Abstract
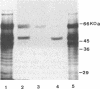
Extracellular amylase in Streptomyces lividans was undetectable in starch-supplemented medium. However, S. lividans produced fivefold-higher levels of amylase than Streptomyces griseus IMRU 3570 when transformed with the S. griseus amy gene. Two major proteins of 57 and 50 kDa with amylase activity accumulated in the culture broths of the donor S. griseus and S. lividans transformed with the amy gene. Both proteins were also present in protoplast lysates in the same relative proportion; they gave a positive reaction with antibodies against the 57-kDa amylase. They did not differ in substrate specificity or enzyme kinetics. The two amylases were purified to homogeneity by a two-step procedure. Both proteins showed the same amino-terminal sequence of amino acids, suggesting that both proteins are derived from the same gene. The deduced signal peptide has 28 amino acids with two positively charged arginines near the amino-terminal end. When an internal NcoI fragment was removed from the amy gene, the resulting S. lividans transformants did not synthesize any of the two amylase proteins and showed no reaction in immunoblotting. Formation of the 50-kDa protein was observed when pure 57-kDa amylase was treated with supernatants of protoplast lysates but not when it was treated with membrane preparations, indicating that the native 57-kDa amylase could be processed intracellularly.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler L. A., Arvidson S. Cloning and expression in Escherichia coli of genes encoding a multiprotein complex involved in secretion of proteins from Staphylococcus aureus. J Bacteriol. 1988 Nov;170(11):5337–5343. doi: 10.1128/jb.170.11.5337-5343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahri S. M., Ward J. M. Cloning and expression of an alpha-amylase gene from Streptomyces thermoviolaceus CUB74 in Escherichia coli JM107 and S. lividans TK24. J Gen Microbiol. 1990 May;136(5):811–818. doi: 10.1099/00221287-136-5-811. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Jones G. H., Joseph R., Buttner M. J., Ward J. M. The agarase gene (dag A) of Streptomyces coelicolor A3(2): affinity purification and characterization of the cloned gene product. J Gen Microbiol. 1987 Aug;133(8):2089–2096. doi: 10.1099/00221287-133-8-2089. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daza A., Gil J. A., Vigal T., Martin J. F. Cloning and characterization of a gene of Streptomyces griseus that increases production of extracellular enzymes in several species of Streptomyces. Mol Gen Genet. 1990 Jul;222(2-3):384–392. doi: 10.1007/BF00633844. [DOI] [PubMed] [Google Scholar]
- Dehottay P., Dusart J., De Meester F., Joris B., Van Beeumen J., Erpicum T., Frère J. M., Ghuysen J. M. Nucleotide sequence of the gene encoding the Streptomyces albus G beta-lactamase precursor. Eur J Biochem. 1987 Jul 15;166(2):345–350. doi: 10.1111/j.1432-1033.1987.tb13521.x. [DOI] [PubMed] [Google Scholar]
- Eckhardt T., Strickler J., Gorniak L., Burnett W. V., Fare L. R. Characterization of the promoter, signal sequence, and amino terminus of a secreted beta-galactosidase from "Streptomyces lividans". J Bacteriol. 1987 Sep;169(9):4249–4256. doi: 10.1128/jb.169.9.4249-4256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentschev I., Hess J., Goebel W. Change in the cellular localization of alkaline phosphatase by alteration of its carboxy-terminal sequence. Mol Gen Genet. 1990 Jul;222(2-3):211–216. doi: 10.1007/BF00633820. [DOI] [PubMed] [Google Scholar]
- Hall J., Hazlewood G. P., Huskisson N. S., Durrant A. J., Gilbert H. J. Conserved serine-rich sequences in xylanase and cellulase from Pseudomonas fluorescens subspecies cellulosa: internal signal sequence and unusual protein processing. Mol Microbiol. 1989 Sep;3(9):1211–1219. doi: 10.1111/j.1365-2958.1989.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Fukusumi S., Ohshima T., Beppu T. Cloning and expression in Escherichia coli of two additional amylase genes of a strictly anaerobic thermophile, Dictyoglomus thermophilum, and their nucleotide sequences with extremely low guanine-plus-cytosine contents. Eur J Biochem. 1988 Sep 15;176(2):243–253. doi: 10.1111/j.1432-1033.1988.tb14275.x. [DOI] [PubMed] [Google Scholar]
- Hoshiko S., Makabe O., Nojiri C., Katsumata K., Satoh E., Nagaoka K. Molecular cloning and characterization of the Streptomyces hygroscopicus alpha-amylase gene. J Bacteriol. 1987 Mar;169(3):1029–1036. doi: 10.1128/jb.169.3.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Kishida H., Okanishi M. Molecular cloning of a xylanase gene from Streptomyces sp. No. 36a and its expression in streptomycetes. J Antibiot (Tokyo) 1986 Jul;39(7):985–993. doi: 10.7164/antibiotics.39.985. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kendall K., Cullum J. Cloning and expression of an extracellular-agarase from Streptomyces coelicolor A3(2) in Streptomyces lividans 66. Gene. 1984 Sep;29(3):315–321. doi: 10.1016/0378-1119(84)90060-x. [DOI] [PubMed] [Google Scholar]
- Kieser T., Melton R. E. Plasmid pIJ699, a multi-copy positive-selection vector for Streptomyces. Gene. 1988 May 15;65(1):83–91. doi: 10.1016/0378-1119(88)90419-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long C. M., Virolle M. J., Chang S. Y., Chang S., Bibb M. J. alpha-Amylase gene of Streptomyces limosus: nucleotide sequence, expression motifs, and amino acid sequence homology to mammalian and invertebrate alpha-amylases. J Bacteriol. 1987 Dec;169(12):5745–5754. doi: 10.1128/jb.169.12.5745-5754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondou F., Shareck F., Morosoli R., Kluepfel D. Cloning of the xylanase gene of Streptomyces lividans. Gene. 1986;49(3):323–329. doi: 10.1016/0378-1119(86)90368-9. [DOI] [PubMed] [Google Scholar]
- Noack D., Geuther R., Tonew M., Breitling R., Behnke D. Expression and secretion of interferon-alpha 1 by Streptomyces lividans: use of staphylokinase signals and amplification of a neo gene. Gene. 1988 Aug 15;68(1):53–62. doi: 10.1016/0378-1119(88)90598-7. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Wirth D. F., Hering C. Expression of the Streptomyces enzyme endoglycosidase H in Escherichia coli. J Biol Chem. 1981 Oct 25;256(20):10640–10644. [PubMed] [Google Scholar]
- Sen S., Oriel P. Multiple amylase genes in two strains of Bacillus stearothermophilus. Gene. 1989 Mar 15;76(1):137–144. doi: 10.1016/0378-1119(89)90015-2. [DOI] [PubMed] [Google Scholar]
- Siggens K. W. Molecular cloning and characterization of the beta-amylase gene from Bacillus circulans. Mol Microbiol. 1987 Jul;1(1):86–91. doi: 10.1111/j.1365-2958.1987.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N., Sakurai K., Sasaki T., Takekawa S., Yamagata H., Tsukagoshi N., Udaka S. A single gene directs synthesis of a precursor protein with beta- and alpha-amylase activities in Bacillus polymyxa. J Bacteriol. 1989 Jan;171(1):375–382. doi: 10.1128/jb.171.1.375-382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virolle M. J., Bibb M. J. Cloning, characterization and regulation of an alpha-amylase gene from Streptomyces limosus. Mol Microbiol. 1988 Mar;2(2):197–208. doi: 10.1111/j.1365-2958.1988.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Virolle M. J., Long C. M., Chang S., Bibb M. J. Cloning, characterisation and regulation of an alpha-amylase gene from Streptomyces venezuelae. Gene. 1988 Dec 30;74(2):321–334. doi: 10.1016/0378-1119(88)90166-7. [DOI] [PubMed] [Google Scholar]