Abstract
Background
Health concerns about the exposure to genotoxic and carcinogenic agents in the air are particularly significant for outdoor workers in less developed countries.
Aims
To investigate the association between personal exposure to a group of air pollutants and severity of DNA damage in outdoor workers from two Mexican cities.
Methods
DNA damage (Comet assay) and personal exposure to volatile organic compounds, PM2.5, and ozone were investigated in 55 outdoor and indoor workers from México City and Puebla.
Results
In México City, outdoor workers had greater DNA damage, reflected by a longer tail length, than indoor workers (median 46.8 v 30.1 μm), and a greater percentage of highly damaged cells (cells with tail length ⩾41 μm); in Puebla, outdoor and indoor workers had similar DNA damage. There were more alkali labile sites in outdoor than indoor workers. The DNA damage magnitude was positively correlated with PM2.5 and ozone exposure. Outdoor and indoor workers with ⩾60% of highly damaged cells (highly damaged workers) had significantly higher exposures to PM2.5, ozone, and some volatile organic compounds. The main factors associated with the highly damaged workers were ozone, PM2.5, and 1‐ethyl‐2‐methyl benzene exposure.
Conclusions
With this approach, the effects of some air pollutants could be correlated with biological endpoints from the Comet assay. It is suggested that the use of personal exposure assessment and biological endpoints evaluation could be an important tool to generate a more precise assessment of the associated potential health risks.
Keywords: single strand breaks, comet assay, outdoor workers, ozone, VOCs, PM2.5
The concentrations of recognised or suspected genotoxic and carcinogenic agents found in the air of large cities in developed and, in particular, developing countries have raised concerns about the potential for chronic health effects for the exposed populations. Health impacts associated with exposure to ambient air contaminants could be particularly significant for the large sectors of the population in the developing countries who work outdoors in both the formal and informal sectors.1 These individuals would be expected to experience elevated exposures resulting from their prolonged outdoor activities in close proximity to emission sources and the relatively high concentrations of air pollutants of outdoor origin. México City, a metropolitan area with approximately 20 million inhabitants, is affected by the emissions generated from the exhaust of 3.5 million vehicles that are in daily circulation. The concentrations of air pollutants have declined across México City over the past decade. However, ambient concentrations of airborne particulate matter (PM) with mass mean diameter ⩽10 μm (PM10) and of ozone (O3) remain above the corresponding recommended level,2 and continue to be elevated compared to levels typical of the urban areas of high income countries. In 2002, PM10 annual mean concentrations across the monitoring network of México City ranged from 41 to 95 μg/m3, exceeding the 50 μg/m3 annual standard, and on 3 out of 10 days PM10 concentrations were above the 150 μg/m3 24‐hour standard. During the same year, O3 annual mean concentrations ranged from 0.16 to 0.41 ppm, with levels above the 0.11 ppm hourly standard on 7 out of 10 days.2
There is very limited information on ambient concentrations or exposures for a broad range of potential genotoxic and carcinogenic air pollutants (benzene, formaldehyde, polycyclic aromatic hydrocarbons, etc). Little is known about the associated health risks posed by these contaminants to the population of México City and of other Mexican urban areas.3,4
A number of studies have described diverse health effects associated with exposure to air pollutants in México City. Borja‐Aburto and colleagues5 reported a 3.4% increase in daily mortality per 10 μg/m3 increase in PM10 concentration, while Loomis and collaborators6 reported a 6.9% increase in daily infant mortality per 10 μg/m3 increment in PM10. Associations between O3 and/or PM concentrations and decreases in children's pulmonary function, and increases in respiratory infections, school absenteeism, and asthma episodes, have been reported by several investigators.7,8
The association between exposure to carcinogenic and genotoxic environmental contaminants and genetic damage has been demonstrated with a broad range of assays and indicators such as chromosomal aberrations, sister chromatid exchange, micronuclei, gene mutations, oncogene activation and suppression, and the single cell gel electrophoresis (SCGE) assay.9,10 The SCGE or Comet assay has the advantage that almost any accessible cell population can be used.11 In addition, the application of different alkaline conditions in the assay provides insights into the mechanism of damage. At pH > 13, the assay detects DNA damage due to single strand breaks, delayed repair sites, and alkali labile sites (ALS) caused by oxidative stress, while at pH = 12.1 ALS are not detected. Thus, a difference in the degree of DNA damage detected under these two pH conditions is indicative of the presence of ALS due principally to oxidative stress. However, ALS could also be caused by other mechanisms such as exposure to DNA alkylating agents.12,13
A number of investigators have used the Comet assay to detect the presence of DNA damage potentially associated with exposure to outdoor air pollutants. Increased DNA damage in whole blood, nasal, buccal, and tear duct epithelial cells has been observed in people living and/or working in the areas of México City where O3 levels are elevated.14,15,16 These previous studies lacked information on actual or estimated personal exposures to the air contaminants of interest, so qualitative surrogates of exposure (for example, area of the city) or ambient air concentrations were used instead. In addition, air contaminants other than the criteria air pollutants were not considered.12,15 In this report, we present the results of an evaluation of the association between actual personal exposures to volatile organic compounds (VOCs), PM2.5, and the estimated exposure to O3 and the presence of DNA damage as detected by the Comet assay for a group of outdoor and indoor workers residing in México City and Puebla.
Materials and methods
Selection of cities
México City (a very large city) and Puebla (a middle sized city) were selected for inclusion in this exploratory study because of their similar geography and air pollutant concentrations. In 2002, the mean annual PM10 concentration in Puebla (60.9 μg/m3) was within the range of mean annual values measured in the monitoring network of México City (41–95 μg/m3). Mean annual concentrations of O3, SO2, and NO2 were approximately 20–30% higher in México City. No PM2.5 measurements were available for comparison between the cities.2,17
Study sample
Potential participants were approached at their workplaces. Workers interested in participating were given an informed consent letter, which they were asked to sign once any additional questions were answered. A sample of 55 male workers ranging from 18 to 60 years of age was recruited. Twenty eight were outdoor workers: 15 taxi drivers and four street vendors in México City, and nine bus drivers in Puebla. Twenty seven were indoor office workers, 20 in México City and seven in Puebla.
Survey of exposure and working conditions
Interviewers administered a questionnaire to collect personal information about the number of hours spent in different indoor and outdoor microenvironments, typical work activities, exercise during the week prior to the study, smoking status, exposure to environmental tobacco smoke, solvents and/or pesticides at their workplace and home, and a medical history. The information derived from this survey was used to explore non‐pollution related variables and factors potentially associated with DNA damage. During the personal monitoring period, the volunteers were also asked to maintain a written 24‐hour time–activity log where they were instructed to record their location (outdoors or indoors), activities, transportation (taxi, bus) and exposure to environmental tobacco smoke while at work or home every 30 minutes.
Blood sample collection and processing
Both exposure and biological monitoring were performed between April and May 2002. Peripheral blood samples (20–40 μl) were collected at the end of the exposure monitoring period by finger tip puncture with automatic disposable lancets using pre‐heparinised micro blood collectors (StatSampler, Northwood, MA, USA) and transferred into vials with RPMI‐1640 medium. The samples were maintained at room temperature to avoid coagulation; they were transported to the Toxicogenomics Laboratory at the Universidad Nacional Autónoma de México and processed the same day.
Viability of the cells was determined by a dual stained cytotoxicity assay (cFDA‐EtBr), performed on a 20 μl blood aliquot immediately after the samples arrived at the laboratory.11 The proportion of live cells in each sample was scored as a viability index. All blood samples had viability indices above 85%.
Comet assay
Samples were processed as previously described by Rojas and colleagues.18 Slides were processed in pairs at two pH conditions, pH > 13 and pH = 12.1. A pH > 13 assay detects single strand breaks, delayed repair sites, and alkali labile sites, while a pH = 12.1 assay does not detect alkali labile sites, therefore a difference of DNA damage under these two pH conditions indicates the presence of oxidative stress related ALS. Aliquots of hydrogen peroxide treated lymphocytes with a known level of DNA damage were included as internal controls for each electrophoresis session.18
The outcome measurement used in the statistical analyses of the data was the comet tail length (TL).10,18 Cells were stained with 20 μl of a 20 μg/ml ethidium bromide solution (Sigma‐Aldrich, St Louis, MO, USA). DNA damage was measured using an Olympus BMX60 epi‐fluorescence microscope and the image analysis software Komet 3.1 (Kinetic Imaging Ltd, Bromborough, UK). One hundred cells (50 cells for each of the duplicate slides) per individual sample, located in the centre of the slide, were measured, the TLs recorded, and the individual's mean value calculated.
For analysis purposes, the cells were also categorised into discrete ranges of DNA damage severity based on their TLs as has been reported elsewhere.19 Cells with TL of 0–20 μm were classified as having “low DNA damage”, those with TLs of 21–40 μm as having “medium DNA damage”, and those with TLs of ⩾41 μm as having “high DNA damage”. Workers with 60% or more cells scored in the “high DNA damage” category were classified as “highly damaged workers” (HDWs). This cut point corresponds to those individuals above the third quartile of the distribution of percentage of cells with a TL of ⩾41 μm.
The unit of statistical analysis was the individual workers; each worker's mean TL and percentage of cells in each DNA damage category were the individual's outcome metrics. For comparison among subgroups, the median TL and median percentage of cells by DNA damage category were used.
Personal exposure monitoring
Personal occupational and non‐occupational monitoring for VOCs were performed using 3500 organic vapour monitors (OVMs) (3M Company, St Paul, MN, USA), placed in the breathing zone of each worker. Personal occupational and non‐occupational exposures to PM2.5 were monitored using 37 mm Teflon filters fitted to a single stage impactor (Model PEM‐761‐203A, SKC Inc., Eighty Four, PA, USA) and personal sampling pumps (Model PCXR4, SKC Inc, Eighty Four, PA, USA. The volunteers wore the OVMs for a two day period, and the filter and pump for one day. The OVMs were extracted and analysed as described by Chung and collaborators.20 PM2.5 samples were weighed according to USEPA guidelines.21
Estimates of personal exposure to ozone
Personal occupational and non‐occupational exposures to O3 were estimated using a simple microenvironmental modelling approach, using the mean hourly O3 concentrations reported by the ambient network monitoring site nearest to each worker location during the day as reported by each worker in the personal activity log. Personal exposures to O3 while indoors or in a vehicle were estimated by multiplying the hour specific O3 concentration at the nearest network monitoring site by mean indoor to outdoor ratios reported in previous studies in México City and other cities.8,22,23
Statistical analysis
The unit of analysis was the individual, not the cells. The SPSS (SPSS Inc., Chicago, IL, USA, Version 10) and STATA (Stata Corp., College Station, TX, USA, Version 7) statistical software packages were used to analyse the data. Because of small sample sizes, and non‐normal distribution of data, non‐parametric tests were used to compare outcomes across workers' subgroups. The Mann–Whitney test was used to compare TL values by workers' occupation and city of residence and to compare the difference in DNA damage related to alkali labile sites (Diff ALS) among subgroups. The χ2 test was used to compare the frequency of cells by extent of DNA damage across worker subgroups. Spearman's correlation analysis was used to explore a relation between personal exposure to air pollutants and TL magnitude. Simple and multiple logistic regression analyses were used to identify work, lifestyle, and environmental risk factors associated with being a HDW.
All protocols and instruments used in this study were reviewed and approved by the University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects.
Results
Worker characteristics
In México City, outdoor workers (mean age = 34.5 years) were younger than indoor workers (mean age = 44.1 years); in Puebla, this relation was reversed but the outdoor and indoor workers were closer in age (mean age = 37.83 and 30.43 years, respectively). Fourteen workers in México City reported being smokers, of which eight were outdoor workers smoking an average of 2.25 (±2.31) cigarettes per day, and six were indoor workers with an average consumption of 6.33 (±4.18) cigarettes per day. In Puebla, three outdoor workers reported being smokers, smoking an average of 2 (±1.73) cigarettes per day, while all the indoor workers were non‐smokers. This level of cigarette consumption is not enough for considering them as smokers and probably explains why we did not find an association between DNA damage and cigarette consumption in this population.15
On average, the indoor workers in México City and Puebla reported being outdoors from 3.9 to 4 hours per day, while the outdoor workers stayed outside from 11.9 to 14.2 hours per day. Twenty one workers exercised outdoors at least 1 hour during the week before the study: eight outdoor and seven indoor workers in México City, and three outdoor and three indoor workers in Puebla. Ventilation in homes and office buildings was natural, with exception of one air conditioned office building in México City. Taxis and buses were also naturally ventilated and because of the season (beginning of the summer) had open windows during most of the daytime.
Comet assay at pH > 13
Overall, the outdoor and indoor workers in this study had a statistically significant difference in TL sizes (median 46.8 and 30.11 μm, respectively). Street vendors had the maximum observed TL (132.41 μm) among all worker subgroups in either city. In México City, outdoor and indoor workers had also a significant difference in TL size (median 46.89 and 27.36 μm, respectively); however, in Puebla outdoor and indoor workers had statistically similar TLs (median 46.68 and 41.44 μm, respectively), as shown in table 1.
Table 1 DNA damage; median tail length (μm) at pH > 13 by exposure group.
| City | Workers | Occupation | n | Median* | Max. |
|---|---|---|---|---|---|
| México City | Outdoor | Total | 19 | 46.89† | 132.41 |
| Street vendors | 4 | 98.88 | 132.41 | ||
| Taxi drivers | 15 | 46.57 | 61.16 | ||
| Indoor | Office workers | 20 | 27.36 | 51.19 | |
| Puebla | Outdoor | Bus drivers | 9 | 46.68 | 52.74 |
| Indoor | Office workers | 7 | 41.44 | 51.47 | |
| Total | Outdoor | 28 | 46.80‡ | 132.41 | |
| Indoor | 27 | 30.11 | 51.47 |
*Mann–Whitney test, outdoor v indoor.
†p⩽0.05; ‡p<0.001.
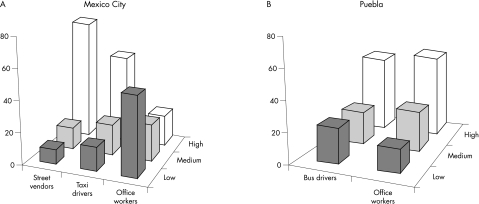
Each individual's percentage of cells by damage category (low, medium, and high DNA damage), was calculated. Overall, the outdoor workers had a higher percentage of highly DNA damaged cells than the indoor workers (68% and 20%, respectively). Figure 1 shows the percentage of cells by damage category, worker occupation, and city. In México City, the outdoor workers had more highly DNA damaged cells, while indoor workers tended to have a lower occurrence of cells in this category of damage. In particular, street vendors, taxi drivers, and office workers in México City had a statistically significant difference in the percentage of highly DNA damaged cells (77%, 57%, and 21%, respectively, χ2 p ⩽ 0.001; fig 1A). Office workers and bus drivers in Puebla had a similar percentage of highly DNA damaged cells (51% and 54%, respectively; fig 1B).
Figure 1 Percentage of cells by DNA damage category, occupation, and city. Cells with TL of 0–20 μm were classified as having “low DNA damage”, those with TLs of 21–40 μm as having “medium DNA damage”, and those with TLs of ⩾41 μm as having “high DNA damage”.
Alkali labile site related DNA damage
Table 2 shows tail length determined at pH > 13 and pH = 12.1 and the difference between them, and DNA damage that would be attributed to ALS (Diff ALS), by each subgroup. Overall, the difference between TL values at pH > 13 and pH = 12.1 was significantly higher for outdoor workers than for indoor workers. In México City, there were significant differences in the Diff. ALS values for the outdoor workers: street vendors (64.92 μm), taxi drivers (16.29 μm), and indoor workers (7.49 μm). However, in Puebla, the Diff. ALS values for outdoor workers (13.69 μm) and indoor workers (10.59 μm) were not significantly different.
Table 2 Induction of DNA damage attributed to alkali labile sites.
| City | Workers | Occupation | n | Median tail length (µm) | ||
|---|---|---|---|---|---|---|
| pH > 13* | pH = 12.1† | Diff. ALS‡ | ||||
| México City | Outdoor | Total | 19 | 46.89 | 32.22 | 14.67 |
| Street vendors | 4 | 98.88 | 34.16 | 64.92§ | ||
| Taxi drivers | 15 | 46.57 | 30.28 | 16.29¶ | ||
| Indoor | Office workers | 20 | 27.36 | 19.86 | 7.49 | |
| Puebla | Outdoor | Bus drivers | 9 | 46.68 | 32.99 | 13.69 |
| Indoor | Office workers | 6 | 41.04 | 30.55 | 10.59 | |
| Total | Outdoor | 28 | 46.80 | 31.52 | 15.28 | |
| Indoor | 26 | 30.11 | 20.41 | 9.70 | ||
*pH > 13, median TL at pH > 13.
†pH = 12.1, median TL at pH = 12.1.
‡Diff. ALS, absolute difference in TL (pH > 13 – TL pH = 12.1) attributed to ALS. Mann–Whitney test.
§Street vendors v Office workers, p ⩽ 0.002.
¶Taxi drivers v office workers, p ⩽ 0.02.
DNA damage and personal exposure to VOCs, PM2.5, and O3
Personal monitoring values observed in this study have been reported elsewhere.24 In México City, total exposures of outdoor workers median to most of a set of 30 VOCs sampled were at least 2.5‐fold higher than indoor worker exposures, showing a similar pattern as in Puebla. However, México City exposures to VOCs were up to twofold higher than Puebla's exposures. Median personal exposure to PM2.5 was also higher for the outdoors workers and comparable in both cities. The outdoor and indoor workers' total exposures to PM2.5 in México City were 133 μ/m3 and 86.6 μg/m3, respectively, while outdoor and indoor workers' exposures in Puebla were 122 μg/m3 and 78.3 μg/m3, respectively. Estimated median O3 exposures during the monitoring period for outdoor and indoor workers in México City were 28.5 ppb and 5.1 ppb, respectively. Outdoor and indoor workers' O3 exposures in Puebla were 36.1 ppb and 19.5 ppb, respectively.
Table 3 presents correlations among air pollutants and tail length at pH > 13. Tail length magnitude at pH > 13 showed a positive correlation with O3 and PM2.5 exposures. Correlation analysis by city and subgroup showed in México City that outdoor workers' PM2.5 exposure was positively correlated to TL magnitude at pH > 13 (R = 0.52, p ⩽ 0.02), while O3 exposure was barely significant (R = 0.42, p ⩽ 0.06). Neither indoor workers in México City nor outdoor and indoor workers in Puebla had any significant correlation between PM2.5 or O3 exposures and TL magnitude. Tail length magnitude and VOC exposures were not correlated at pH > 13.
Table 3 Correlations between tail length and air pollutant exposure.
| Air pollutants | Tail length* | |
|---|---|---|
| R† | p value | |
| Ozone | 0.47 | 0.00 |
| PM2.5 | 0.57 | 0.00 |
| n‐hexane | 0.12 | 0.37 |
| Methyl cyclopentane | 0.10 | 0.46 |
| Benzene | 0.09 | 0.50 |
| Ethyl benzene | 0.12 | 0.37 |
| m,p‐xylene | 0.13 | 0.36 |
| o‐xylene | 0.13 | 0.36 |
| 1,3,5‐trimethyl benzene | 0.16 | 0.25 |
| 1‐ethyl‐2‐methyl benzene | 0.16 | 0.26 |
| 1,2,4‐trimethyl benzene | 0.19 | 0.17 |
| 1,2,3‐trimethyl benzene | 0.18 | 0.18 |
*At pH > 13.
†Spearman correlation.
Exposures and factors associated with being a HDW
Risk factors for being a HDW were examined using simple logistic regression analysis. The odds ratios (ORs) for being a HDW (table 4) for those who work outdoors, work in the south area of México City, use solvents at work, and performed physical exercise the week before the study exceeded five. Exposures to the trimethyl‐benzenes and 1‐ethyl‐1,2‐methyl benzene have ORs equal to or greater than 2; exposures to O3 and PM2.5 had smaller but significant ORs. Among these workers, age ⩾40 years was as a protective factor for being a HDW.
Table 4 Simple logistic regression; odds ratios for being a HDW.
| Odds ratio | 95% CI | p value* | |
|---|---|---|---|
| Outdoor workers | 10.83 | (2.34–54.76) | ⩽0.01 |
| Exercise previous week | 9.17 | (2.21–37.14) | ⩽0.01 |
| Solvent occupational exposure | 6.80 | (1.23–36.53) | 0.02 |
| México City, south area | 5.67 | (1.11–31.54) | 0.04 |
| Age 30–39 years | 3.03 | (0.87–10.51) | 0.08 |
| Environmental tobacco smoke | 2.57 | (0.63–10.06) | 0.19 |
| 1,2,3‐trimethyl benzene | 2.38 | (1.10–5.13 ) | 0.03 |
| 1,2,4‐trimethyl benzene | 2.22 | (1.07–4.64 ) | 0.03 |
| 1‐ethyl‐1,2‐methyl benzene | 2.16 | (1.03–4.51 ) | 0.04 |
| 1,3,5‐trimethyl benzene | 2.08 | (1.01–4.25 ) | 0.05 |
| o‐xylene | 2.02 | (0.88–4.62 ) | 0.10 |
| Ethyl benzene | 1.88 | (0.89–3.95 ) | 0.10 |
| m,p‐xylene | 1.86 | (0.82–4.18 ) | 0.13 |
| Carbon tetrachloride | 1.77 | (0.90–3.46 ) | 0.09 |
| n‐decane | 1.52 | (0.94–2.42 ) | 0.08 |
| Ozone occupational | 1.05 | (1.02–1.08 ) | ⩽0.01 |
| PM2.5 occupational | 1.02 | (1.01–1.04 ) | 0.03 |
| Vitamins | 0.20 | (0.25–1.73 ) | 0.11 |
| Age ⩾40 years | 0.09 | (0.01–0.58 ) | 0.01 |
*χ2.
To identify those factors more closely associated with being a HDW, a multiple logistic regression model was developed, including factors with a p value ⩽0.2 in the simple analysis. The most parsimonious model for being a HDW included exposure to 1‐ethyl‐1,2‐methyl benzene (OR = 1.13, p ⩽ 0.03) and occupational exposure to ozone (OR = 1.06, p ⩽ 0.03) and PM2.5 (OR = 1.03, p ⩽ 0.07) as explanatory variables (model χ2 p ⩽ 0.002, Hosmer–Lemeshow test; p = 0.66, pseudo R2 = 0.25). Even though the performance of physical exercise had a high OR, as its estimate had a low precision expressed by a broad standard error band (OR = 18.92 and SE = 24.64), it was not included in the final model.
Discussion
Chronic exposure to ambient air pollutants experienced by inhabitants of large urban areas has been linked to different health outcomes (asthma, respiratory and cardiovascular diseases, mortality, etc). In this pilot study, we assessed the relation between personal exposures to ambient air pollutants and ALS DNA strand breaks. Seeking people at high risk of these effects, we recruited individuals expected to experience elevated exposures to outdoor air pollutants, such as the outdoor workers of these Mexican cities. Our results showed that these outdoor workers spent up to 300% more time outdoors than the indoor workers did. In addition, the outdoor workers were exposed to higher concentrations of air pollutants of outdoor origin. We found that consistent with the higher exposures to air pollutants of ambient origin, the outdoor workers also evidenced higher DNA damage as indicated by longer TLs overall, and a greater percent of leucocytes classified in the higher DNA damage category in comparison to the indoor workers (table 1, figs 1 and 2).
We found a significant difference in the ALS single strand breaks between pH conditions that may be related to oxidative DNA damage (table 2), a result that agrees with others and our previous results, where DNA damage correlated with outdoor O3 concentrations.14,15 There is increasing evidence that outdoor O3 and other airborne oxidants can cause DNA damage through the production of reactive oxygen species.25,26 Reactive oxygen species may lead to the presence of DNA apurinic and apyrimidic lesions evidenced as ALS, as measured by the alkaline Comet assay shown in table 2. In particular, we found a positive correlation between the level of exposure to O3 and PM2.5 and DNA damage magnitude (table 3).
Exposure to airborne particles has also been related to oxidative stress, principally shown in vitro. Bonner et al exposed human cells, mouse monocytes, and mouse fibroblasts to a suspension of PM10 particles sampled from México City air, and found an increase in DNA strand breaks.27 The same kind of approach was used by Alfaro‐Moreno et al who found that PM samples collected in the northern and central zones of México City produced longer TLs in human lymphocytes than PM samples from the southern area.28 Some authors suggest that transitional metals present in the particles can induce oxidative DNA damage directly, impair DNA repair, or cause damage mediated by inflammation as a possible mechanism of this association.29 In addition, our study found a positive correlation between PM2.5 personal exposure data and DNA single strand breaks in outdoor workers.
Main messages
There is a lack of information about the health impacts of environmental pollutants in urban outdoor workers in developing countries.
Urban outdoor workers in developing countries experienced a higher risk of DNA damage related to occupational exposures to environmental air pollutants than indoor workers in the service sector.
The positive association between DNA damage and some alkyl benzenes probably expresses a different mechanism of DNA damage of these substances than PM and O3 damage mechanisms. For example, an evaluation of the genotoxic effect of alkyl benzenes reported the presence of a mutagenic effect on S typhimurium and an increase of sister chromatid exchange.30 The independence of the alkyl benzenes effect from the O3 and PM2.5 effects is reflected in the significant association of 1‐ethyl‐1,2‐methyl benzene and tri‐methyl benzenes exposures with an increase risk of being HDW.
Surprisingly, a known genotoxic agent, benzene, was not statistically associated with the DNA single strand breaks even when outdoor workers' exposure was relatively high (25 μg/m3). This lack of association may be explained by an antagonism of benzene with toluene that also was present at high air concentrations (115.57 μg/m3).24,31
For population studies using the Comet assay, a parameter for consideration is physical activity. Moderate to high physical activity has been associated with an increase of DNA damage.9 Tsai and collaborators32 reported an increase in the urinary excretion of 8‐OHdG and greater frequency of DNA strand breaks in lymphocytes of individuals who performed exercise at moderate intensity. In this study, the increased oxygen and air pollutant intake due to physical exercise was a risk factor for being a HDW. However, our instrument limitations prevented a more precise estimation of this exposure magnitude, causing a considerable SE in its risk estimate (table 4).
Smoking was not associated in this study with the observed DNA damage. This result may be due to low cigarette consumption among these workers (2–6 cigarettes per day). That result is in agreement with other reports of lack of association between smoking and the DNA damage in leucocytes as detected by the Comet assay.33,34 A recent study analysing whole blood cells and our group's previous report using buccal cells found an association between smoking and DNA damage in individuals with a consumption of 10 or more cigarettes per day, but not at lower consumptions.15,35
In the univariate analysis, being over 40 years old appears to be a protective factor, which is not in agreement with previous reports.36 However, age was not significant when controlling for the other variables in the multiple logistic regression analysis.
It is not possible to perform a direct comparison across similar studies of DNA damage in populations exposed to air pollutants because of differences in population characteristics, agents analysed, exposure metrics, and differences in some of the Comet assay parameters. Even so, it is possible to develop a relative index of DNA damage that permits a qualitative comparison across studies. Tail length ratios for exposed versus non‐exposed groups reported by other investigations and this study were estimated (table 5). Ratios greater than 1 indicate a higher DNA damage in the exposed group compared with the less exposed or control group. The ratios found in the present study were comparable in magnitude to those estimated from data reported by previous occupational studies,26,37,38,39,40 and similar to the ratios for a non‐occupationally exposed group in México City studied previously by our group.16 The ratio for street vendors was greater than those seen in other studies, and comparable to the ratio of DNA damage estimated from data reported by Calderón‐Garcidueñas and colleagues14 for a group of security guards in México City who were highly exposed to ambient air pollutants during their work shift.
Table 5 Comparison with results of previous studies.
| Exposure | TL ratio* | |
|---|---|---|
| Other studies | ||
| Carriere et al37 | Air pollutants | 1.27 |
| Cheng et al40 | Ozone | 1.11 |
| Glück and Gebbers38 | Cigarette smoke | 1.89 |
| Lam et al39 | Benzene | 1.39 |
| Pitarque et al40 | Fuel | 1.15 |
| México City | ||
| Calderón et al14 | Air pollutants | 5.38 |
| Rojas et al16 | Air pollutants | 1.30 |
| Valverde et al15 | Gasoline and vehicle emissions | |
| This study | Air pollutants | 1.20 |
| Vendors v office | 3.61 | |
| Taxi v office | 1.77 |
*Tail length ratio = TL of exposed/TL of non‐exposed or control subjects.
Policy implications
Exposure mitigation programmes should be developed for outdoor workers exposed to high levels of ambient air pollutants.
Evaluation of biological endpoints and personal exposure assessment together help to indicate the most affected groups.
This approach could be an important tool to generate a more precise assessment of the associated potential health risks.
In the urban areas of many developing countries, there are large numbers of workers who perform activities under similar conditions to those found in Mexican cities. The potential health impact of these environmental exposures for these workers can be significant. Compared to previous reports this pilot study results offer evidence of the role that concurrent exposures to other pollutants besides O3, such as PM2.5 and VOCs present in urban air may play in the generation of DNA damage in highly exposed individuals. This pilot study approach suggests that the combined use of personal exposure and biological endpoints evaluation is an important tool to generate a more precise assessment of the air pollution potential health risks.
Acknowledgements
This project was supported by grants CDC/NIOSH T42/CCT610417 and NIH/Fogarty International Center 5 D43TW00644. The authors also acknowledge the contribution of Ernesto Soto, Maria del Carmen López, Masoud Afshar, and Francisco Mandujano in the preparation and analysis of the samples.
Abbreviations
ALS - alkali labile sites
DNA - deoxyribonucleic acid
OVMs - organic vapour monitors
OR - odds ratio
O3 - ozone
PM - particulate matter
PM2.5 - particulate matter with mass mean diameter ⩽2.5 μm
PM10 - particulate matter with mass mean diameter ⩽10 μm
SCGE - single cell gel electrophoresis
TL - tail length
VOCs - volatile organic compounds
Footnotes
Competing interests: none
References
- 1.Delclos G, Betancourt O, Marquez F.et al Globalización y salud laboral. Arch Prev Riesgos Labo 200363–8. [Google Scholar]
- 2.Secretaria de Medio Ambiente (Environment Department) Informe del Estado de la Calidad del Aire. Tendencias 2002 para la Zona Metropolitana del Valle de México. México: Gobierno del Distrito Federal, 2003
- 3.Ortiz E, Alemon E, Romero D.et al Personal exposure to benzene, toluene and xylene in different microenvironments at the México City metropolitan zone. Sci Total Environ 2002287241–248. [DOI] [PubMed] [Google Scholar]
- 4.Bravo H, Sosa R, Sanchez P.et al Concentrations of benzene and toluene in the atmosphere of the Southwestern area at the México City Metropolitan Zone. Atmos Environ 2002363843–3849. [Google Scholar]
- 5.Borja‐Aburto V H, Castillejos M, Gold D R.et al Mortality and ambient fine particles in southwest México City, 1993–1995. Environ Health Perspect 1998106849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loomis D, Castillejos M, Gold D R.et al Air pollution and infant mortality in México City. Epidemiology 199910118–123. [PubMed] [Google Scholar]
- 7.Romieu I, Meneses F, Ruiz S.et al Effects of intermittent ozone exposure on peak expiratory flow and respiratory symptoms among asthmatic children in México City. Arch Environ Health 199752368–376. [DOI] [PubMed] [Google Scholar]
- 8.Gold D R, Allen G, Damokosh A.et al Comparison of outdoor and classroom ozone exposures for school children in México City. J Air Waste Manage Assoc 199646335–342. [PubMed] [Google Scholar]
- 9.Hartmann A, Fender H, Speit G. Comparative biomonitoring study of workers at a waste disposal site using cytogenetic tests and the comet (single‐cell gel) assay. Environ Mol Mutagen 19983217–24. [DOI] [PubMed] [Google Scholar]
- 10.Albertini R J, Anderson D, Douglas G R.et al IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res 2000463111–172. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann A, Agurell E, Beevers C.et al Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis 20031845–51. [DOI] [PubMed] [Google Scholar]
- 12.Rojas E, Valverde M, Kala S V.et al Accumulation of DNA damage in the organs of mice deficient in gamma‐glutamyltranspeptidase. Mutat Res 2000447305–316. [DOI] [PubMed] [Google Scholar]
- 13.Anderson D, Plewa M J. The International Comet Assay Workshop. Mutagenesis 19981367–73. [DOI] [PubMed] [Google Scholar]
- 14.Calderon‐Garcidueñas L, Osnaya‐Brizuela N, Ramirez‐Martinez L.et al DNA strand breaks in human nasal respiratory epithelium are induced upon exposure to urban pollution. Environ Health Perspect 1996104160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valverde M, Lopez M D C, Lopez I.et al DNA damage in leukocytes and buccal and nasal epithelial cells of individuals exposed to air pollution in México City. Environ Mol Mutagen 199730147–152. [PubMed] [Google Scholar]
- 16.Rojas E, Valverde M, Lopez M C.et al Evaluation of DNA damage in exfoliated tear duct epithelial cells from individuals exposed to air pollution assessed by single cell gel electrophoresis assay. Mutat Res Genet Toxicol Environ Mutagen 200046811–17. [DOI] [PubMed] [Google Scholar]
- 17.SEDURBECOP Reporte de Contaminantes en la Ciudad de Puebla. 2002. Puebla: Gobierno del Estado de Puebla, 2003
- 18.Rojas E, Lopez M C, Valverde M. Single cell gel electrophoresis assay: methodology and applications. J Chromatogr B 1999722225–254. [DOI] [PubMed] [Google Scholar]
- 19.Altamirano‐Lozano M, Valverde M, Alvarez‐Barrera L.et al Genotoxic studies of vanadium pentoxide (V2O5) in male mice, II. Effects in several mouse tissues. Teratog Carcinog Mutagen 199919243–255. [DOI] [PubMed] [Google Scholar]
- 20.Chung C W, Morandi M T, Stock T H.et al Evaluation of a passive sampler for volatile organic compounds at ppb concentrations, varying temperatures, and humidities with 24‐h exposures. 1. Description and characterization of exposure chamber system. Environ Sci Technol 1999333661–3665. [Google Scholar]
- 21.CENICA Preparacion y pesaje de filtros en monitoreo atmosférico de bajo volumen. Método de referencia CENICA‐PT‐APF‐01 (Preparation and weighing of filters for low volume atmospheric monitoring). México City: Centro Nacional de Investigación y Capacitación Ambiental‐INE, 2001
- 22.Chao C Y H. Comparison between indoor and outdoor air contaminant levels in residential buildings from passive sampler study. Build Environ 200136999–1007. [Google Scholar]
- 23.Riediker M, Williams R, Devlin R.et al Exposure to particulate matter, volatile organic compounds, and other air pollutants inside patrol cars. Environ Sci Technol 2003372084–2093. [DOI] [PubMed] [Google Scholar]
- 24.Tovalin H, Morandi M T, Rojas E.et al The association between exposures to VOCs and PM2.5 and indicators of DNA damage among outdoor workers. Proceedings of the 13th Annual Conference of the International Society of Exposure Analysis. Stresa, Italy, 21–25 September 2003361
- 25.Bornholdt J, Dybdahl M, Vogel U.et al Inhalation of ozone induces DNA strand breaks and inflammation in mice. Mutat Res Genet Toxicol Environ Mutagen 200252063–72. [DOI] [PubMed] [Google Scholar]
- 26.Cheng P, Yao J K. A systematic evaluation of oxidative stress indices in biological samples. Schizophr Res 20036061 [Google Scholar]
- 27.Bonner J C, Rice A B, Lindroos P M.et al Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from México City. Am J Respir Cell Mol Biol 199819672–680. [DOI] [PubMed] [Google Scholar]
- 28.Alfaro‐Moreno E, Martinez L, Garcia‐Cuellar C.et al Biologic effects induced in vitro by PM10 from three different zones of México City. Environ Health Perspect 2002110715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prahalad A K, Inmon J, Dailey L A.et al Air pollution particles mediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem Res Toxicol 200114879–887. [DOI] [PubMed] [Google Scholar]
- 30.Spiechowicz J, Wyszynska E, Dziubaltowska K. Genotoxicity evaluation of trimethylbenzenes. Mutat Res 1998412299–305. [DOI] [PubMed] [Google Scholar]
- 31.Andreoli C, Leopardi P, Crebelli R. Detection of DNA damage in human lymphocytes by alkaline single cell gel electrophoresis after exposure to benzene or benzene metabolites. Mutat Res 199737795–104. [DOI] [PubMed] [Google Scholar]
- 32.Tsai K, Hsu T G, Hsu K M.et al Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med 2001311465–1472. [DOI] [PubMed] [Google Scholar]
- 33.Speit G, Witton‐Davies T, Heepchantree W.et al Investigations on the effect of cigarette smoking in the comet assay. Mutat Res 200354233–42. [DOI] [PubMed] [Google Scholar]
- 34.Hininger I, Chollat‐Namy A, Sauvaigo S.et al Assessment of DNA damage by comet assay on frozen total blood: method and evaluation in smokers and non‐smokers. Mutat Res 200455875–80. [DOI] [PubMed] [Google Scholar]
- 35.Ellahueñe M F, Pérez‐Alzola L P, Farfán‐Urzue M.et al Preliminary evaluation of DNA damage related with the smoking habit measured by the Comet assay in whole blood cells. Cancer Epidemiol Biomarkers Prev 2004131223–1229. [PubMed] [Google Scholar]
- 36.Singh N P, Danner D B, Tice R R.et al Basal DNA damage in individual human‐lymphocytes with age. Mutat Res 19912561–6. [DOI] [PubMed] [Google Scholar]
- 37.Carrier P, Maroni M, Alcini D.et al Assessment through environmental and biological measurements of total daily exposure to volatile organic compounds of office workers in Milan, Italy. Indoor Air 200010258–268. [DOI] [PubMed] [Google Scholar]
- 38.Gluck U, Gebbers J O. The comet assay of nasal epithelia: measurement of DNA damage for the assessment of genotoxic air pollution. Laryngoscope 2000110123–125. [DOI] [PubMed] [Google Scholar]
- 39.Lam T H, Zhu C Q, Jiang C Q. Lymphocyte DNA damage in elevator manufacturing workers in Guangzhou, China. Mutat Res 2002515147–157. [DOI] [PubMed] [Google Scholar]
- 40.Pitarque M, Vaglenov A, Nosko M.et al Evaluation of DNA damage by the Comet assay in shoe workers exposed to toluene and other organic solvents. Mutat Res 1999441115–127. [DOI] [PubMed] [Google Scholar]