Abstract
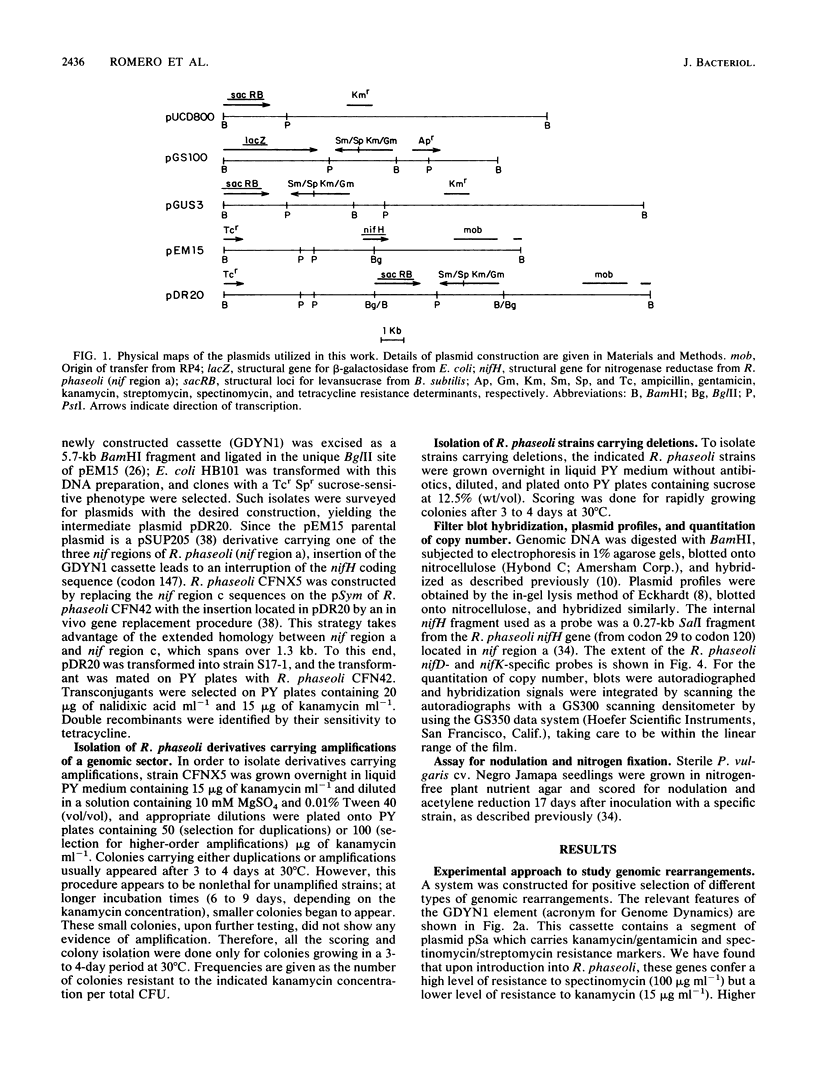
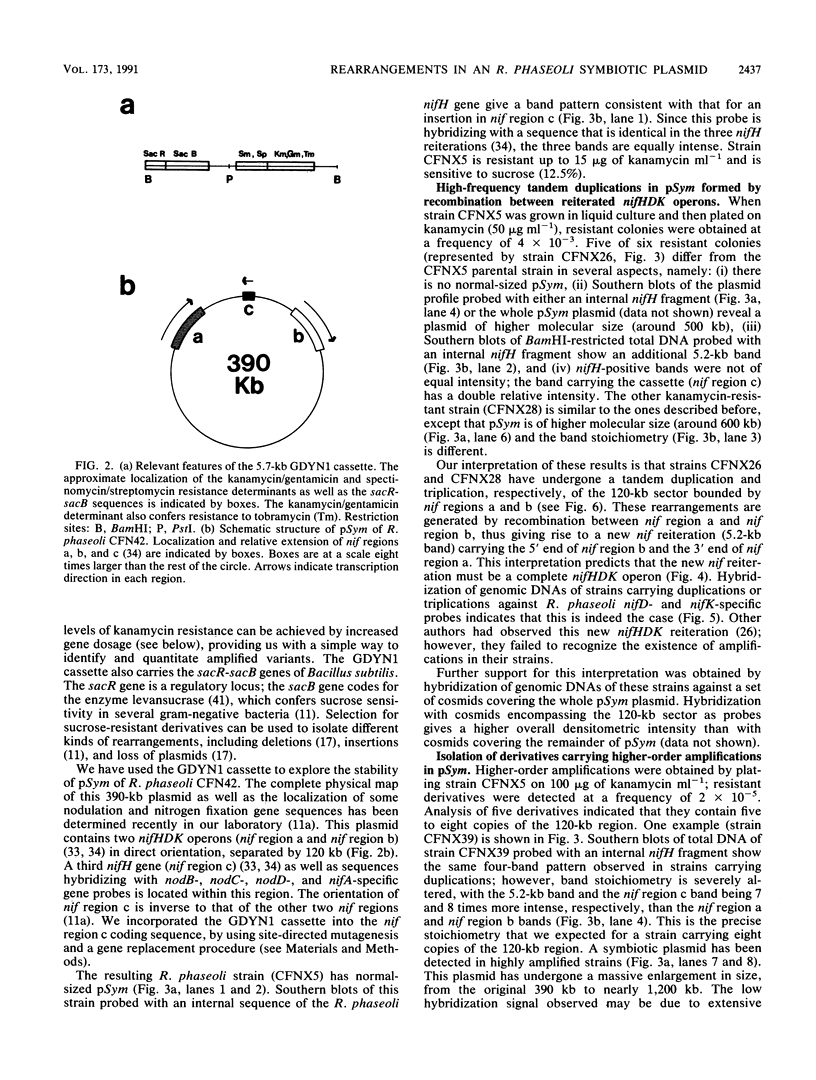
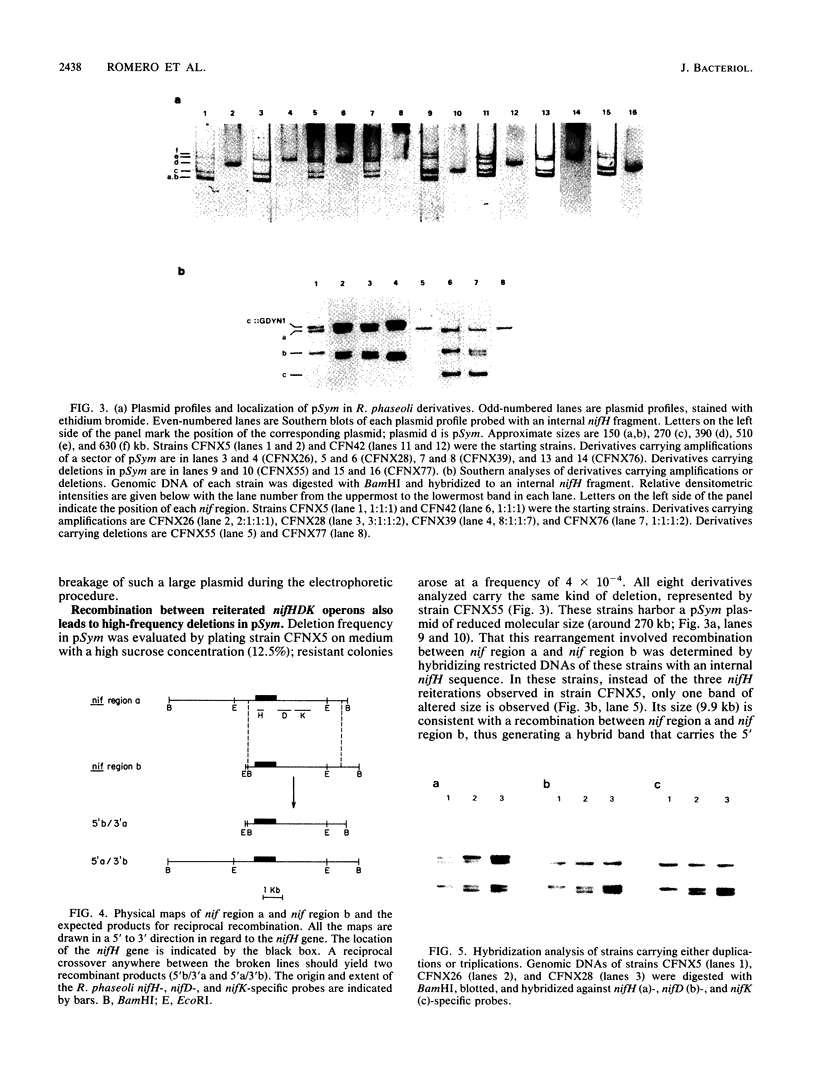
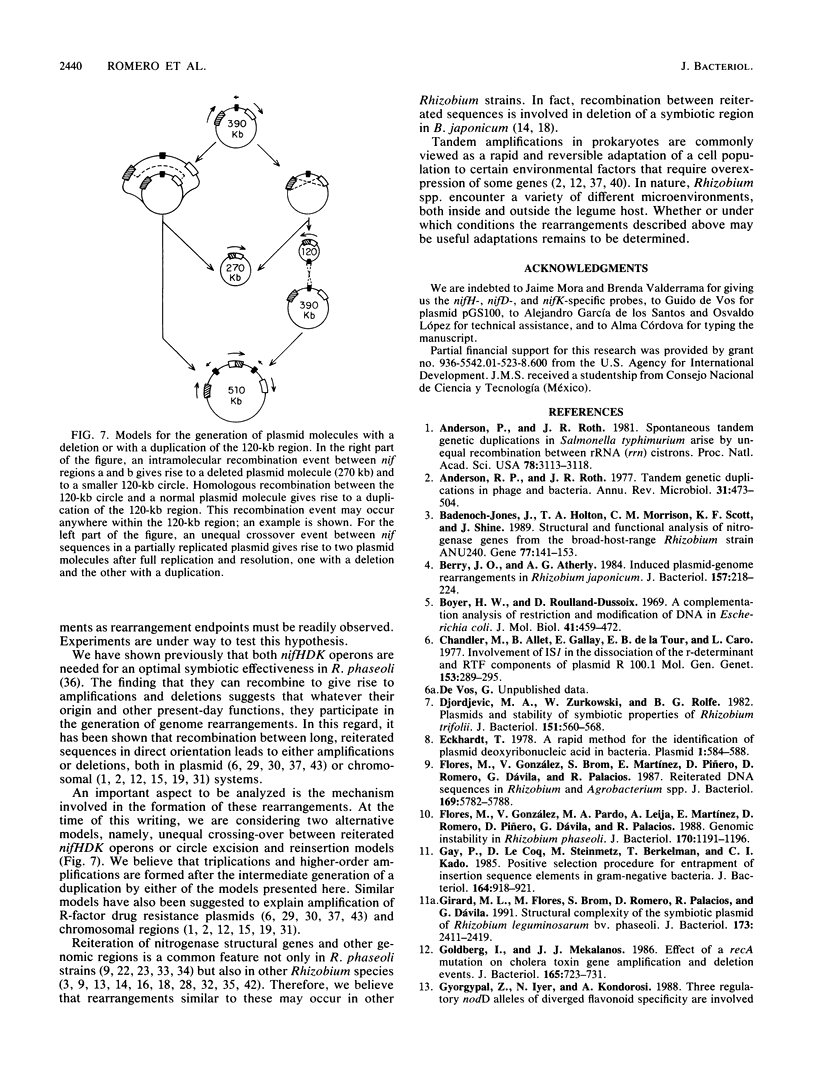
One remarkable characteristic of the genomes of some Rhizobium species is the frequent occurrence of rearrangements. In some instances these rearrangements alter the symbiotic properties of the strains. However, no detailed molecular mechanisms have been proposed for the generation of these rearrangements. To understand the mechanisms involved in the formation of rearrangements in the genome of Rhizobium phaseoli, we have designed a system which allows the positive selection for amplification and deletion events. We have applied this system to investigate the stability of the symbiotic plasmid of R. phaseoli. High-frequency amplification events were detected which increase the copy number of a 120-kb region carrying nodulation and nitrogen fixation genes two to eight times. Deletion events that affect the same region were also found, albeit at a lower frequency. Both kinds of rearrangements are generated by recombination between reiterated nitrogenase (nifHDK) operons flanking the 120-kb region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A. 1981 May;78(5):3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Badenoch-Jones J., Holton T. A., Morrison C. M., Scott K. F., Shine J. Structural and functional analysis of nitrogenase genes from the broad-host-range Rhizobium strain ANU240. Gene. 1989 Apr 15;77(1):141–153. doi: 10.1016/0378-1119(89)90368-5. [DOI] [PubMed] [Google Scholar]
- Berry J. O., Atherly A. G. Induced plasmid-genome rearrangements in Rhizobium japonicum. J Bacteriol. 1984 Jan;157(1):218–224. doi: 10.1128/jb.157.1.218-224.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chandler M., Allet B., Gallay E., Boy de La Tour E., Caro L. Involvement of IS1 in the dissociation of the r-determinant and RTF components of the plasmid R100.1. Mol Gen Genet. 1977 Jun 24;153(3):289–295. doi: 10.1007/BF00431594. [DOI] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Rolfe B. G. Plasmids and stability of symbiotic properties of Rhizobium trifolii. J Bacteriol. 1982 Aug;151(2):560–568. doi: 10.1128/jb.151.2.560-568.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Flores M., González V., Brom S., Martínez E., Piñero D., Romero D., Dávila G., Palacios R. Reiterated DNA sequences in Rhizobium and Agrobacterium spp. J Bacteriol. 1987 Dec;169(12):5782–5788. doi: 10.1128/jb.169.12.5782-5788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Berkelman T., Kado C. I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985 Nov;164(2):918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M. L., Flores M., Brom S., Romero D., Palacios R., Dávila G. Structural complexity of the symbiotic plasmid of Rhizobium leguminosarum bv. phaseoli. J Bacteriol. 1991 Apr;173(8):2411–2419. doi: 10.1128/jb.173.8.2411-2419.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986 Mar;165(3):723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Hennecke H. Mapping of a Bradyrhizobium japonicum DNA Region Carrying Genes for Symbiosis and an Asymmetric Accumulation of Reiterated Sequences. Appl Environ Microbiol. 1987 Sep;53(9):2247–2252. doi: 10.1128/aem.53.9.2247-2252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Grafstrom R. H., Harnish B. W., Hillman B. S. Tandem duplications resulting from recombination between ribosomal RNA genes in Escherichia coli. J Mol Biol. 1977 Nov 5;116(3):407–428. doi: 10.1016/0022-2836(77)90077-8. [DOI] [PubMed] [Google Scholar]
- Honma M. A., Ausubel F. M. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8558–8562. doi: 10.1073/pnas.84.23.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. F., Quandt J., O'Connell M. P., Pühler A. Direct selection for curing and deletion of Rhizobium plasmids using transposons carrying the Bacillus subtilis sacB gene. Gene. 1989 May 15;78(1):111–120. doi: 10.1016/0378-1119(89)90319-3. [DOI] [PubMed] [Google Scholar]
- Kaluza K., Hahn M., Hennecke H. Repeated sequences similar to insertion elements clustered around the nif region of the Rhizobium japonicum genome. J Bacteriol. 1985 May;162(2):535–542. doi: 10.1128/jb.162.2.535-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. J., Capage M., Hill C. W. A repetitive DNA sequence, rhs, responsible for duplications within the Escherichia coli K-12 chromosome. J Mol Biol. 1984 Jul 25;177(1):1–18. doi: 10.1016/0022-2836(84)90054-8. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium genetics. Annu Rev Genet. 1989;23:483–506. doi: 10.1146/annurev.ge.23.120189.002411. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Homologous sequences other than insertion elements can serve as recombination sites in plasmid drug resistance gene amplification. J Bacteriol. 1983 Oct;156(1):177–185. doi: 10.1128/jb.156.1.177-185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Recombination sites in plasmid drug resistance gene amplification. J Bacteriol. 1985 Dec;164(3):1359–1361. doi: 10.1128/jb.164.3.1359-1361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Prakash R. K., Atherly A. G. Reiteration of genes involved in symbiotic nitrogen fixation by fast-growing Rhizobium japonicum. J Bacteriol. 1984 Nov;160(2):785–787. doi: 10.1128/jb.160.2.785-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renalier M. H., Batut J., Ghai J., Terzaghi B., Gherardi M., David M., Garnerone A. M., Vasse J., Truchet G., Huguet T. A new symbiotic cluster on the pSym megaplasmid of Rhizobium meliloti 2011 carries a functional fix gene repeat and a nod locus. J Bacteriol. 1987 May;169(5):2231–2238. doi: 10.1128/jb.169.5.2231-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero David, Singleton Paul W., Segovia Lorenzo, Morett Enrique, Bohlool B. Ben, Palacios Rafael, Dávila Guillermo. Effect of Naturally Occurring nif Reiterations on Symbiotic Effectiveness in Rhizobium phaseoli. Appl Environ Microbiol. 1988 Mar;54(3):848–850. doi: 10.1128/aem.54.3.848-850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberón-Chávez G., Nájera R., Olivera H., Segovia L. Genetic rearrangements of a Rhizobium phaseoli symbiotic plasmid. J Bacteriol. 1986 Aug;167(2):487–491. doi: 10.1128/jb.167.2.487-491.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonti R. V., Roth J. R. Role of gene duplications in the adaptation of Salmonella typhimurium to growth on limiting carbon sources. Genetics. 1989 Sep;123(1):19–28. doi: 10.1093/genetics/123.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S., Gonzy-Tréboul G., Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200(2):220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Identification and characterization of a small sequence located at two sites on the amplifiable tetracycline resistance plasmid pAMalpha1 in Streptococcus faecalis. J Bacteriol. 1977 Jan;129(1):400–406. doi: 10.1128/jb.129.1.400-406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkowski W. Molecular mechanism for loss of nodulation properties of Rhizobium trifolii. J Bacteriol. 1982 Jun;150(3):999–1007. doi: 10.1128/jb.150.3.999-1007.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]





