Abstract
Objective
The main goal was to investigate the potential of a probabilistic approach for exposure assessment and use this information to evaluate the impact of a complex of policy actions/interventions on dermal exposure to antineoplastic agents among oncology nurses. The central theme of this study was to make optimal use of existing data, supplemented only with limited additional information from a questionnaire survey.
Methods
A task based exposure model was used to estimate dermal exposure of the hands among oncology nurses in non‐academic hospitals in the Netherlands. Monte Carlo simulation was used to integrate information from available (exposure) studies and generate exposure distributions for the total population of oncology nurses in both pre‐ and post‐intervention situation. Graphs and descriptive statistics of the simulated exposure distributions were used to evaluate trends in population exposure.
Results
The inventory showed that important intervention occurred in the preparation and administering of antineoplastic agents and in the handling of urine. Hardly any changes were identified in de nursing tasks. The use of gloves seemed to have decreased for a number of tasks. The results of the analysis show that the interventions did not affect the median exposure. However frequencies of occurrence of individuals with very high and very low total dermal exposures decreased substantially in the post‐intervention situation. Analysis of the effect of pregnancy showed that pregnancy is very unlikely to influence exposure or any of the key input variables.
Conclusions
The present study shows that the probabilistic approach adds valuable information to deterministic exposure assessment, especially when extrapolating data on a subpopulation to populations of individuals at large. The results show that the identified changes in the past decade in Dutch non‐academic hospitals resulted in changes in the exposure distribution of antineoplastic agents among oncology nurses.
Keywords: Monte Carlo simulation, interventions, occupational exposure, antineoplastic agents, nurses
Trends in exposure have been observed in occupational settings at the level of sectors or entire industries for a substantial number of years now.1,2,3,4,5,6 Except for a few cases1 the general perception is that we are lacking in our knowledge of underlying factors that drive these trends.7,8 When looking at small scale intervention studies, Goldenhar and Schulte, Zwerling et al, and more recently Roelofs et al concluded that many of these studies showed methodological shortcomings resulting in an inability to perform and evaluate the impact of specific interventions in a structured and effective manner.9,10,11,12 Obviously, the evaluation of industry‐wide interventions involving a spectrum of control measures is even more an untouched topic in occupational health research. A rare example of such a study is the Minnesota Wood Dust Study.13,14,15 In this study the effectiveness of different levels of intervention on wood dust exposure was studied using a group randomised trial looking at both quantitative and qualitative outcomes.
There is a need for understanding exposure trends in branches or industrial settings, if we want to effectively evaluate or predict the impact of interventions at population level. In the Netherlands this is stimulated by the introduction of covenants which are agreements between employers' organisations, trade unions, and the government with the aim to improve working conditions in specific branches.16 Covenants can comprise detailed agreements to reduce exposures, introduce exposure control measures, or inform and educate workers. In the context of these covenants effectiveness of implemented control measures receives special attention.
Occupational exposure to antineoplastic drugs is a focal point of both the covenants for general and academic hospitals in the Netherlands. As a precursor to these covenants, several policy actions and research initiatives have taken place in order to reduce exposure to antineoplastic agents among hospital personnel. These research initiatives and policy actions presumably led to a strong increase in the awareness of the potential risk of occupational exposure to antineoplastic agents and the need for interventions to control exposure. The actual implementation of control measures and impact on the exposure levels in the population of nurses at large has never been evaluated in a structured manner.
The main goal of this study was to investigate the potential of a probabilistic approach to assess exposure, and use this information to evaluate shifts in the population exposure distribution due to interventions in the past decade. The central theme of this study was to make optimal use of existing (exposure) data for both pre‐ and post‐intervention periods, supplemented only with limited additional information from a questionnaire survey on present task frequency and glove use. A simple task based exposure model in combination with Monte Carlo simulations17 was used to integrate this information and generate exposure distributions for the population of oncology nurses “at large” for both the current situation and the situation one decade ago. In Monte Carlo simulations, distributions of output variables (in this case exposure to antineoplastic agents) are simulated by drawing random values from distributions of input variables, according to a given algorithm (in this case a task based exposure model) that describes that output variable.
For logistical reasons academic hospitals did not participate in this study and results only directly apply to the population of oncology nurses working in non‐academic (or general) hospitals.
Material and methods
The population of interest for this study are oncology nurses working in non‐academic Dutch hospitals on departments with activities with antineoplastic agents or with patients receiving chemotherapy, and are at risk of exposure to antineoplastic agents.
An overview was made of research and policy actions that occurred in relation to antineoplastic agents' exposure between 1990 and 2004. To get an overview of actual implementation of interventions resulting from the performed studies and policy actions we asked nurses to indicate specific changes in their work performance in the past decade related to antineoplastic agents. Additionally some key people from hospitals and branch organisations were asked to indicate what changes they observed in general in work performance around antineoplastic agents. All this information was used to create an overview of the most important interventions that might have influenced exposure to antineoplastic agents and the period in which they took place.
Information with respect to input data was obtained from existing exposure studies and an additional questionnaire survey among a random sample of nurses from our study population performed in this study.18,19,20,21,22,23,24 In order to study the impact of policy initiatives taken during the last decade, Monte Carlo simulation was used to generate population exposure distributions for pre‐ and post‐intervention situation.
Dermal exposure model
Equation 1 shows the model for dermal exposure of the hands to all antineoplastic agents. Since work pattern varies slightly between days within a week we used a cumulative exposure over a week to ensure a representative time frame for long term exposure.
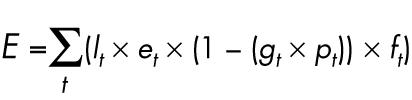 |
where E = total dermal exposure of the hands (ng/week), lt = task performance (yes/no), et = potential dermal contamination after performing task t (ng), gt = glove use during tasks t (yes/no), pt = glove protection at task t (%), ft = frequency of task t (times/week).
The tasks taken into account in the model are: preparation of antineoplastic agents, administering of chemotherapy, washing a patient, changing bedding, handling patient's urine, and cleaning activities. A short description of each task is given in table 1.
Table 1 Description of tasks taken into account in the exposure model.
| Task | Description |
|---|---|
| Preparation | Incorporated activities like dissolving or diluting antineoplastic agents in vials and transferring the contents (highly concentrated antineoplastic agents) between vials and syringes or IV bag. Exposure during these activities might occur through leakage or via contaminated objects |
| Administering | The tasks incorporates connection and disconnection of the IV system to the patients central IV system (if present) and other activities like unwrapping the IV system if packed and disposing it after administration. Exposure can occur through contact with contamination on the IV system. |
| Washing patient | Washing a patient that received chemotherapy within the past 48 hours with use of a bowl of water and washing towel. Patients sweat is known to be contaminated with antineoplastic agents. |
| Changing bedding | Changing of bed sheets from patients that have received chemotherapy within the past 48 hours. Bed sheets are contaminated through patients' sweat or other excreta. |
| Handling urine | Can incorporate transporting the pan/urinal emptying it in the washing machine or additional handlings like weighing the pot/urinal or transferring urine to a measuring cup. Exposure can occur through splashes or deposition of aerosols during handling or through contact with contaminated objects. |
| Cleaning | Includes cleaning of all known potentially contaminated object, these can be in the (bath)room of the patient or in the general cleaning room where eg pots and urinals are stored and cleaned. |
Input data
A short description of all model parameters is given in table 2. A detailed description of the different studies and the extracted data is given below, as well as imputations and extrapolations that had to be performed for missing data.
Table 2 Description of model variables and data sources for input data.
| Input variable | Description | Distributions used in the simulation | Source | |
|---|---|---|---|---|
| 1990–97 | 2003–04 | |||
| Task performance | Gives whether or not a certain task is performed for each iteration (scenario). Probability depends on the % of nurses performing the task in the study population (table 3) | Binomial ((1,0); (# performed, # not performed)) | Peelen et al, 199921 | Questionnaire survey conducted this study |
| Task frequency | For each iteration a value is generated (times/week) for each task from its respective input distribution | Lognormal (mean, SD) | Peelen et al, 199921 | Questionnaire survey conducted this study |
| Potential dermal contamination | The dermal contamination of the hands or gloves. At each iteration a value is drawn from the binomial to decide if exposure is above or below LOD (table 4). Secondly an exposure value is drawn from the respective distribution | Binomial ((1, 2); (# samples <LOD, # samples >LOD)) | Fransman et al, 2005*24 | Fransman et al, 200524 |
| 1 = Uniform (0, LOD) | Peelen et al, 1999†21 | |||
| 2 = Lognormal ((mean, SD); truncated (LOD)) | ||||
| Glove use | Gives whether or not gloves are worn for each iteration (scenario). Probability depends on the % of nurses wearing gloves in the study populations (table 5) | Binomial ((1,0); (# wear gloves, # no gloves)) | Peelen et al, 199921 | Questionnaire survey conducted this study |
| Glove protection | The protective effect of gloves is expressed as being between 0–100% of potential exposure | Triangular (min, mean, max) | Fransman et al, 200524 | Fransman et al, 200524 |
LOD, limit of detection.
*For tasks; washing patients, changing bedsheets, cleaning.
†For tasks; preparation, urine handling, administering.
Pre‐intervention
Data on model parameters for the pre‐intervention situation were obtained from a Dutch epidemiological study on reproductive effects among hospital personnel.21 The dataset contained measurement data of the dermal exposure of the hands to cyclophosphamide, measured on gloves for three tasks—preparation, administering, and handling urine. The measurements were performed in 1997 in seven different hospitals. For three tasks (washing patients, changing bed sheets, cleaning) no measurement data on dermal exposure were available. Data from the study of Fransman et al used for the post‐intervention (mentioned below) were extrapolated and also used for the pre‐intervention period.24 The results of our inventory on changes in work characteristics discussed later in this paper showed that no large changes occurred in these tasks suggesting no large difference in dermal exposure for these tasks.
A second dataset from the epidemiological study contained questionnaire information from a large sample of nurses (n = 5546) with a response rate of 79% (n = 4393).21 From this dataset nurses working in non‐academic hospitals in departments where antineoplastic agents were used were selected. A total of 507 records of the questionnaire dataset contained sufficient information to be used for the analysis. From this population information was available on work performance characteristics, task frequency, and glove use. The questionnaire information was related to activities with all antineoplastic agents. Each subject had provided this information for the first month of their most recent pregnancy or for the period they were trying to get pregnant. The majority of the population (n = 345, 69%) provided information from a one month period between 1995–97. Since this questionnaire dataset only contained information from nurses who were pregnant or were trying to get pregnant, we evaluated the potential impact of pregnancy on exposure to antineoplastic agents. Because only the post‐intervention dataset contained data on both pregnant and non‐pregnant women, these data were used to perform analysis with respect to the effect of pregnancy on the exposure to antineoplastic agents. The distribution for dermal exposure was simulated for the (sub)population of nurses being pregnant at the time of answering the questionnaire. This distribution was compared with the exposure distribution of the total population.
Post‐intervention
For the post‐intervention situation data on dermal task exposure of the hands were obtained from a dataset collected partially within a large European study RISKOFDERM described by Fransman et al.24 This dataset contains glove and hand wash samples, supplying data on both potential and actual exposure of the hands to cyclophosphamide for four tasks (washing patients, changing bedding, handling urine, and cleaning). All samples were obtained from nurses during their normal working activities. Measurement data were not available for dermal exposure during administering. Dermal exposure estimates were imputed using data from surface wipes of IV infusion bags.24 It was assumed that a 100% transfer occurs from the bags onto the hands, creating a worst case scenario for the dermal contamination during administering.
Information on work performance, task frequency, and glove use for the post‐intervention situation was obtained through an additional questionnaire survey. A random sample of 33 hospitals was selected stratified for size and geographical location, of these 23 (70%) agreed to participate. In these hospitals 1863 nurses were selected for participation in the questionnaire survey. A total of 999 nurses completed and returned the questionnaire resulting in a 54% response rate.
Glove protection in our model was assumed to be equal for both time periods and was estimated using the dermal exposure data from Fransman et al.24 The ratio of actual and potential exposure was calculated. This ratio was used as a measure of the protective effect of gloves (% of contamination “blocked” by the gloves). No data were available for glove protection during administering. Protection was assumed to be in the same range as for preparation so a comparable distribution was imputed for protection during administering of antineoplastic agents.
In our study cyclophosphamide was used as a marker; estimates of dermal exposure were assumed to be representative for antineoplastic agents in general during the performance of the measured tasks.
Input distributions
Distributions of input data for model parameters were determined using descriptive analysis performed in SAS v8.2 (SAS Institute, Cary, NC, USA) and the software package Best Fit (Palisade Corporation, Ithaca, NY, USA) and information from scientific literature.
Dermal exposure, in the simulation, is for each task represented by three distributions to take into account the limit of detection in the measured data. A uniform distribution represents data points below the limit of detection (LOD) with zero as lower bound and the limit of detection as upper bound. Data points above LOD are represented by a lognormal distribution based on the geometric mean and the geometric standard deviation of the values above LOD in each dataset. A third, binomial distribution is used to simulate the probability of sampling a value below or above the LOD (based on the number of measurements above LOD in each dataset). Table 2 gives the distributions used for all input variables.
Data analysis and simulation
The model for total dermal exposure of the hands and input distributions for all model variables were programmed into an Excel (Microsoft Corporation) worksheet.
The total exposure was simulated using @Risk 4.5 (Pallisade Corporation), an add‐in for probabilistic modelling and simulation in Excel, using Monte Carlo simulations.25,34 A stable output distribution for total exposure was obtained with 10000 iterations of the model. Correlations between input variables were studied using PROC CORR procedure in SAS v8.2. When correlation between input variables is observed @Risk offers the possibility of running MC simulations taking into account a correlation matrix.
Population distributions of the actual dermal exposure to antineoplastic agents accumulated during a week were created for the pre‐ and post‐intervention situation. Simulated data were then transported to Excel to create cumulative probability plots (on a log scale for exposure). Percentile values were generated to study shifts within the total range of the population dermal exposure distributions.
Uncertainty
A probabilistic uncertainty analysis was performed to obtain insight into effects of imprecise input parameters. As datasets reflecting dermal exposure contained the smallest number of data points and incorporated the largest variability, the uncertainty analysis focussed on these input parameters.
First, bootstrapping26,27 of the original data for dermal exposure was performed in SAS v8.2 (SAS Institute) for all datasets. One hundred bootstraps were performed for each task, creating one hundred datasets of equal size compared to the original dataset. Secondly one hundred Monte Carlo simulations of total dermal exposure were performed using one set of bootstrapped input datasets for each simulation. This resulted in 100 different output distributions for total dermal exposure of the hands.
Scenario analysis
To explore the possibility of performing a scenario analysis we did a prospective assessment of a fictive intervention scenario, in which we assumed that the use of gloves could be increased for all tasks to 90% of the population. The input distributions for glove use were changed and a Monte Carlo simulation of 10 000 iterations was run. Data were then analysed as described above and output distribution plots were created to look at differences in the exposure distributions.
Results
Policy, research initiatives, and interventions
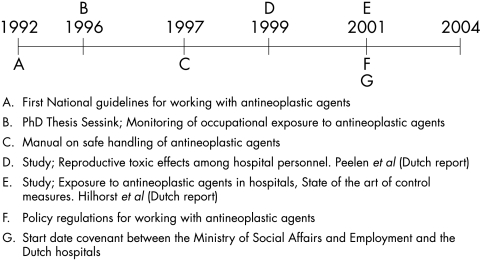
Figure 1 shows the timeline with the most important studies and policy actions that occurred in the past 15 years in the Netherlands. Antineoplastic agents first became a topic in occupational hygiene in the Netherlands with the introduction of the first guidelines (1992). Around this time focus was primarily on preparation of antineoplastic agents and the health risks of pharmacy personnel. From 1997 onwards the focus more and more shifted towards nursing staff and the patient as a possible source of exposure and work environment contamination.
Figure 1 Timeline of activities between 1990 and 2004 related to nurses exposure to antineoplastic agents.
The results of the inventory on work characteristic and interventions from the questionnaire survey indicated that nurses experienced changes in several tasks. Preparation was largely eliminated from the wards and moved to the pharmacy. For administering of antineoplastic agents, most hospitals introduced the use of closed infusion systems to decrease the chance of highly concentrated antineoplastic agents entering the (work) environment during this task. When handling urine the use of different work protocol and technical equipment, such as automated urinal and pot washers was introduced in most of the hospitals. For the other tasks no obvious changes (for example, control measures) were reported.
Most of the changes occurred in the late 1990s after the results of the epidemiological study21 were published and again after the introduction of the guidelines in 2001. The effect of the first guidelines primarily was the complete elimination of preparation of antineoplastic agents.
Input data
Tables 3, 4, and 5 give data of input distributions for all model variables. The bold values in the tables are the imputed values based on surrogate data and/or expert judgement as explained in the methods section.
Table 3 Input distribution data for task performance and task frequency.
| Variable | Task performance | Task frequency times/week | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐intervention | Post‐intervention | Pre‐intervention | Post‐intervention | |||||
| Task | Yes* | No | Yes | No | GM† | GSD‡ | GM† | GSD‡ |
| Preparation | 6% | 94% | 0% | 100% | 2.17 | 3.11 | – | – |
| Administering | 52% | 48% | 76% | 24% | 2.26 | 3.18 | 3.62 | 2.71 |
| Washing patients | 54% | 46% | 53% | 47% | 2.32 | 2.82 | 1.88 | 2.29 |
| Changing bed sheets | 54% | 46% | 78% | 22% | 1.74 | 2.55 | 3.13 | 3.11 |
| Handling urine | 46% | 54% | 76% | 24% | 2.22 | 2.98 | 5.24 | 2.25 |
| Cleaning | 53% | 47% | 58% | 42% | 1.62 | 1.76 | 2.57 | 2.57 |
*Percentage of nurses in the study population performing this task.
†Geometric mean of task frequency per week.
‡Geometric standard deviation for task frequency per week.
Table 4 Input distribution data for dermal exposure.
| Task | Potential dermal exposure of hands | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐intervention | Post‐intervention | |||||||
| GM† | GSD‡ | LOD§ | >LOD (total)¶ | GM† | GSD‡ | LOD§ | >LOD (total)¶ | |
| Preparation | 52338 | 2.25 | 10 | 100% (8) | – | – | – | – |
| Administering | 1078 | 3.99 | 10 | 52% (29) | 390 | 3.30 | 16 | 25% (20) |
| Washing patients | 217* | 1.90 | 53 | 93% (28) | 217 | 1.90 | 53 | 93% (28) |
| Changing bed sheets | 82 | 1.80 | 53 | 61% (28) | 82 | 1.80 | 53 | 61% (28) |
| Handling urine | 1030 | 3.06 | 10 | 64% (11) | 70 | 1.75 | 53 | 54% (26) |
| Cleaning | 264 | 2.08 | 53 | 53% (19) | 264 | 2.09 | 53 | 53% (19) |
*Bold values in the table indicate imputed data.
†Geometric mean of potential dermal exposure of hands (in ng/ performance).
‡Geometric standard deviation of potential dermal exposure of hands.
§Limit of detection in ng.
¶Number of samples above the limit of detection (total number of samples).
Table 3 shows the percentages of nurses in the study populations both pre‐ and post‐intervention who performed the six identified tasks. It also gives the geometric means and geometric standard deviations of the task frequency in times per week. The most important change is that preparation has completely disappeared from the work contents of oncology nurses. The frequency of other tasks except for washing patients increased. Correlation between input variables was only observed for task frequencies, correlation coefficients being all below 0.6 for both pre‐ and post‐intervention data. Incorporation of a correlation matrix in the MC simulation for task frequency did not show large changes in the simulated output distributions.
Table 4 shows the values used to estimate the input distributions for the dermal exposure of each task. The geometric means and the geometric standard deviation are the parameters of the lognormal distribution based on the measurements above LOD from the respective input datasets of dermal exposure, as explained in the methods section. The column “LOD” gives the respective limits of detection for each dataset, which was also set as the upper bound of the uniform distribution representing the measurements below LOD in our simulation. The last column in each section (pre‐ and post‐intervention) indicates the percentage and number of samples above LOD and the total number of samples in each dataset.
The mean values in table 4 show that dermal contamination has decreased substantially for administering and urine handling. For administering the imputed data related to contamination of infusion bags also indicates a decrease in dermal exposure during this task.
Table 5 shows that use of gloves has increased for cleaning and handling urine with respectively 24% and 8%; for administering there was a small decrease of glove use from 89% to 81%. A more substantial decrease of glove use was observed for the nursing tasks of washing patients and changing bed sheets with more than 30% decrease of glove use.
Table 5 Input distribution data for glove use and glove protection.
| Variable | Glove use | Glove protection | |||||
|---|---|---|---|---|---|---|---|
| Pre‐intervention | Post‐intervention | ||||||
| Task | Yes† | No | Yes | No | Mean‡ | Min§ | Max¶ |
| Preparation | 84% | 16% | – | – | 0.93 | 0.74 | 0.99 |
| Administering | 89% | 11% | 81% | 19% | 0.90* | 0.75 | 0.99 |
| Washing patients | 67% | 33% | 30% | 70% | 0.83 | 0.40 | 0.97 |
| Changing bed sheets | 67% | 33% | 36% | 64% | 0.52 | 0.07 | 0.80 |
| Handling urine | 82% | 18% | 90% | 10% | 0.46 | 0.09 | 0.91 |
| Cleaning | 35% | 65% | 59% | 41% | 0.92 | 0.81 | 0.98 |
*Bold values in the table indicate imputed data.
†Percentage of nurses in the study wearing gloves at this task.
‡Most likely value for glove protection in this case the mean was chosen.
§Minimum value for the protection of gloves.
¶Maximum value for the protection of gloves.
Monte Carlo simulations of total dermal exposure of the hands
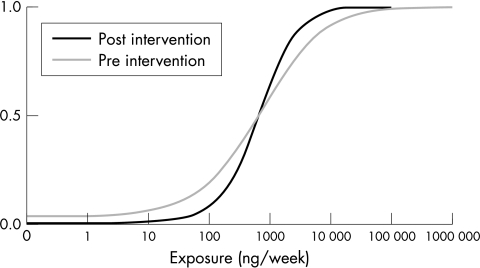
Figure 2 shows the cumulative probability plots of distributions of the simulated total dermal exposure of the hands on a weekly basis for the population of oncology nurses at large for both the pre‐ and post‐intervention situation.
Figure 2 Distribution of dermal exposure for the total population pre‐ and post‐intervention.
The plot shows that the median value of exposure has hardly changed, being approximately 650 ng/week. Exposure to antineoplastic agents in the pre‐intervention situation showed a larger variability than post intervention, where exposure seems to have converged towards the median exposure. In other words, the numbers of individuals with extreme “high” and “low” values of total dermal exposures have decreased substantially. This is also shown by the fourfold increase in the 10th percentile for the two distributions, from 31 to 124 ng/week and the 2.5 fold decrease of the 90th percentile from 8200 to 3200 ng/week.
Comparison of cumulative probability plots for the (sub)population of pregnant nurses (not presented in this paper) and the total population of oncology nurses showed that the two graphs almost completely overlap. Hence pregnancy is very unlikely to influence exposure or any of the key input variables.
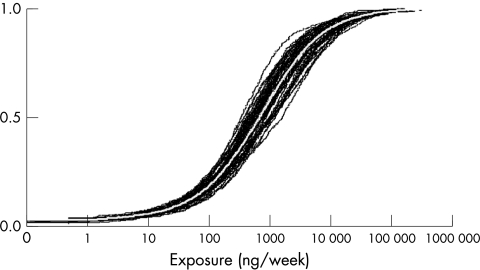
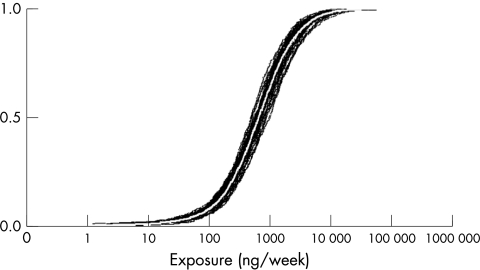
Uncertainty analysis
Figures 3 and 4 show the cumulative probability plots of the population distributions for the pre‐ and post‐intervention situation with their respective uncertainty bands caused by uncertainty in our dermal contamination data. When creating an overlay plot of both distributions (not presented), it shows that there is a large overlap between the uncertainty bands of both distributions. Nevertheless the tails of the post‐intervention distribution falls outside the pre‐intervention uncertainty area.
Figure 3 Uncertainty plot of the distribution of dermal exposure for pre‐intervention situation.
Figure 4 Uncertainty plot of the distribution of dermal exposure for the post‐intervention population.
Scenario analysis
The simulated scenario of increase of glove use to 90% during all six tasks results in a shift to the left of the exposure distribution. The median weekly dermal exposure of the hands decreases on average with approximately 30% from 650 to 432 ng/week. At the left tail of the distribution only a small decrease is observed as expected. The 90th percentile of exposure decreases almost twofold from 3200 to 1775 ng/week.
Discussion
The cumulative distributions of the population dermal exposure to antineoplastic agents indicate that a trend occurred over the past decade. The elimination of the highest exposure values is primarily associated with the complete disappearance of preparation from the work package. The strong decrease in dermal exposure when handling urine or administering antineoplastic agents, causes a further decrease of the number of oncology nurses with “high” exposures. A shift towards fewer nurses with “low” exposures seems to be associated with the centralisation of cancer treatments and patients on specialised oncology wards, which was indicated by many hospitals to have occurred over the past decade. The observed decrease in glove use, when washing patients and changing bedding, is contrary to what was expected in light of the suspected increase in awareness of potential exposure. No reason for this could be found.
One may assume that these results will also apply to nurses performing the same tasks in academic hospitals. In general, work performance and characteristic do not vary substantially between academic and general hospitals in the Netherlands.
The use of a probabilistic approach enabled the integration of data from many different sources to simulate the exposure distribution for the population at large. This provided the opportunity to take into account the full range of potential exposure scenarios and exposure levels and study the trend in the population exposure distribution.
Nevertheless, some issues have to be considered. When performing Monte Carlo simulations it is important that “all” exposure scenarios based on possible combinations of input variables can be generated, especially when running a high number of iterations.28 This means that extremely high exposures might be generated due to the combination of rare events and the choice could be made to restrict the model (for example, by truncation of input distribution). In our case there were no reasons to restrict our model. Correlation between input variables also plays an important role in generating realistic exposure scenarios. In our analysis correlations were only observed in task frequencies. Incorporation of correlations matrix into our Monte Carlo simulation did not show any large change in our simulated output distribution. Eventually the “extreme” scenarios generated—although not very likely to occur—were not eliminated from the analysis.
The simulation could have been biased in several ways. Firstly, six tasks were taken into account in our exposure model while exposure might also occur outside these tasks. Secondly, we only assessed the dermal exposure of the hands. Nevertheless both national and international studies show that the six tasks considered are responsible for the majority of exposure. They also show that the primary route of exposure is via the skin with more than 90% of exposure found on the hands.18,22,23,24,29,30,31
Another factor for potential bias was that all dermal exposure measurements focused on exposure to cyclophosphamide. As no information is available on exposure pattern for other antineoplastic drugs, the exposure pattern of cyclophosphamide was assumed to be representative for dermal exposure of the hands to antineoplastic drugs. The results of the available measurements were used in our assessment to estimate dermal exposure of the hands to antineoplastic agents in general.
A fourth important factor introducing uncertainty is the fact that we lack data for some variables of our model for one or both of our simulations. For our pre‐intervention simulation, data were not available on dermal exposure during the tasks washing patients, changing bed sheets, and cleaning. As mentioned earlier, the results of the questionnaire survey did not indicate important changes (control measures, technical) with respect to these tasks, therefore dermal exposure was assumed to be equal for pre‐intervention and post‐intervention situation.
For the post‐intervention situation no data were available for dermal contamination when administering. Exposure through leakage of the intravenous infusion systems is generally believed to be very minimal after introduction of “closed” infusion systems and strict work protocols. Therefore exposure during this task is believed to mainly occur through transfer from contaminated IV infusion bags. By assuming a 100% transfer we created a worst case scenario overestimating the “true” dermal exposure.
A last source of uncertainty introduced by lack of data is the fact that no specific data were available on glove protection for the pre‐intervention. It might have been that an increase in awareness did influence the protective effect in a positive way. Yet this could not be incorporated in our modelling approach.
Another important factor is the quality of the input data used in our simulation—that is, the number of samples available and representativeness of the data for the population at large. For the model variables task frequency, task performance, and glove use we had questionnaire information from a large, random sample of our study population, probably resulting in low uncertainty in the input distributions for these variables. A source of uncertainty for the post‐intervention data might have been a relatively low response (54%) in the questionnaire survey compared to the response for the pre‐intervention data (79%).
The dermal exposure datasets were significantly smaller (<30 data points) with in some cases a substantial number of values below LOD. Particularly when the variability in these datasets was large, this resulted in uncertainty in estimates of input distributions for dermal exposure. As our uncertainty analysis showed this was more the case for the pre‐intervention assessment than for our post‐intervention assessment.
For glove protection data were too limited to fit any distribution and no a priori information was available on a parametric distribution; therefore the triangular distribution was selected with an optimum (mean) minimum and maximum protection defined.
The analysis performed on the post‐intervention data with regard to the influence of pregnancy on exposure showed that pregnancy did not have an effect on the work characteristics and respectively the exposure distribution for this population. This is reassuring and indicates that the questionnaire survey used here is representative for the total population of oncology nurses subject to our evaluation. It also indicates that the expected raised awareness of the past decade around reproductive toxic effects associated with exposure to antineoplastic agents did not clearly result in a change in the work practice for pregnant nurses working on oncology departments.
In general it can be stated that the data used were of good quality. Therefore the results of this probabilistic assessment are reliable and give a good insight in shifts of exposure to antineoplastic agents over the past decade. The main goal of the performed uncertainty analysis was to explore its potential in this type of assessment. Additional uncertainty and/or sensitivity analysis could have been conducted; however we believe this would not necessarily have added significant information to our impact assessment and was therefore considered beyond the scope of our study.
Probabilistic modelling is increasingly used as an approach to assess exposure for regulatory purposes.32,33 This study shows that the probabilistic approach can also add valuable information to trend analysis, especially when extrapolating data on a subpopulation to populations at large.
It enables researchers or policy makers to—prospectively or retrospectively—investigate and quantify the impact of policy and/or interventions on exposure in a population of workers. The important benefit is that probabilistic assessments enable the use of the fragmented data available to researchers. The use of uncertainty and/or sensitivity analysis can subsequently give a good insight into the effect of the quality of the data on the estimated outcome distribution, enabling a transparent interpretation of the assessment results. Semple et al also showed that Monte Carlo simulation provides a tool to examine the influence of uncertainty on an exposure model34
Probabilistic evaluations, such as the one presented in this paper, are to our opinion applicable to a wide variety of prevention and/or intervention programmes in the workplace. Obviously this increases the necessity of collecting and storing input data in a structured manner and making them available to researchers. This will increase the quality and informativeness of future probabilistic evaluations of intervention programmes.
Acknowledgements
The authors would like to acknowledge all contact persons and nurses from the participating hospitals who cooperated in the questionnaire survey, and Carina Rubingh and James A Deddens for their advice on the statistical analysis. This research was granted by the Ministry of Social Affairs and Employment, the Netherlands.
Abbreviations
LOD - limit of detection
Footnotes
Competing interest: none declared.
References
- 1.Vermeulen R, de Hartog J, Swuste P.et al Trends in exposure to inhalable particulate and dermal contamination in the rubber manufacturing industry: effectiveness of control measures implemented over a nine‐year period. Ann Occup Hyg 200044343–354. [PubMed] [Google Scholar]
- 2.Burstyn I, Kromhout H, Cruise P J.et al Designing an international industrial hygiene database of exposures among workers in the asphalt industry. Ann Occup Hyg 20004457–66. [PubMed] [Google Scholar]
- 3.Burstyn I, Kromhout H. Trends in inhalation exposure to hydrocarbons among commercial painters in The Netherlands. Scand J Work Environ Health 200228429–438. [DOI] [PubMed] [Google Scholar]
- 4.van Tongeren M J, Kromhout H, Gardiner K. Trends in levels of inhalable dust exposure, exceedance and overexposure in the European carbon black manufacturing industry. Ann Occup Hyg 200044271–280. [PubMed] [Google Scholar]
- 5.Symanski E, Chang C C, Chan W. Long‐term trends in exposures to nickel aerosols. AIHAJ 200061324–333. [DOI] [PubMed] [Google Scholar]
- 6.Okun A, Cooper G, Bailer A J.et al Trends in occupational lead exposure since the 1978 OSHA lead standard. Am J Ind Med 200445558–572. [DOI] [PubMed] [Google Scholar]
- 7.Kromhout H, Vermeulen R. Long‐term trends in occupational exposure: Are they real? What causes them? What shall we do with them? Ann Occup Hyg 200044325–327. [DOI] [PubMed] [Google Scholar]
- 8.Symanski E, Kupper L L, Rappaport S M. Comprehensive evaluation of long‐term trends in occupational exposure: Part 1. Description of the database. Occup Environ Med 199855300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenhar L M, Schulte P A. Intervention research in occupational health and safety. J Occup Med 199436763–775. [PubMed] [Google Scholar]
- 10.Goldenhar L M, Schulte P A. Methodological issues for intervention research in occupational health and safety. Am J Ind Med 199629289–294. [DOI] [PubMed] [Google Scholar]
- 11.Zwerling C, Daltroy L H, Fine L J.et al Design and conduct of occupational injury intervention studies: a review of evaluation strategies. Am J Ind Med 199732164–179. [DOI] [PubMed] [Google Scholar]
- 12.Roelofs C R, Barbeau E M, Ellenbecker M J.et al Prevention strategies in industrial hygiene: a critical literature review. AIHA J (Fairfax, Va) 20036462–67. [DOI] [PubMed] [Google Scholar]
- 13.Brosseau L M, Parker D L, Lazovich D.et al Designing intervention effectiveness studies for occupational health and safety: The Minnesota Wood Dust Study. Am J Ind Med 20024154–61. [DOI] [PubMed] [Google Scholar]
- 14.Lazovich D, Parker D L, Brosseau L M.et al Effectiveness of a worksite intervention to reduce an occupational exposure: the Minnesota wood dust study. Am J Public Health 2002921498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazovich D, Murray D M, Brosseau L M.et al Sample size considerations for studies of intervention efficacy in the occupational setting. Ann Occup Hyg 200246219–227. [DOI] [PubMed] [Google Scholar]
- 16.SZW Occupational health and safety covenants: just what we need. The Hague: Ministry of Social Affairs and Employment, 2004
- 17.Cullen A C, Frey H C.Probabilistic techniques in exposure assessment: a handbook for dealing with variability and uncertainty in models and inputs. New York: Plenum Press, 1999
- 18.Sessink P J, Boer K A, Scheefhals A P.et al Occupational exposure to antineoplastic agents at several departments in a hospital. Environmental contamination and excretion of cyclophosphamide and ifosfamide in urine of exposed workers. Int Arch Occup Environ Health 199264105–112. [DOI] [PubMed] [Google Scholar]
- 19.Sessink P J, Bos R P, Kerkhof M C A.et al Environmental contamination and Assessment of exposure to antineoplastic agents by determination of cyclophosphamide in urine of exposed pharmacy technicians: Is skin absorption an important exposure route. Arch environ health 199449165–169. [DOI] [PubMed] [Google Scholar]
- 20.Sessink P J, Wittenhorst B C J, Anzion R B M.et al Exposure of pharmacy technicians to antineoplastic agents after additional protective measures. Arch Environ Health 199752240–244. [DOI] [PubMed] [Google Scholar]
- 21.Peelen S, Roeleveld N, Heederik D.et al Reproductie toxische effecten bij ziekenhuispersoneel. Den Haag: Ministerie van Sociale Zaken en Werkgelegenheid, 1999
- 22.Kromhout H, Hoek F, Uitterhoeve R.et al Postulating a dermal pathway for exposure to antineoplastic drugs among hospital workers. Applying a conceptual model to the result of three workplace surveys. Ann Occup Hyg 200044551–560. [DOI] [PubMed] [Google Scholar]
- 23.Fransman W, Vermeulen R, Kromhout H. Occupational dermal exposure to cyclophosphamide in Dutch hospitals: a pilot study. Ann Occup Hyg 200448237–244. [DOI] [PubMed] [Google Scholar]
- 24.Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health 200578403–412. [DOI] [PubMed] [Google Scholar]
- 25.Vose D.Risk analysis: a quantitative guide. New York: John Wiley and Sons, 2000
- 26.Frey H C, Burmaster D E. Methods for characterizing variability and uncertainty: comparison of bootstrap simulation and likelihood‐based approaches. Risk Anal 199919109–130. [Google Scholar]
- 27.Zhao Y, Frey H C. Quantification of variability and uncertainty for censored data sets and application to air toxic emission factors. Risk Anal 2004241019–1034. [DOI] [PubMed] [Google Scholar]
- 28.Hamey P Y. An example to illustrate the potential use of probabilistic modelling to estimate operator exposure to pesticides. Ann Occup Hyg 200145(Suppl 1)55–64. [DOI] [PubMed] [Google Scholar]
- 29.Sessink P J M, Anzion R B M, Broek P H H vd.et al Detection of contamination with anti‐neoplastic agents in a hospital pharmacy department. Pharm Weekbl Sci 19921416–22. [DOI] [PubMed] [Google Scholar]
- 30.Sessink P J M.Monitoring of occupational exposure to antineoplastic agents. Nijmegen: University of Nijmegen, 1996
- 31.Turci R, Sottani C, Ronchi A.et al Biological monitoring of hospital personnel occupationally exposed to antineoplastic agents. Toxicol Lett 200213457–64. [DOI] [PubMed] [Google Scholar]
- 32.Keenan R E, Finley B L, Price P S. Exposure assessment: then, now, and quantum leaps in the future. Risk Anal 199414225–230. [DOI] [PubMed] [Google Scholar]
- 33.Cox L A., Jr Reassessing benzene risks using internal doses and Monte–Carlo uncertainty analysis. Environ Health Perspect 1996104(Suppl 6)1413–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semple S, Proud L A, Cherrie J W. Use of Monte Carlo simulations to investigate uncertainty in exposure modeling. Scand J Work Environ Health 200329347–353. [DOI] [PubMed] [Google Scholar]