Abstract
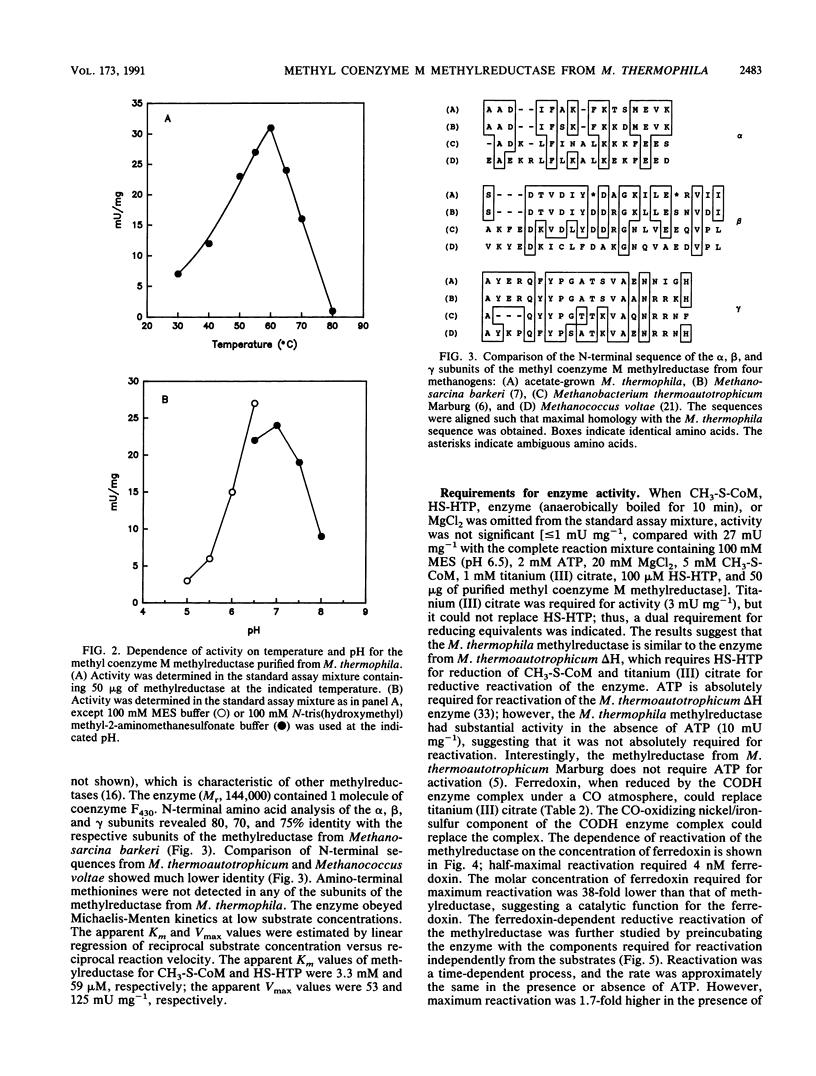
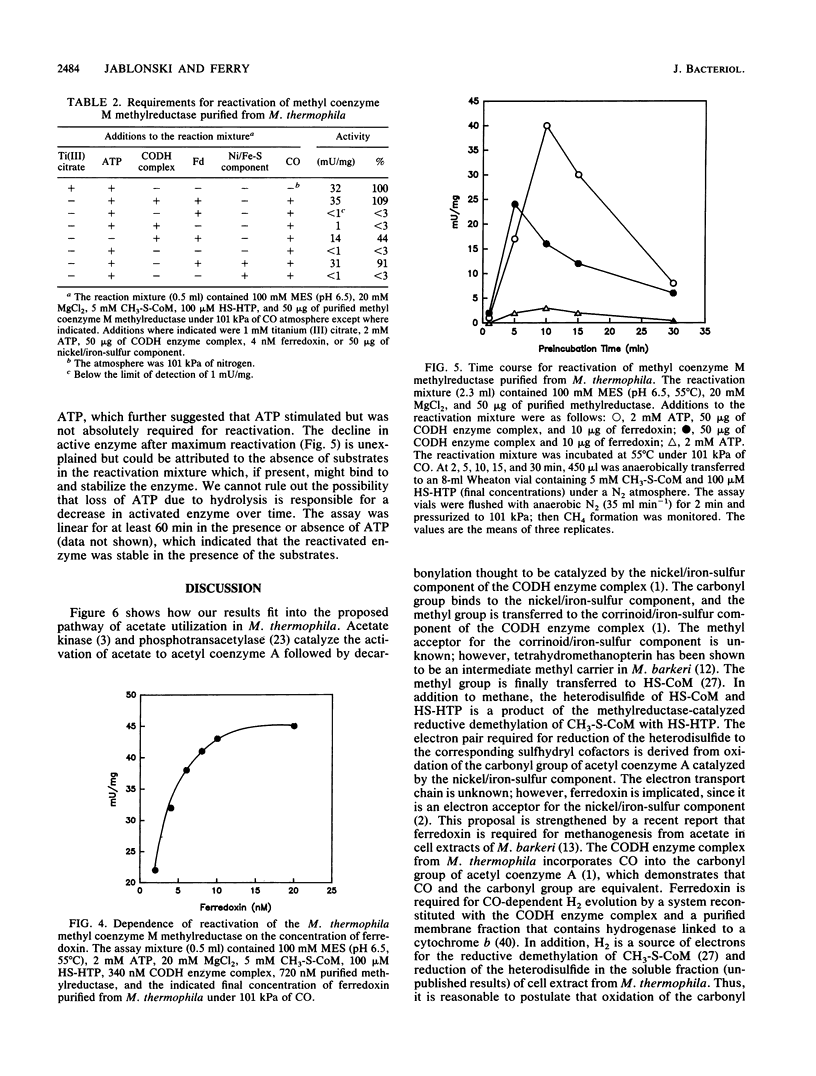
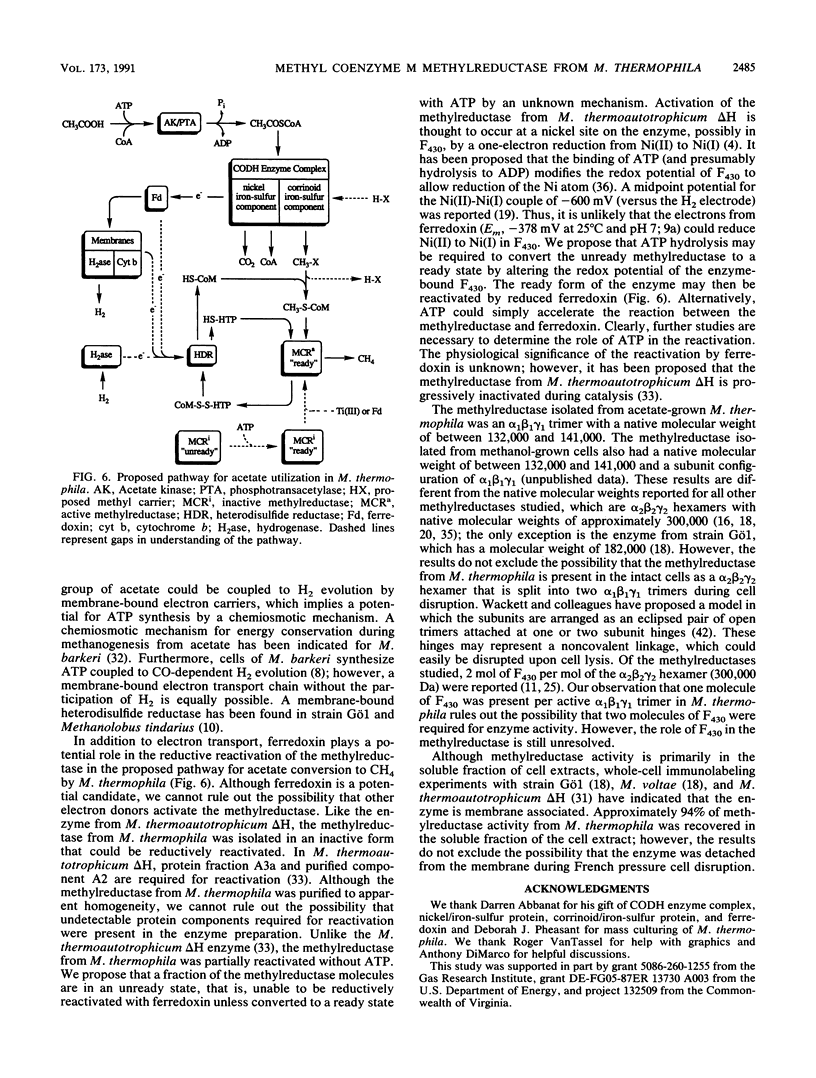
Methyl coenzyme M methylreductase from acetate-grown Methanosarcina thermophila TM-1 was purified 16-fold from a cell extract to apparent homogeneity as determined by native polyacrylamide gel electrophoresis. Ninety-four percent of the methylreductase activity was recovered in the soluble fraction of cell extracts. The estimated native molecular weight of the enzyme was between 132,000 (standard deviation [SD], 1,200) and 141,000 (SD, 1,200). Denaturing polyacrylamide gel electrophoresis revealed three protein bands corresponding to molecular weights of 69,000 (SD, 1,200), 42,000 (SD, 1,200), and 33,000 (SD, 1,200) and indicated a subunit configuration of alpha 1 beta 1 gamma 1. As isolated, the enzyme was inactive but could be reductively reactivated with titanium (III) citrate or reduced ferredoxin. ATP stimulated enzyme reactivation and was postulated to be involved in a conformational change of the inactive enzyme from an unready state to a ready state that could be reductively reactivated. The temperature and pH optima for enzyme activity were 60 degrees C and between 6.5 and 7.0, respectively. The active enzyme contained 1 mol of coenzyme F430 per mol of enzyme (Mr, 144,000). The Kms for 2-(methylthio)ethane-sulfonate and 7-mercaptoheptanoylthreonine phosphate were 3.3 mM and 59 microM, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbanat D. R., Ferry J. G. Synthesis of acetyl coenzyme A by carbon monoxide dehydrogenase complex from acetate-grown Methanosarcina thermophila. J Bacteriol. 1990 Dec;172(12):7145–7150. doi: 10.1128/jb.172.12.7145-7150.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceti D. J., Ferry J. G. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. Evidence for regulation of synthesis. J Biol Chem. 1988 Oct 25;263(30):15444–15448. [PubMed] [Google Scholar]
- Ankel-Fuchs D., Böcher R., Thauer R. K., Noll K. M., Wolfe R. S. 7-Mercaptoheptanoylthreonine phosphate functions as component B in ATP-independent methane formation from methyl-CoM with reduced cobalamin as electron donor. FEBS Lett. 1987 Mar 9;213(1):123–127. doi: 10.1016/0014-5793(87)81476-x. [DOI] [PubMed] [Google Scholar]
- Bokranz M., Bäumner G., Allmansberger R., Ankel-Fuchs D., Klein A. Cloning and characterization of the methyl coenzyme M reductase genes from Methanobacterium thermoautotrophicum. J Bacteriol. 1988 Feb;170(2):568–577. doi: 10.1128/jb.170.2.568-577.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokranz M., Klein A. Nucleotide sequence of the methyl coenzyme M reductase gene cluster from Methanosarcina barkeri. Nucleic Acids Res. 1987 May 26;15(10):4350–4351. doi: 10.1093/nar/15.10.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott M., Eikmanns B., Thauer R. K. Coupling of carbon monoxide oxidation to CO2 and H2 with the phosphorylation of ADP in acetate-grown Methanosarcina barkeri. Eur J Biochem. 1986 Sep 1;159(2):393–398. doi: 10.1111/j.1432-1033.1986.tb09881.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Whitman W. B., Wolfe R. S. Nickel-containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3707–3710. doi: 10.1073/pnas.79.12.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Fischer R., Thauer R. K. Ferredoxin-dependent methane formation from acetate in cell extracts of Methanosarcina barkeri (strain MS). FEBS Lett. 1990 Sep 3;269(2):368–372. doi: 10.1016/0014-5793(90)81195-t. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hoppert M., Mayer F. Electron microscopy of native and artificial methylreductase high-molecular-weight complexes in strain Gö 1 and Methanococcus voltae. FEBS Lett. 1990 Jul 2;267(1):33–37. doi: 10.1016/0014-5793(90)80281-m. [DOI] [PubMed] [Google Scholar]
- Klein A., Allmansberger R., Bokranz M., Knaub S., Müller B., Muth E. Comparative analysis of genes encoding methyl coenzyme M reductase in methanogenic bacteria. Mol Gen Genet. 1988 Aug;213(2-3):409–420. doi: 10.1007/BF00339610. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Ferry J. G. Activation of acetate by Methanosarcina thermophila. Purification and characterization of phosphotransacetylase. J Biol Chem. 1989 Nov 5;264(31):18392–18396. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Ferry J. G. Carbon monoxide-dependent methyl coenzyme M methylreductase in acetotrophic Methosarcina spp. J Bacteriol. 1984 Nov;160(2):526–532. doi: 10.1128/jb.160.2.526-532.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K. M., Donnelly M. I., Wolfe R. S. Synthesis of 7-mercaptoheptanoylthreonine phosphate and its activity in the methylcoenzyme M methylreductase system. J Biol Chem. 1987 Jan 15;262(2):513–515. [PubMed] [Google Scholar]
- Noll K. M., Wolfe R. S. Component C of the methylcoenzyme M methylreductase system contains bound 7-mercaptoheptanoylthreonine phosphate (HS-HTP). Biochem Biophys Res Commun. 1986 Sep 30;139(3):889–895. doi: 10.1016/s0006-291x(86)80261-3. [DOI] [PubMed] [Google Scholar]
- Noll K. M., Wolfe R. S. The role of 7-mercaptoheptanoylthreonine phosphate in the methylcoenzyme M methylreductase system from Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1987 May 29;145(1):204–210. doi: 10.1016/0006-291x(87)91307-6. [DOI] [PubMed] [Google Scholar]
- Ossmer R., Mund T., Hartzell P. L., Konheiser U., Kohring G. W., Klein A., Wolfe R. S., Gottschalk G., Mayer F. Immunocytochemical localization of component C of the methylreductase system in Methanococcus voltae and Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5789–5792. doi: 10.1073/pnas.83.16.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann S., Müller V., Blaut M., Gottschalk G. Bioenergetics of methanogenesis from acetate by Methanosarcina barkeri. J Bacteriol. 1988 Mar;170(3):1369–1372. doi: 10.1128/jb.170.3.1369-1372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Bobik T. A., Wolfe R. S. Reductive activation of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1988 Sep;170(9):3946–3952. doi: 10.1128/jb.170.9.3946-3952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Escalante-Semerena J. C., Wolfe R. S. Component A2 of the methylcoenzyme M methylreductase system from Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Apr;162(1):61–66. doi: 10.1128/jb.162.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Wolfe R. S. Component A3 of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H: resolution into two components. J Bacteriol. 1989 Sep;171(9):4556–4562. doi: 10.1128/jb.171.9.4556-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlesky K. C., Ferry J. G. Ferredoxin requirement for electron transport from the carbon monoxide dehydrogenase complex to a membrane-bound hydrogenase in acetate-grown Methanosarcina thermophila. J Biol Chem. 1988 Mar 25;263(9):4075–4079. [PubMed] [Google Scholar]
- Terlesky K. C., Ferry J. G. Purification and characterization of a ferredoxin from acetate-grown Methanosarcina thermophila. J Biol Chem. 1988 Mar 25;263(9):4080–4082. [PubMed] [Google Scholar]
- Terlesky K. C., Nelson M. J., Ferry J. G. Isolation of an enzyme complex with carbon monoxide dehydrogenase activity containing corrinoid and nickel from acetate-grown Methanosarcina thermophila. J Bacteriol. 1986 Dec;168(3):1053–1058. doi: 10.1128/jb.168.3.1053-1058.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Hartwieg E. A., King J. A., Orme-Johnson W. H., Walsh C. T. Electron microscopy of nickel-containing methanogenic enzymes: methyl reductase and F420-reducing hydrogenase. J Bacteriol. 1987 Feb;169(2):718–727. doi: 10.1128/jb.169.2.718-727.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Presence of nickel in factor F430 from Methanobacterium bryantii. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1196–1201. doi: 10.1016/0006-291x(80)90413-1. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]