. Our results lend support to such a mechanism and may help to explain the delayed effects observed in several previous air pollution morbidity and mortality studies, such as those conducted in Erfurt, Germany,26 and in the Utah Valley, Utah.27
We identified several factors that increased the odds of air pollution related SVE within our cohort. Participants reporting previous cardiovascular conditions, including MI or hypertension, were most susceptible to pollution induced SVE. Participants on cardiac medications, including beta blockers or Ca2+ channel blockers, also showed sensitivity to air pollution mediated SVE. The association of these cardiac medications and increased sensitivity to air pollution is likely due to these medications serving as a surrogate for the presence of the clinical cardiovascular conditions and not to the pharmacological effect of the drug per se.
It has also been hypothesised that individuals with chronic lung disease comprise a susceptible subgroup, given their tendency for loss of vagal restraint, thereby leading to an increased heart rate and conditions suitable for ectopic tachycardias.28 However, air pollution did not influence the odds of SVE in these participants. Our results differ from previous studies reporting significant effects of air pollution on SVE for groups of COPD patients.8,9,11 A study of 16 COPD patients in Vancouver, whose mean rate of SVE was 33 beats/hour, reported a 22% increased rate of SVE for each 5.8 μg/m3 increase in PM2.5.11 As the number of participants with bronchitis, COPD and/or asthma (n = 5–6) in our analyses was low, the lack of observed effects for these groups is likely a function of weak statistical power. Moreover, the small size (n = 23) of the cohort used in the SVE models may limit the generalisability of our findings regarding effect modification by diagnosis.
Effect sizes of air pollution on the odds of VE were extremely small. The lack of observed effects may have been due to a lack of vulnerability of our small (n = 18), and therefore limited, study population. Dockery and colleagues (2004) demonstrated in their study of ICD patients that ventricular arrhythmias within three days of a prior event presented significantly higher effects (OR = 1.60, 95% CI 1.25 to 2.04) for two day mean PM2.5 than those more than three days since a previous event (OR = 0.98, 95% CI 0.84 to 1.14).22 In a discussion of their findings, the authors suggest that air pollution may act in combination with acutely predisposing conditions, which increase ventricular electrical instability and that could lead to arrhythmia occurrence.22 It is plausible that our study population did not experience such acutely predisposing conditions that would enhance the effects of air pollution on VE.
Our results are consistent with a previous study examining the effects of air pollution on both SVE and VE in the elderly, where significant effects were found with SVE but not VE.8 In contrast, Riediker and colleagues (2004) found a significant effect of air pollution on both SVE and VE in their study of highway patrolmen, reporting a 19.1% increased rate of VE for each 10 μg/m3 increase in PM2.5.10 Studies of associations of PM2.5 with risk of VE require a sufficient number of observations on a vulnerable study population and may, as in the case of the patrolmen, require stress during the period of observation.
The relatively brief period of observation (that is, 30 minute ECG recording per participant visit) may have further limited our ability to capture the air pollution effect on the odds of VE, a relatively rare outcome. Recordings of longer length (for example, 24 hour) as conducted in other panel studies8,9,10 may provide outcome data for which associations with air pollution are better detected.
We also recognise that the occurrence of at least one SVE or VE is a subclinical outcome. Ectopy or electrical instability may result from inflammation, autonomic dysfunction, ischaemia or pressure/volume overload of the heart. While ventricular fibrillation and ventricular tachycardia can lead to sudden death,29,30 and atrial fibrillation and other atrial tachycardias can increase risk of hospitalisation, stroke, and, sometimes, death,31,32 the clinical importance of isolated ventricular or atrial (supraventricular) ectopic beats is unknown. As previously discussed, the arrhythmia outcome we observed to be linked to pollution, “any SVE”, may result from subclinical electrical instability due to either autonomic dysfunction or inflammation.
While we were able to detect associations between pollution levels and SVE in analyses when considering the outcome as a discrete binary variable (that is, occurrence of at least one arrhythmia during the protocol), we did not detect significant associations between increased pollution levels and increased counts of arrhythmias when considering SVE as a continuous variable. Associations with the counts data remained consistent with the null when limiting the analysis to those subjects included in the logistic analyses. The large amount of overdispersion in the non‐zero counts could have reduced our power to detect associations between pollution levels and the arrhythmia counts. For example, it is likely that the effects of pollution on arrhythmia were small relative to the large amount of variability in the arrhythmia counts themselves; we were not able to detect air pollution effects when using a model (that is, the negative binomial model) that reflected this large variability.
Conclusion
In summary, our results suggest that increased levels of ambient air pollution, particularly for regional pollutants, including SO42− and O3, may increase the risk of supraventricular arrhythmia in the elderly. Findings of cumulative air pollution effects (⩾five day moving average concentrations before the health assessment) as the highest and most significant, may suggest that a long acting mechanism promoted the ectopic beats in our population. Furthermore, the results suggest that individuals with a history of clinically significant cardiac disease may be at particular risk of air pollution health effects.
Main messages
Increased levels of ambient pollutants, including regional pollutants such as sulfate and ozone, increase the risk of supraventricular arrhythmia in the elderly in Steubenville.
The effect of regional air pollution on ectopic beats may occur through relatively long acting pathways such as inflammation.
Individuals with a history of clinically significant cardiac disease may be at particular risk of air pollution health effects.
Policy implications
Sulfate and other regional pollutants may increase the risk of arrhythmia in susceptible populations.
Acknowledgements
The authors wish to thank all of the participants of the study as well as those involved in project coordination (Monique Verrier, Meghan Syring, Marisa Barr), data collection (field team members from the Franciscan University of Steubenville), and data processing (Bruce Nearing, Gail McCallum, Marina Jacobson‐Canner). The authors are also grateful for CONSOL Energy Inc Research & Development's laboratory analysis of air pollutant samples and for the provision of continuous ambient monitoring data. Finally, the authors are appreciative of the insightful comments provided by Jeremy Sarnat and Petros Koutrakis.
Abbreviations
BMI - body mass index, BP, blood pressure
COPD - chronic obstructive pulmonary disease
ECG - electrocardiogram
HMPM - Harvard multi‐pollutant monitor
HRV - heart rate variability
ICD - implantable cardioverter defibrillators
IQR - interquartile range
MI - myocardial infarction
SVE - supraventricular ectopy
VE - ventricular ectopy
Footnotes
Funding: This work is supported by funding from the National Institute of Environmental Health Sciences (ES‐09825), the US Environmental Protection Agency (R826780‐01‐0, R827353‐01‐0), the Ohio Coal Development Office within the Ohio Air Quality Development Authority (CDO/D‐98‐2), the Electric Power Research Institute (EP‐P4464/C2166), the American Petroleum Institute (#78142), and the United States Department of Energy's National Energy Technology Laboratory (DE‐FC26‐00NT40771). These sponsors did not play a role in the study design, collection, analysis, nor interpretation of the data, writing of the report, nor the decision to submit the paper for publication. Any opinions, findings, conclusions, or recommendations expressed herein are those of the authors and do not necessarily reflect the views of these funding sources.
Competing interests: none.
References
- 1.Schwartz J. Air pollution and hospital admissions for heart disease in eight U.S. counties. Epidemiology 19991017–22. [PubMed] [Google Scholar]
- 2.Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect 2000108841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann J K, Tager I B, Lurmann F.et al Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ Health Perspect 20021101247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger K B, Tolbert P E, Klein M.et al Ambient air pollution and cardiovascular emergency department visits. Epidemiology 20041546–56. [DOI] [PubMed] [Google Scholar]
- 5.Samet J M, Dominici F, Curriero F C.et al Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 20003431742–1749. [DOI] [PubMed] [Google Scholar]
- 6.Hoek G, Brunekreef B, Fischer P.et al The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology 200112355–357. [DOI] [PubMed] [Google Scholar]
- 7.Pope CA I I I, Burnett R T, Thurston G D.et al Cardiovascular mortality and long‐term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 200410971–77. [DOI] [PubMed] [Google Scholar]
- 8.Linn W S, Gong H, Jr, Clark K W.et al Day‐to‐day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manage Assoc 199949108–115. [DOI] [PubMed] [Google Scholar]
- 9.Brauer M, Ebelt S T, Fisher T V.et al Exposure of chronic obstructive pulmonary disease patients to particles: Respiratory and cardiovascular health effects. J Expo Anal Environ Epidemiol 200111490–500. [DOI] [PubMed] [Google Scholar]
- 10.Riediker M, Cascio W E, Griggs T R.et al Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med 2004169934–940. [DOI] [PubMed] [Google Scholar]
- 11.Ebelt S T, Wilson W E, Brauer M. Exposure to ambient and nonambient components of particulate matter: A comparison of health effects. Epidemiology 200516396–405. [DOI] [PubMed] [Google Scholar]
- 12.Demokritou P, Kavouras I G, Ferguson S T.et al Development and laboratory performance evaluation of a personal multipollutant sampler for simultaneous measurements of particulate and gaseous pollutants. Aerosol Sci Technol 200135741–752. [Google Scholar]
- 13.Sarnat S E, Coull B A, Schwartz J.et al Factors affecting the association between ambient concentrations and personal exposures to particles and gases. Environ Health Perspect 2006114649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connell D P, Withum J A, Winter S E.et al The Steubenville Comprehensive Air Monitoring Program (SCAMP): Analysis of short‐term and episodic variations in PM2.5 concentrations using hourly air monitoring data. J Air Waste Manage Assoc 200555559–573. [DOI] [PubMed] [Google Scholar]
- 15.Connell D P, Withum J A, Winter S E.et al The Steubenville Comprehensive Air Monitoring Program (SCAMP): Associations among fine particulate matter, co‐pollutants, and meteorological conditions. J Air Waste Manage Assoc 200555481–496. [DOI] [PubMed] [Google Scholar]
- 16.Peters A, Liu E, Verrier R L.et al Air pollution and incidence of cardiac arrhythmia. Epidemiology 20001111–17. [DOI] [PubMed] [Google Scholar]
- 17.Pope CA I I I, Verrier R L, Lovett E G.et al Heart rate variability associated with particulate air pollution. Am Heart J 1999138890–899. [DOI] [PubMed] [Google Scholar]
- 18.Gold D R, Litonjua A, Schwartz J.et al Ambient pollution and heart rate variability. Circulation 20001011267–1273. [DOI] [PubMed] [Google Scholar]
- 19.Creason J, Neas L, Walsh D.et al Particulate matter and heart rate variability among elderly retirees: The Baltimore 1998 PM study. J Expo Anal Environ Epidemiol 200111116–122. [DOI] [PubMed] [Google Scholar]
- 20.Liao D P, Duan Y K, Whitsel E A.et al Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population‐based study. Am J Epidemiol 2004159768–777. [DOI] [PubMed] [Google Scholar]
- 21.Ho J E, Stevenson W G, Strichartz G R.et al Mechanisms of cardiac arrhythmias. In: Lilly LS (ed). Pathophysiology of heart disease: a collaborative project of medical students and faculty, 3rd edition. Baltimore, MD: Lippincott Williams & Wilkins, 2003253–268.
- 22.Dockery D W, Luttmann‐Gibson H, Rich D Q.et al Particulate air pollution and nonfatal cardiac events. Part II: Association of air pollution with confirmed arrhythmias recorded by implanted defibrillators. Health Effects Institute Research Report Number 124 200583–126. [PubMed]
- 23.Seaton A, MacNee W, Donaldson K.et al Particulate air pollution and acute health effects. Lancet 1995345176–178. [DOI] [PubMed] [Google Scholar]
- 24.Stone P H, Godleski J J. First steps toward understanding the pathophysiologic link between air pollution and cardiac mortality. Am Heart J 1999138804–807. [DOI] [PubMed] [Google Scholar]
- 25.Brook R D, Brook J R, Rajagopalan S. Air pollution: The “heart” of the problem. Curr Hypertens Rep 2003532–39. [DOI] [PubMed] [Google Scholar]
- 26.Wichmann H E, Spix C, Tuch T.et al Daily mortality and fine and ultrafine particles in Erfurt, Germany: Part I. Role of particle number and particle mass. Res Rep Health Eff Inst 2000985–86. [PubMed] [Google Scholar]
- 27.Pope C A. Particulate pollution and health: a review of the Utah valley experience. J Expo Anal Environ Epidemiol 1996623–34. [PubMed] [Google Scholar]
- 28.Levine P A, Klein M D. Mechanisms of arrhythmias in chronic obstructive lung disease. Geriatrics 19763147–56. [PubMed] [Google Scholar]
- 29.Myerburg R J, Kessler K M, Castellanos A. Sudden cardiac death. Structure, function, and time‐dependence of risk. Circulation 199285I2–10. [PubMed] [Google Scholar]
- 30.Bayes de Luna A, Coumel P, Leclercq J F. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J 1989117151–159. [DOI] [PubMed] [Google Scholar]
- 31.Wolf P A, Mitchell J B, Baker C S.et al Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998158229–234. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin E J, Wolf P A, D'Agostino R B.et al Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 199898946–952. [DOI] [PubMed] [Google Scholar]