Abstract
Objectives
To evaluate lifetime exposure to trihalomethanes (THM) through ingestion, inhalation, and dermal absorption in a hospital based case‐control study of bladder cancer conducted between 1998 and 2001 in five areas of Spain. The study base was comprised of subjects living in the catchment areas of the participating hospitals.
Methods
Individual information on water related habits was obtained from personal interviews of 1219 cases and 1271 controls: residential and occupational history, drinking water source at each residence and job, amount of water consumption, frequency and duration of showering, bathing, and swimming pool attendance. THM levels, water source history, and year when chlorination started in study areas were ascertained through measurements in drinking water samples and questionnaires to water companies and local authorities. Estimates of THM levels covered 79% of the subjects' person‐years of exposure.
Results
Current and historical average THM levels in water were correlated. Control subjects reported that drinking water source in the last residence was municipal for 63%, bottled for 22%, private well for 2%, and other sources for 13%. For the time window between age 15 and the time of interview, average residential THM level was 32.2 μg/l. THM exposure through ingestion was 23.7 μg/day on average, and was correlated with the ingestion THM level in the workplace. Overall, 79% usually took showers, 16% usually took baths, and 13% had ever attended a swimming pool. Between 21% and 45% of controls unexposed to THM through ingestion were evaluated as moderately or highly exposed through showering or bathing, and 5–10% were exposed through swimming in pools.
Conclusion
The importance of evaluating different routes is underscored by findings from experimental studies showing substantial differences in THM uptake and internal distribution by route.
Keywords: disinfection by‐products, trihalomethanes, exposure assessment, exposure routes, case‐control study
Disinfection by‐products (DBP) have been associated with elevated risk of several types of cancer, with evidence most consistent for the urinary bladder. This is particularly true among studies that incorporated a detailed evaluation of exposure to trihalomethanes (THM, the most prevalent group of chlorination by‐products) as an index of exposure to DBP.1,2,3,4 Relative risks in these studies were generally below 2.0, and despite the consistency of the evidence, concerns remain about the validity of these findings, particularly regarding the evaluation of exposure.
DBP constitute a complex mixture of hundreds of compounds with heterogeneous physical and chemical properties, different mutagenic and carcinogenic potential, and with temporal and spatial variability.5,6 Different exposure pathways (ingestion, inhalation, dermal absorption) depend on the type of DBP and the exposure setting, such as drinking the water or beverage made up with water, showering, bathing, swimming in pools, and to a lesser extent other water related activities such as washing dishes, cooking, and washing children.7,8,9,10 Recent studies show that inhalation and dermal absorption are important routes of population exposure to THMs.6,11,12 In a case‐control study of bladder cancer in Spain, we evaluated lifetime exposure to THM through ingestion, inhalation, and dermal absorption among participants. We describe here the methods used to assess exposure, and evaluate how different exposure routes affect estimated markers of individual THM levels.
Methods
We conducted a multicentre case‐control study of bladder cancer from 1998 to 2001 in Spain. Subjects were identified in 18 hospitals from five areas: Asturias, Alicante, Tenerife, Barcelona, and Vallès (including Sabadell and Manresa cities). Cases were patients aged 20–80 diagnosed with primary bladder cancer who lived in the catchment area of the participating hospitals. Controls were hospital patients with diagnoses considered to be unrelated to the known risk factors for bladder cancer. They were matched individually 1:1 to cases by gender, age group (5‐year strata), and residence area. Control patients were admitted to hospitals for the following reasons: 37% hernias, 11% other abdominal surgery, 23% fractures, 7% other orthopaedic problems, 12% hydrocoele, 4% circulatory disorders, 2% dermatological disorders, 1% ophthalmological disorders, and 3% other diseases. Response rates were 84% for cases and 88% for controls, with 1219 cases and 1271 controls included in the study. The study was approved by the ethics committees from all the participating centres (universities and research institutes), and all participants provided informed and written consent.
Estimation of past THM levels
Questionnaires were sent to approximately 200 water utilities and local authorities requesting current and historical information: proportion of ground/surface source over the years, type of disinfectant, annual average THM levels in treated water since the late 1970s (total and chloroform, bromodichloromethane, dibromochloromethane, and bromoform), annual average level of organic matter, pH and temperature in raw water, chlorine dose since 1950, and year when chlorination started. The questionnaire is available on the OEM website (http://www.occenvmed.com/supplemental). The data collected differed widely among municipalities. Water source history and the year chlorination was initiated were available for 123 municipalities, accounting for 79% of the person‐years accumulated during the lifetime residential history of the study population. Among these, 48 municipalities had additional data on THM levels. Six medium size municipalities (0.9% of the person‐years) completed the questionnaire but did not provide information on water source history and/or year of initiation of chlorination. Data were not available from 634 very small study municipalities. Chlorine was the most widely used disinfectant for drinking water. The number of available THM measurements from each municipality ranged from less than 10 to more than 100. We collected 113 tap water samples from the study areas in 1999 to measure specific THMs.13 THM measurements collected from questionnaires were grouped by study area and we calculated the average. The Pearson correlation coefficient between average current THM levels from our measurements and the past average THM levels by area, was calculated.
We used data on THM levels, water source history (proportion of ground/surface over the years), and the year chlorination was initiated to estimate current and past THM levels. Under the assumption of constant THM level for a constant water source by municipality, historical THM levels were estimated. For each water purveyor, the average of available THM levels in recent years was calculated and back extrapolated (approximately back to year 1920). If the water source changed, the proportion of surface water was used as a weight to this average. THM level before chlorination started was assumed to be zero. The year when chlorination started varied widely among study municipalities, from 1933 in Barcelona to the 1990s in many small municipalities in Asturias. For municipalities using only ground water in the past, THM estimates were based on those of nearby municipalities currently using ground water with available THM data. Estimation of past THM levels in Barcelona was done at the zip code level, since the city is supplied by two rivers with distinct raw water characteristics.
Individual data
Trained interviewers administered a computer assisted personal interview (CAPI) to subjects in the hospitals (see http://www.imim.es/epicuro/epicuro%20questionnaires.htm). Among respondents, subjects who refused to answer the CAPI were administered a reduced interview of critical items (20%) that did not include all questions on water related variables. Relevant information for the assessment of exposure to DBP was:
Residential history from birth. All residences during at least one year: year started, year stopped, full street address, city, province, region, and country. Full address was used to ascertain zip code in Barcelona.
Occupational history from age 16. All jobs worked at least during six consecutive months: year started, year stopped, city, province, region, and country.
Water source at each residence and job. The actual question as asked (translated from Spanish) was: “What (is/was) the primary source of drinking water (where you live/when you lived there)?” Five possible responses: municipal water supply/private well/bottled/other (specify)/don't know. For the occupational history, the question was the same, referring to job instead of residence.
Average daily water consumption (litres/day), including water and water based beverages, from a food frequency questionnaire.
Frequency, duration, and water temperature (hot/cold/both) of bathing/showering. The actual questions were: (1) “How did you usually bathe? Did you generally take a bath or did you use a shower?” Five possible responses: shower/bath/both/washbowl/don't know. (2) “How often did you take a shower/bath?” Response: no. times per day/week/month/year. “How long, on average, did it last?” (3) “Did you use hot water, cold water, or warm water (mixing both hot and cold)?”.
Frequency, duration, and location (indoor, outdoor) of swimming pool attendance. The actual questions were: (1) “During your adult life, did you ever go to a swimming pool for a swim?” (2) “How many times a year did you go to a swimming pool for a swim?” (3) “What year/age did you start going to a swimming pool?” (4) “What year/age did you stop going to a swimming pool?” (5) “When you went to the swimming pool, for how long did you usually remain in the water?” (6) “Was it generally an indoor or an outdoor swimming pool?”.
Individual THM exposure indices
Four indices of exposure to DBP were calculated for the period between age 15 and the time of interview. This time window is long enough to capture an aetiologically meaningful period and minimised the proportion of missing exposure data in the population, since the typical missing periods corresponded to early years in life. Fifty one per cent of study subjects had a known ingestion THM level for the whole exposure window. Analyses included only subjects with at least 70% of years with an estimated THM level. Subjects with less than 70% known THM exposure comprised subjects with incomplete residential histories (including those interviewed through an abbreviated critical items interview), unknown drinking water source, or missing THM data in the municipalities where they lived.2,4
Residential THM exposure. Time weighted average THM level in water supplied to residences (μg/l).
Ingestion THM level (μg/day). Exposure to THM through drinking the water at home or work. A null THM level was attributed to subjects who reported drinking bottled water, water from a private well, or other non‐municipal source (for example, spring). The questionnaire did not distinguish between type of bottled water and we assumed a null THM level for any bottled water consumption. Residential THM levels (μg/l) were multiplied by amount of daily water consumption (l/day). Average ingestion THM level at the job was calculated separately following the same procedure.
THM exposure from bathing and showering (μg/l*min/day). Exposure through dermal absorption and inhalation while showering and bathing in the home. Residential THM levels (μg/l) were multiplied by frequency and average duration of bathing or showering per day (min/day).
Exposure to DBP from swimming in pools. Exposure to DBP through dermal absorption and inhalation (and to a lesser extent ingestion) from swimming in pools. Lifetime duration of attendance calculated by combining frequency of attendance and reported average time in the water.
Twenty two per cent of respondents reported use of bottled water at their last residence. We evaluated through a sensitivity analysis whether changes of source of drinking water within each residence would produce significant errors in our estimates of ingested THMs. This could occur since subjects had few lifetime residences and we did not record changes of water source within each residence. We used two alternative scenarios. For subjects whose last residence or next to last residence was longer than 10 years and reported drinking bottled water, we assumed municipal water consumption (1) before 1980 and (2) before 1990.
Results
THM levels in drinking water started to be measured in Barcelona in 1979, while in most other regions routine analysis started after 1990. Average past THM levels from utility records were correlated with average THM levels from our measurements in 1999 (Pearson correlation coefficient, 0.87). Among controls, the average duration at a given residence was 19 years (SD 17, range 1–81, centiles 25, 50, 75: 5, 15, 27). Mean duration at the last residence was 30 years (SD 19, range 1–82, centiles 25, 50, 75: 16, 28, 48). The mean number of residences per person was 3.1 (SD 2.0, range 1–20, centiles 25, 50, 75: 1, 3, 4). The average duration at the longest place of residence was 35 years (SD 16, range 1–81, centiles 25, 50, 75: 25, 32, 43). Municipal water was the drinking water source at the last residence for 63% of the controls; private well water, 2%; bottled water, 22%; and water from other sources, such as springs, rain water, streams, 13%. The comparable distribution in 1960 was 59%, 7%, 7%, and 27% respectively. The distribution of water sources differed by area.
Showering was common for 79% of control subjects, 16% usually took a bath, 4% took a shower or a bath, and 1% usually washed using a washbowl. Ever swimming in a pool was reported by 13% of control subjects. A total of 70% attended outdoor, 17% indoor, and 13% both types of swimming pools. Average frequency of swimming pool attendance was 5 times per month (SD 7, range: <1 to 30), and average swimming duration was 30 minutes (SD 30, range: 1 minute to 3 hours). Lifetime cumulative duration of swimming was on average 24.5 days (SD 30.0, range 0.1–92) for those attending indoor swimming pools and 17.2 days (SD 32.9, range 0.01–188) for outdoor pools.
The average residential THM level, 32.2 μg/l (Table 1), differed by area. Levels were highest among subjects living in Alicante at the time of interview (75.1 μg/l) and lowest in Tenerife (4.9 μg/l). Chloroform was the most prevalent THM (13.8 μg/l), followed by bromodichloromethane (9.0 μg/l). The proportion of the four THMs differed by area.13
Table 1 Description of the study control population (n = 1271).
| no. (%) | |
|---|---|
| Gender | |
| Men | 1105 (86.9%) |
| Women | 166 (13.1%) |
| Age (years) | |
| Mean (SD) | 65 (10) |
| Range | 19–88 |
| Centiles 25, 50, 75 | 59, 67, 72 |
| Area | |
| Barcelona | 247 (19.4%) |
| Vallès | 190 (14.9%) |
| Alicante | 84 (6.6%) |
| Tenerife | 226 (17.8%) |
| Asturias | 524 (41.3%) |
| Average residential THM (μg/l) | |
| Mean (SD) | 32.2 (28.1) |
| Range | 0–142.8 |
| Average ingestion THM in the home (μg/day) | |
| Mean (SD) | 23.7 (33.8) |
| Range | 0–243.3 |
| Duration of shower/bath × average residential THM (min/day)*(μg/l) | |
| Mean (SD) | 296.3 (532.4) |
| Range | 0–8062.4 |
Table 2 shows a cross classification of the subjects by different exposure indices, grouped in quartiles. Among subjects considered as unexposed to THM through ingestion in the household (for example, subjects living in the area of Barcelona but consuming bottled water), 46% were classified as medium–highly exposed through showering or bathing, and 10% were classified as exposed through swimming in pools. Most subjects (80%) highly exposed through ingestion in the home were also evaluated as medium–highly exposed through baths or showers. Agreement rate between quartiles of ingested THM and THM exposure through showering and bathing was 32.2%.
Table 2 Number of subjects (controls) by level of exposure to different indices of exposure to THM*.
| Level of exposure | Water consumption at home (ingestion), μg/day | ||||
|---|---|---|---|---|---|
| Unexposed | Low | Medium | High | Total | |
| 0 | >0–10.0 | >10.0–35.0 | >35.0 | ||
| Bathing/showering (dermal absorption/inhalation), μg/l*min/day | |||||
| Low exposed (<50) | 29 (6%) | 56 (12%) | 13 (3%) | 5 (1%) | 103 (22%) |
| Medium–low (50–<167) | 27 (6%) | 41 (9%) | 51 (11%) | 17 (4%) | 136 (30%) |
| Medium–high (167–<333) | 17 (4%) | 17 (4%) | 25 (5%) | 39 (8%) | 98 (21%) |
| High (⩾333) | 32 (7%) | 11 (2%) | 27 (6%) | 53 (12%) | 123 (27%) |
| Total | 105 (23%) | 125 (27%) | 116 (25%) | 114 (25%) | 460 (100%) |
| Swimming pool attendance (dermal absorption/inhalation)† | |||||
| Never | 128 (23%) | 125 (23%) | 121 (22%) | 100 (18%) | 474 (87%) |
| Ever | 14 (3%) | 16 (3%) | 16 (3%) | 26 (5%) | 72 (13%) |
| Total | 142 (26%) | 141 (26%) | 137 (25%) | 126 (23%) | 546 (100%) |
*Analysis limited to controls with ⩾70% of known ingestion and residential THM exposure. Ingestion and bathing/showering THM exposure metrics are the average of the exposure window from age 15 until the time of interview. THM exposure categories are defined by quartiles.
†Missing information on swimming pools for 268 subjects.
The prevalence of bottled water consumption among controls that left their residence in the 1990s, 1980s 1970s, and 1960s, was 22%, 13%, 9%, and 4%, respectively. These proportions were higher, however, in those areas with the highest THM levels. In the alternative scenario which assumed that bottled water consumption occurred only after 1990, almost half the subjects classified as non‐exposed on the basis of their report in the questionnaire, were re‐categorised as moderately or highly exposed to THM by ingestion. In this instance, 21% of persons unexposed to ingested THM were classified as medium–highly exposed via bathing/showering. The 1980 scenario suggested lower rates of misclassification. This type of misclassification would not affect subjects highly exposed to THM through ingestion.
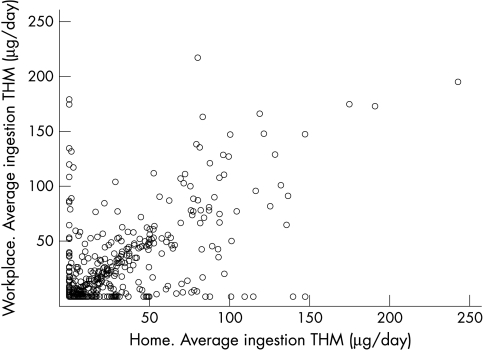
Average ingestion THM exposures at home and at the workplace were correlated with a Pearson correlation coefficient of 0.61 (based on 524 control subjects) (fig 1). Correlation restricted to subjects who never drank bottled water was 0.74 (based on 304 control subjects).
Figure 1 Correlation between work and home ingestion THM levels (μg/day).
Discussion
We evaluated long term exposure to DBP using different exposure indices in a population with a relatively stable residential history and showed that the level of individual exposure to these compounds depends on the route of exposure.
Experimental research has shown different uptake and biological effects of DBP by route of exposure.14 Assumption that ingestion is the main exposure route could lead to biased exposure estimates and may distort results in epidemiological studies. A modelling study indicated that swimming had a large impact on an individual's level of chloroform uptake, compared to bathing, showering, or drinking water.11 The ingestion route likely dominates for non‐volatile DBP (for example, haloacetic acids, MX). However, since the putative agent(s) for the increased cancer risk is unknown, the evaluation of different exposure pathways can provide clues for the identification of the relevant chemicals and possible mechanisms of action.
We evaluated ingestion THM exposure at workplaces and in homes. The correlation between the two was moderate, indicating that considering only home exposure could introduce some misclassification in the overall ingestion estimate. This measurement error is probably non‐differential with regard to case/control status and would attenuate the magnitude of risk estimates. Similarly, the fact that bottled water use was related to high THM levels in public water supplies likely did not introduce differential misclassification.
The retrospective evaluation of lifetime exposure depends on assumptions in the estimation of past THM levels. Some of these assumptions are unlikely to introduce any major misclassification; for example, the distinction between no/low exposed and medium/high exposed subjects. The source of water (surface or ground) and the application of chlorine are useful predictors of DBP levels and can help distinguish between exposure groups.13 The assumption that THM level over the years remained constant in a municipality so long as water source remained the same, may have introduced some misclassification because many factors influence THM levels apart from source and chlorination status: season, changes in raw water parameters, treatment, etc. We selected municipality as the minimum geographical unit with homogeneous water characteristics. Although seasonal variability is relevant for certain diseases with a short exposure window (for example, pregnancy outcomes), it becomes a minor source of exposure variability for diseases with a long latency period (such as cancer), and annual averages are reasonable exposure markers. Data from the area of Barcelona covering 25 years indicate a significant annual variation but no evident long term trend in levels of THM until the time the study was conducted.15 The selected exposure window (from age 15 until the time of interview) has covered on average 50 years of study subjects (mean age at interview was 65). This time window probably captured the relevant period of exposure, defined to be at least 25 years before diagnosis.4
A concern in developing the ingestion exposure index was the assumption that the source of drinking water remained constant for a given residence, since we did not ask about changes of water source within each residence. This problem is a limitation of our study, and stresses the need for a more detailed record of source of drinking water at each residence, particularly in populations, such as this, with long average residence periods. Data on filter use was not collected. Although it could potentially be a source of bias, it is not probably a major one since filtration of tap water is not a common practice in Spain, particularly in the past. Although some bottled waters might be prepared from tap water with additional sophisticated treatment, THM levels are probably low and it probably has not introduced a significant bias in the ingestion THM estimate.
Controls were matched to cases by age group, gender, and residence area. Since residence area was related to THM exposure, but not to disease status, matching on this factor probably reduced statistical efficiency (widening confidence intervals) but did not introduce bias in the estimation of risks.16
Disinfection by‐products are a complex mixture of compounds. Composition depends on factors such as water source, raw water characteristics, and water treatment. THM are prevalent and ubiquitous by‐products of chlorination. They are the DBP regularly analysed by water authorities in Spain, where chlorine has been the most commonly used disinfectant for drinking water. As other disinfectants such as chlorine dioxide, ozone, and chloramines are substituted for chlorine, non‐THM DBP may become more prevalent, and in the future, THM levels may not be adequate surrogates of exposure to DBP.
Main messages
Exposure to trihalomethanes (THMs) and other disinfection by‐products (DBPs) may occur through ingestion, inhalation, and dermal absorption during any water related activity, such as water consumption, showering, bathing, swimming pool attendance, etc.
Evaluation of one single exposure route or situation (e.g. ingestion) may lead to misclassification of the total THM exposure.
Epidemiological studies should collect individual data on different exposure habits, in addition to extensive data on DBP levels in water, to assess total DBP exposure.
In conclusion, an extensive collection of data on THM levels and of lifetime personal information on water related habits allowed us to estimate the average exposure to THM over the lengthy exposure window of aetiological relevance for cancer. The evaluation of different routes and situations of exposure is important when evaluating exposure to THM since route of exposure has been associated in experimental studies with substantial differences in uptake and internal distribution of these compounds.
Supplementary Material
Acknowledgements
We thank Natalia Blanco and Marta Huguet for her help with the collection of THM data from municipalities, and the technical officers in the municipalities and treatment plants who completed the questionnaires. Thanks are extended to F Fernández, C Murta, R Jaramillo, A Amorós, J Lloreta, S Hernández, A Gelabert, J Carles, L Cecchini, JM Saladié, L Ibarz, M Nadal, M Céspedes, D García, J Pujadas, R Hernando, E Martínez, A Cabezuelo, M Nogué, M Domènech, J Badal, J Malet, P Hernández, J Rodriguez de Vera, AI Martín, F Taño, Galbis, F Cáceres, F García‐López, M Ull, A Teruel, E Andrada, A Bustos, A Castillejo, A Menéndez, JL Guate, JM Lanzas, J Velasco, JM Fernández, JJ Rodríguez, A Herrero, R Abascal, C Manzano, T Miralles, M Rivas, M. Argüelles, M Díaz, J Sánchez, O Diaz, A Mateos, V Frade, P Muntañola, C Pravia, AM Huescar, F Huergo, and J Mosquera for participating in the study at different levels.
Abbreviations
DBP - disinfection by‐products
THM - trihalomethane
Footnotes
Funding: This project was funded by the Spanish Ministry of Health (FIS 2001–2002), the EPICUR‐red (ISIII‐GO3/174), the Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics (NCI Contract No. NO2‐CP‐11015), and the European Union (Environment and genetic factors in bladder cancer: a multicentric case‐control study in Europe. BIOMED. 1998–2001).
Competing interests: none
References
- 1.King W D, Marrett L D. Case‐control study of bladder cancer and chlorination by‐ products in treated water (Ontario, Canada). Cancer Causes Control 19967596–604. [DOI] [PubMed] [Google Scholar]
- 2.Cantor K P, Lynch C F, Hildesheim M E.et al Drinking water source and chlorination byproducts. I. Risk of bladder cancer. Epidemiology 1998921–28. [PubMed] [Google Scholar]
- 3.Doyle T J, Zheng W, Cerhan J R.et al The association of drinking water source and chlorination by‐products with cancer incidence among postmenopausal women in Iowa: a prospective cohort study. Am J Public Health 1997871168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanueva C M, Cantor K P, Cordier S.et al Disinfection byproducts and bladder cancer. A pooled analysis. Epidemiology 200415357–367. [DOI] [PubMed] [Google Scholar]
- 5.Symanski E, Savitz D A, Singer P C. Assessing spatial fluctuations, temporal variability, and measurement error in estimated levels of disinfection by‐products in tap water: implications for exposure assessment. Occup Environ Med 20046165–72. [PMC free article] [PubMed] [Google Scholar]
- 6.King W D, Dodds L, Armson B A.et al Exposure assessment in epidemiologic studies of adverse pregnancy outcomes and disinfection byproducts. J Expo Anal Environ Epidemiol 200414466–472. [DOI] [PubMed] [Google Scholar]
- 7.Lin F L, Hoang S W. Inhalation exposure to THMs from drinking water in south Taiwan. Sci Total Environ 200024641–49. [DOI] [PubMed] [Google Scholar]
- 8.Kaur S, Nieuwenhuijsen M J, Ferrier H.et al Exposure of pregnant women to tap water related activities. Occup Environ Med 200461454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggazzotti G, Fantuzzi G, Righi E.et al Blood and breath analyses as biological indicators of exposure to trihalomethanes in indoor swimming pools. Sci Total Environ 1998217155–163. [DOI] [PubMed] [Google Scholar]
- 10.Jo W K, Weisel C P, Lioy P J. Routes of chloroform exposure and body burden from showering with chlorinated tap water. Risk Anal 199010575–580. [DOI] [PubMed] [Google Scholar]
- 11.Whitaker H, Nieuwenhuijsen M J, Best N. The relationship between water concentrations and individual uptake of chloroform: a simulation study. Environ Health Perspect 2003111688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuckols J R, Ashley D L, Lyu C.et al Influence of tap water quality and household water use activities on indoor air and internal dose levels of trihalomethanes. Environ Health Perspect 2005113863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villanueva C M, Grimalt J O, Kogevinas M. Haloacetic acids and trihalomethane concentrations in finished drinking waters from different sources. Water Res 200337954–959. [DOI] [PubMed] [Google Scholar]
- 14.DeMarini D M. Mehanistic information on disinfection by‐products for risk assessment. International Symposium on Genotoxicity and Immunotoxicity: Unwelcome effects in aquatic systems, Koblenz (Germany), 22–24 April 2004
- 15.Villanueva C. Chlorination by‐products and other drinking water pollutants: environmental levels and public health implications. Master thesis [in Catalan]. Universitat Autonoma de Barcelona 2000
- 16.Rothman K J, Greenland S.Modern epidemiology. Philadelphia: Lippincott‐Raven, 1998
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.