Abstract
Aims
To examine the effect of silica exposure, in the absence of silicosis, on the prevalence of pulmonary tuberculosis (PTB), which is epidemic among South African gold miners.
Methods
Cross‐sectional study of 520 gold miners over 37 years of age. Length of service, and cumulative and average dust and quartz exposure indices were derived for each miner. Chest radiographs were read for PTB by two NIOSH “B” readers. PTB was defined as a self‐reported history of PTB or PTB on chest radiograph. Logistic regression was used to adjust for age, smoking, and silicosis. PTB effects of different exposure metrics for silica, scaled on their interquartile range (IQR), were compared.
Results
Means (ranges) were: age 46.7 (37.1–59.9) years; length of service 21.8 (6.3–34.5) years; average intensity of respirable quartz 0.053 (0–0.095) mg/m3. PTB prevalence was 19.4% (95% CI 16.0 to 22.8) on history alone, and 35.2% (95% CI 31.1 to 39.3) on history or on chest radiograph. Length of service was poorly predictive of PTB, while all exposure indices which included dust or quartz yielded prevalence odds ratios (PORs) of approximately 1.4 (95% CI ∼1.1 to 1.8) for changes of one interquartile range in exposure. Controlling for silicosis—by adjustment or restriction—did not modify these results. Drillers and winch operators had the highest PTB prevalences and the highest dust and silica exposures.
Conclusion
Older in‐service gold miners in South Africa have a high prevalence of PTB, which is significantly associated with dust and silica exposure, even in the absence of silicosis. Limitations include a survivor workforce and the use of cumulative exposures based on current exposures. Dust control is an important component in control of the PTB epidemic in South African gold mines.
Keywords: dust, mining, quartz, silicotuberculosis, tuberculosis
Pulmonary tuberculosis (PTB) is currently epidemic on South African goldmines, being associated with both silicosis and HIV infection.1 These latter conditions are themselves running at high prevalences in the gold mines. PTB incidence rates among gold miners are very high at 3000/100 000,2 when compared with PTB incidence rates among coal and platinum miners, and that of the general South African population—344/100 000.3 It is notable that TB associated mortality has increased in absolute terms to become the leading cause of death in in‐service gold miners since 1996, mainly because of AIDS.2
It is accepted that the presence of silicosis increases the risk of PTB.2,4,5,6,7 However, the question of whether silica exposure in the absence of silicosis can cause PTB is less clear, and has been identified as a research need.8
Several South African researchers have studied the association between silicosis and PTB.1,7,9,10,11,12,13,14,15 None used directly measured silica exposure, but all found the presence of silicosis to be a strong determinant of PTB, at least in their unadjusted analyses. Kleinschmidt and Churchyard9 used occupation and cumulative service as proxies for silica exposure, but only age and certain occupations like drilling were found to be significantly associated with PTB after adjusting for period, cumulative service, and silicosis. Corbett and colleagues14 found that age was the only predictor of PTB, and although they mentioned surface versus underground work (that is, occupation) as having a protective effect on PTB, they did not analyse any silica exposure or proxy variable in relation to PTB incidence. With regard to HIV, they found that the relative risk of PTB conferred by silica exposure was similar in HIV positive and HIV negative miners and that there were stable PTB rates in HIV negative miners over time. A recently published finding by Sonnenberg and colleagues,21 however, showed increasing PTB rates from 1995 onwards in HIV negative miners. Corbett and colleagues1 showed that the PTB incidence was elevated for increasing grade of silicosis and for exposed occupations (underground versus surface workers) but not with increasing duration of employment. They also showed in a case‐control study from 19997 an effect on PTB incidence of high grade silicosis, focal radiological scarring, and dusty job at diagnosis but not with increasing job duration.
The relationship between PTB and directly measured silica exposure independent of the presence of silicosis has been examined by few authors.16,17,18,19 While findings have been suggestive of such an association, these studies have been hampered by methodological flaws arising from low subject numbers, design problems potentially leading to bias in favour of an association, or have not been set up specifically to examine PTB in relation to silica dust as more than a binary variable.
More generally, all published studies to date have had to rely on silica exposure proxies, which are likely to be misclassified and consequently to dilute silica effects on PTB occurrence. Where actual silica measures have been used, as in Hnizdo and Murray,16 these were estimated gravimetric measures derived from prior konimeter readings from studies of Page‐Shipp and Harris.20
The aim of the current study was to examine whether silica exposure in the absence of silicosis can cause pulmonary tuberculosis, using gravimetric measures of silica exposure, which are more precise than previously employed measures. In addition, a methodological aim was to use odds ratios based on interquartile ranges to explore the relative effects of different exposure metrics on the prevalence of PTB, and to explore the role of silicosis.
Methods
Detailed methods have been published elsewhere.22 Briefly, 520 gold miners over 37 years of age were studied cross‐sectionally when they returned from annual leave in 2000–01 for a history of PTB, smoking habits, and past chest illnesses. Chest radiographs were taken and complete job histories obtained.
A job exposure matrix was derived from time weighted average respirable dust concentrations from two sources: (1) research measurements came from a specifically commissioned occupational hygiene sub‐study; and (2) routine measurements came from government mandated dust surveillance at the mine. The latter were used to estimate exposures for those jobs not covered by the research measurements. Quartz fractions were measured by x ray diffraction. Cumulative respirable dust and quartz exposures were calculated for each miner using length of service and the hygiene data.
Prevalence odds ratios were used to compare the relative effects of different exposure metrics, and to explore possible exposure misclassification. For comparability across exposure metrics, the interquartile range (IQR) of each metric was used as the unit for calculation of odds ratios. Odds ratios are reported here for 1 unit changes in exposure, both on the raw observed scale and for changes of magnitude of one IQR. In the latter case, for any specific exposure metric the odds ratio represents the ratio of the odds of PTB in individuals whose exposure under that metric differs by one IQR.
The main outcome variable was the prevalence of past or present PTB either on self‐reported history or on chest radiograph. PTB changes on the chest radiograph were defined as present if they were identified by either of two readers, using the ILO classification by marking the “tb” box under “other symbols”.23 The chest radiographs were read independently and separately by two NIOSH “B” trained readers. The kappa statistic was used to test for agreement between the readers, and between their readings and a positive history of PTB. For the purposes of the multivariate analysis, silicosis was defined as those with chest radiographs of >1/0. For the purposes of the restricted analysis, silicosis was defined as 1/0 or greater as classified by either of the readers.
Data were analysed using Stata 8.24 Associations between PTB prevalence and exposure, age, smoking, and silicosis were explored and examined graphically. Logistic regression was used to examine adjusted exposure‐response relationships and effect measure modification. A 5% criterion for statistical significance was used.
Ethics approval was obtained from the Research Ethics Committee of the Health Sciences Faculty of the University of Witwatersrand and from the Medical Research Ethics Committee of Anglogold Health Services. Written informed consent was obtained from each subject. Those found to have significant abnormalities were referred to the company clinical services for further evaluation and management.
Results
Characteristics of the study sample are shown in tables 1 and 2. As it was an age restricted sample, the lower end of the age range starts at 37 years with a relatively long mean service duration.
Table 1 Exposure characteristics of study sample (n = 520).
| Mean | SD | Median | Range (IQR) | |
|---|---|---|---|---|
| Age in years | 46.7 | 4.4 | 46.1 | 37.1–59.9 (43.1–50.1) |
| Number of jobs | 5.3 | 2.6 | 5 | 1–16 (3–7) |
| Length of service in years | 21.8 | 5.3 | 21.9 | 6.3–34.5 (18.7–25.3) |
| Cumulative respirable dust (mg.years/m3) | 8.2 | 2.88 | 8.0 | 0–22.68 (6.2–9.9) |
| Average intensity respirable dust (mg/m3) | 0.37 | 0.096 | 0.36 | 0–0.70 (0.31–0.45) |
| Cumulative respirable quartz (mg.years/m3) | 1.15 | 0.43 | 1.12 | 0–3.08 (0.84–1.42) |
| Average intensity respirable quartz (mg/m3) | 0.053 | 0.015 | 0.051 | 0–0.095 (0.033–0.064) |
SD, standard deviation; IQR, interquartile range.
Table 2 Radiological and smoking characteristics of study sample (n = 520).
| Number | % | 95% CI | |
|---|---|---|---|
| PTB on history | 101 | 19.4 | 16.0 to 22.8 |
| CXR prevalence of PTB—Reader 1 | 143 | 27.5 | 23.6 to 31.4 |
| CXR prevalence of PTB—Reader 2 | 90 | 17.3 | 14.0 to 20.6 |
| Prevalence of PTB on history or CXR—either reader | 183 | 35.2 | 31.1 to 39.3 |
| Silicosis 1/1 or greater—Reader 1 | 94 | 18.3 | 15.0 to 21.9 |
| Silicosis 1/1 or greater—Reader 2 | 102 | 19.9 | 16.5 to 23.6 |
| Silicosis 1/0 or greater—either reader | 147 | 28.2 | 24.4 to 32.2 |
| Ever smokers | 267 | 51.3 | 47.0 to 55.7 |
| Current smokers | 150 | 28.8 | 24.9 to 32.8 |
| Ex‐smokers | 117 | 22.5 | 18.9 to 26.1 |
95% CI, 95% confidence interval; CXR, chest x ray.
Agreement between the readers on radiological presence of PTB was 86.7% (expected 64.7%, kappa 0.62, p < 0.0001), between Reader 1's classification and a history of PTB was 78.46% (expected 63.8%, kappa 0.41, p < 0.0001), and between Reader 2's classification and a history of PTB was 82.88% (expected 70.0%, kappa 0.43, p < 0.0001). These kappas represent moderate (0.41–0.60) to substantial (0.61–0.80) agreement beyond chance.25
Among current underground occupations, six jobs accounted for 86% of the workers' current employment (table 3). Two of the jobs contained a higher proportion of workers with PTB than the overall prevalence—driller and winch operator. These jobs were also those with the highest quartz and dust concentrations.
Table 3 Dust concentration and prevalence of PTB by current job title.
| Current job title | Mean respirable dust (mean respirable quartz) | n | PTB on history | PTB on history or radiograph |
|---|---|---|---|---|
| (both in mg/m3) | n (%) | n (%) | ||
| Driller | 0.48 (0.075) | 94 | 25 (27%) | 43 (46%) |
| Winch operator | 0.48 (0.065) | 73 | 18 (25%) | 28 (38%) |
| Team leader | 0.35 (0.054) | 53 | 9 (17%) | 21 (40%) |
| Underground assistant | 0.34 (0.048) | 111 | 15 (14%) | 31 (28%) |
| Crew supervisor | 0.27 (0.038) | 53 | 7 (13%) | 16 (30%) |
| Loco operator | 0.25 (0.038) | 61 | 11 (18%) | 18 (30%) |
| All six jobs | 0.37 (0.055) | 445 | 85 (19%) | 157 (35%) |
Job title was not used as a proxy exposure metric in the analysis because workers had held a number of jobs in their course of employment and cumulative metrics were both calculable and preferred.
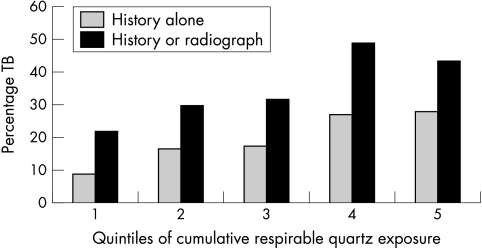
Figure 1 illustrates the prevalence of PTB by cumulative respirable quartz exposure, showing a strong increasing trend across quintiles. All exposure variables show similar significant trend increases in PTB prevalence, as measured by the score test for trend of odds across groups. The strongest trends were for cumulative respirable quartz (fig 1) and for cumulative respirable dust (not shown), with p values <0.001 for both metrics by either definition of PTB—that is, history alone, or history or radiograph.
Figure 1 Prevalence of the two PTB outcome measures by quintiles of cumulative respirable quartz exposure.
There were strong unadjusted associations between PTB prevalence and all exposure metrics except for length of service. PTB was not significantly associated with either age or smoking, but was significantly associated with silicosis.
Prevalence odds ratios (PORs) for the different exposure metrics, adjusted for age and smoking, are shown in table 4, including an additional adjustment for silicosis, plus a restricted analysis excluding the silicotics—that is, those with silicosis on chest radiograph of 1/0 or greater as classified by either reader. The PORs shown in curved brackets are those with adjustment for silicosis, and those in square brackets are those of the restricted analysis. Note that all these PORs correspond to a 1 unit difference in exposure; for example, for cumulative respirable dust and quartz it will be 1 mg.year/m3. Because of the different ranges of the metrics (see table 1) these PORs are not directly comparable. In order to allow for direct comparisons between the various metrics, table 4 also shows PORs corresponding to IQR differences in exposure. All metrics show similar associations with PTB, and apart from length of service, PORs were markedly similar whether adjusting for silicosis or restricting to non‐silicotics.
Table 4 Association between PTB on history or radiograph and different exposure metrics.
| Exposure metric | Prevalence odds ratio | 95% CI | Prevalence odds ratio, scaled in IQR units | 95% CI |
|---|---|---|---|---|
| Length of service in years | 1.042* | 1.000 to 1.086 | 1.31 | 1.00 to 1.72 |
| (1.032)† | (0.990 to 1.077) | (1.24) | (0.94 to 1.63) | |
| (1.026)‡ | (0.984 to 1.070) | (1.19) | (0.90 to 1.57) | |
| [1.033]§ | [0.983 to 1.085] | [1.24] | [0.89 to 1.71] | |
| Cumulative respirable dust (mg.years/m3) | 1.107 | 1.036 to 1.183 | 1.45 | 1.14 to 1.85 |
| (1.093) | (1.021 to 1.170) | (1.38) | (1.08 to 1.77) | |
| (1.080) | (1.008 to 1.156) | (1.32) | (1.03 to 1.70) | |
| [1.097] | [1.010 to 1.191] | [1.40] | [1.04 to 1.89] | |
| Average intensity respirable dust (μg/m3)¶ | 1.003 | 1.001 to 1.005 | 1.48 | 1.13 to 1.95 |
| (1.003) | (1.001 to 1.005) | (1.45) | (1.10 to 1.91) | |
| (1.002) | (1.000 to 1.004) | (1.41) | (1.06 to 1.86) | |
| [1.003] | [1.000 to 1.005] | [1.51] | [1.07 to 2.12] | |
| Cumulative respirable quartz (mg.years/m3) | 1.950 | 1.257 to 3.024 | 1.47 | 1.14 to 1.89 |
| (1.811) | (1.158 to 2.831) | (1.41) | (1.09 to 1.82) | |
| (1.678) | (1.070 to 2.634) | (1.35) | (1.04 to 1.75) | |
| [1.863] | [1.080 to 3.215] | [1.43] | [1.05 to 1.99] | |
| Average intensity respirable quartz (μg/m3)¶ | 1.017 | 1.005 to 1.029 | 1.44 | 1.11 to 1.87 |
| (1.016) | (1.004 to 1.028) | (1.42) | (1.09 to 1.84) | |
| (1.015) | (1.003 to 1.028) | (1.39) | (1.07 to 1.81) | |
| [1.018] | [1.003 to 1.033] | [1.47] | [1.07 to 2.03] |
*Adjusted for age and smoking, n = 520.
†(Adjusted for age, smoking, and silicosis >1/0 as classified by Reader 1, n = 520).
‡(Adjusted for age, smoking, and silicosis >1/0 as classified by Reader 2, n = 520).
§[In non‐silicotics, adjusted for age and smoking, n = 373].
¶Units for average intensities of exposure are in micrograms to display more easily interpretable prevalence odds ratios.
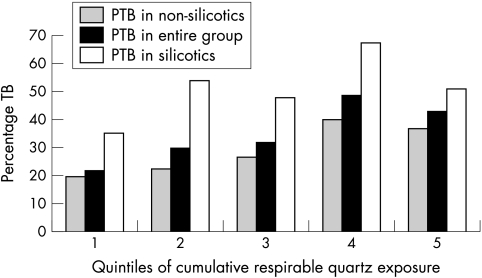
Figure 2 shows the trend in the prevalence of PTB among non‐silicotics, among the entire group, and among silicotics by quintile of cumulative respirable quartz exposure. All three show increasing trends for prevalence of PTB while only those for non‐silicotics and for the entire group were significant at the 5% level.
Figure 2 Prevalence of PTB on history or radiograph by quintiles of cumulative respirable quartz exposure, among non‐silicotics, in the entire group, and among silicotics alone.
When we used other outcome measures, namely PTB on history alone, PTB on history or Reader 1's PTB reading, and PTB on history or Reader 2's PTB reading, the findings were all similar.
Effect measure modification was examined by including interaction terms for exposure with other measured variables, including the presence of silicosis, in regression analyses. No significant interactions were found.
Discussion
The very high prevalences of PTB in this workforce of older gold miners as indicated by any of the criteria used in this study are similar to those found in studies of comparable Southern African mineworker populations.26 A 35% prevalence of PTB based on the most sensitive definition was observable despite the likelihood that sicker workers had been selected out of the workforce.
Our analysis has shown significant associations between measures of silica and dust exposure and PTB prevalence. The higher PTB prevalences found in occupational groups such as drillers and winch operators are consistent with these effects and concur with Kleinschmidt's finding that drilling is strongly associated with a high incidence of PTB.9
This study of PTB prevalence in active mineworkers was the first in South Africa to use individually attributable exposure variables based on gravimetrically measured dust and silica. This has allowed comparisons between the effects of different exposure metrics (including proxies such as length of service or occupation) on the prevalence of PTB. Significant unadjusted relationships were found between PTB prevalence and these metrics. All measures were similar in effect except for length of service, which was of borderline significance. Again with the exception of length of service, these exposure effects persisted after adjustment for age and smoking.
Exposure misclassification occurs when length of service is used as a proxy for exposure, and this causes dilution of the effect compared with effects obtained using gravimetrically based exposure metrics. This explains how the published literature might have been interpreted as showing that increasing exposure based on length of service alone on the gold mines does not confer higher risk of PTB,1,7,9,14,21 or does so only after many years.
A second source of exposure misclassification and effect dilution is suggested by the virtually identical IQR based PORs for all the exposure variables based on gravimetric measurements. As a known aetiological agent of PTB, quartz based exposure metrics would be expected to have stronger effects than other metrics, but this does not show up in our results.
The effect of silica on PTB prevalence in this study is not accounted for by the presence of silicosis. Even after adjustment for the presence of radiological silicosis, and in the analysis restricted to the subgroup of non‐silicotics, there is almost as strong and significant a residual association between silica exposure and PTB prevalence. This confirms what Hnizdo and Murray16 found in white miners—that silica was a risk factor for PTB even in the absence of both radiological and necropsy evidence of silicosis.
Silicosis has been shown to be neither a confounder nor a mediator in these data, nor has it been shown to be an effect measure modifier. It is thus most likely to be a simple marker of substantial silica exposure, explaining the frequent reports in the literature of silicosis as a risk factor for PTB. Given the reliance on proxies for exposure and associated exposure misclassification that characterises much of the literature, it is understandable that the silica effect on PTB independently of the presence of silicosis has been less clearly linked to silica exposure than the relationship between silicosis and PTB.
The value of using odds ratios expressed in comparable IQR units is clear from a comparison of columns in table 4. Odds ratios corresponding to one unit changes in exposure on the raw scale are associated with significant point estimates that are, however, very close to the null, and are not easily interpretable with respect to size or relevance. In addition, the confidence limits could be indeterminable. An example of this is found in table 4, where the units of average intensities of dust and quartz were scaled to micrograms/m3, because using the original units in milligrams/m3 resulted in very large upper confidence limits that were difficult to interpret. This occurred because the entire ranges of the average intensities of dust and quartz were well below 1, rendering a one unit increase meaningless. Expressing odds ratios in IQR units meant that the point estimates were directly relevant to the typical variation in exposure, as was borne out in the last columns of table 4. Columns 2 and 4 differ merely in the definition of a unit change in exposure and this has no impact on the strength of association. The method of IQR based odds ratios has been used in recent studies on ambient air pollution, for example in Beeson and colleagues,27 and should increasingly find its way into use in occupational health research.
This study had some limitations. Being a prevalence study of a survivor population may have led to underestimates of PTB prevalence and dilution of the silica effect, as measured by any metric, including length of service. A more appropriate design for causal interpretation would have been a prospective study of PTB incidence with direct estimation of relative risk. However, the finding of a silica effect despite a potential bias towards the null strengthens the findings in the current study. Nevertheless, there is a clear need to conduct a prospective cohort study of PTB incidence and its determinants on the mines. Although such studies should be relatively easy to undertake because of the high PTB incidence, which would yield sufficient numbers of PTB cases over a short period of time, achieving adequate working life exposure attribution would remain a problem given the current poor state of exposure surveillance on South African mines.
Main messages
The prevalence of pulmonary tuberculosis in currently employed older South African gold miners is very high at 35%.
Pulmonary tuberculosis is significantly associated with dust and with silica exposure, independently of the presence of silicosis.
Length of service is a poor proxy for exposure compared with indices that include gravimetric measures of intensity.
Another limitation of the current study is that exposure levels measured in 2000 and 2001 when the study was conducted may not be representative of exposures throughout the working life of these miners. If exposures have declined over time, there may be overestimation of the PTB risk if cumulative exposure estimates are based on current exposures. There is a paucity of reliable published occupational hygiene data for the South African mines, which are further complicated by differences in measurement technology over time.22 However, evidence exists that historical dust levels have indeed been constant over a period of many years, with the first gravimetric dust measurements performed in the 1990s being in the same range as that of the current study, and no technical changes that could have led to reduced exposures.22,28 If anything, underground dust exposure may have increased in both intensity and duration due to deeper level mining and longer service of miners. This potential bias is therefore likely to be insignificant.
A third possible limitation is that the main outcome measure—PTB on history or radiograph—may have predated the period of occupational exposure to silica. Such “non‐occupational TB” could result in misclassification of “occupational TB”, but there is no reason to believe that this is differential with respect to exposure. Hence it is more likely that pre‐existent PTB would dilute the exposure‐response finding, adding credibility to these findings.
The observation that length of service is a poor proxy for exposure for tuberculosis while it is a good proxy for exposure in relation to silicosis22 could be explained in two ways. The healthy survivor effect may operate differentially for silica effects on silicosis and tuberculosis, as workers are sicker with PTB than with silicosis and thus less likely to survive in the workforce. Additionally, as the presence of PTB and especially PTB together with silicosis, could lead to unemployability on the mines, the miners may be inclined not to admit a past history of PTB.
It is notable that the measured exposure levels mostly fell below the South African occupational exposure limit (OEL) of 0.1 mg/m3. However, there is evidence that quartz exposures down to below 0.05 mg/m3 may not be protective for silicosis22,29,30 and the ACGIH intends to lower its threshold limit value to 0.025 mg/m3 in 2005.31 It is notable that 75% of the quartz intensities in table 1 are above the ACGIH intended change.
Since silica exposure is associated with both an increased risk of PTB and silicosis, and since the exposure at the levels outlined above is not protective for silicosis, they may well be insufficiently protective for PTB. More precise estimates of the silica effect on PTB are needed and could be obtained by means of a specifically designed prospective study.
Because the current silica OELs at 0.1 mg/m3 is known to cause silicosis, there is an urgent need for dust as well as TB control measures to halt the PTB epidemic on the goldmines. Such measures will require heightened medical surveillance for PTB in miners exposed to silica, and heightened hygiene surveillance for silica exposures above 0.025 mg/m3, followed by preventive action.
Policy implications
There is a need for increased tuberculosis surveillance in groups exposed to higher dust and silica levels on the mines.
Dust and quartz control must be part of the pulmonary tuberculosis control strategy.
This study has highlighted the need for better occupational hygiene and medical surveillance practice on the mines.
Acknowledgements
We would like to thank the staff of Aurum Health Research, West Vaal Hospital Occupational Health Centre, and West Vaal Region Occupational Hygiene Department for their assistance in conducting this study. We acknowledge Prof. Mary Ross for her support during the conduct of this study, Marco Biffy for carrying out the quartz fraction analysis, Anglogold and Anglogold Health Service for their permission to publish the data, and Prof. Tom Robins for his help with the method of using IQRs. This study was commissioned and funded by the South African Government Department of Minerals and Energy Affairs Mine Health and Safety Council as HEALTH 606 within the research programme of the Safety in Mines Research Advisory Committee (SIMRAC).
Footnotes
Competing interests: none declared
References
- 1.Corbett E L, Churchyard G J, Clayton T C.et al HIV infection and silicosis: the impact of two potent risk factors on the incidence of mycobacterial disease in South African miners. AIDS 2000142759–2768. [DOI] [PubMed] [Google Scholar]
- 2.Churchyard G J, Corbett E L. Tuberculosis and associated diseases. In: Guild R, Ehrlich RI, Johnston JR, Ross MH, eds. A handbook on occupational health practice in the South African mining industry. Johannesburg: Safety in Mines Research Advisory Committee, 2001
- 3.World Health Organisation Global tuberculosis control: surveillance, planning, financing. Geneva, Switzerland: WHO, 2004
- 4.Steen T W, Gyi K M, White N W.et al Prevalence of occupational lung disease among Botswana men formerly employed in the South African mining industry. Occup Environ Med 19975419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trapido A S, Mqoqi N P, Williams B G.et al Prevalence of occupational lung disease in a random sample of former mineworkers, Libode District, Eastern Cape Province, South Africa. Am J Ind Med 199834305–313. [DOI] [PubMed] [Google Scholar]
- 6.Corbett E L, Churchyard G J, Moyake T.et al HIV infection, silicosis and mycobacterial disease incidence in South African miners. Int J Tuberc Lung Dis 19982S301 [Google Scholar]
- 7.Corbett E L, Churchyard G J, Clayton T.et al Risk factors for pulmonary mycobacterial disease in South African gold miners: a case‐control study. Am J Respir Crit Care Med 199915991–97. [DOI] [PubMed] [Google Scholar]
- 8.NIOSH Hazard Review. Health effects of occupational exposure to respirable crystalline silica. DHHS (NIOSH) Publication No. 2002–129. Cincinnati, OH: Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, April, 2002
- 9.Kleinschmidt I, Churchyard G. Variation in incidences of tuberculosis in subgroups of South African gold miners. Occup Environ Med 199754636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray J, Kielkowski D, Reid P. Occupational disease trends in black South African gold miners. An autopsy‐based study. Am J Respir Crit Care Med 1996153706–710. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg P, Murray J, Glynn J R.et al Risk factors for pulmonary disease due to culture‐positive M. tuberculosis or nontuberculous mycobacteria in South African gold miners. Eur Respir J 200015291–296. [DOI] [PubMed] [Google Scholar]
- 12.Churchyard G J, Kleinschmidt I, Corbett E L.et al The epidemiology of mycobacterial disease in South African gold miners in the era of HIV‐infection. Int J Tuberc Lung Dis 19993791–798. [PubMed] [Google Scholar]
- 13.Churchyard G J, Kleinschmidt I, Corbett E L.et al Factors associated with an increased case‐fatality rate in HIV‐infected and non‐infected South African gold miners with pulmonary tuberculosis. Int J Tuberc Lung Dis 20004705–712. [PubMed] [Google Scholar]
- 14.Corbett E L, Charalambous S, Fielding K.et al Stable incidence rates of tuberculosis (TB) among human immunodeficiency virus (HIV)‐negative South African gold miners during a decade of epidemic HIV‐associated TB. J Infect Dis 20031881156–1163. [DOI] [PubMed] [Google Scholar]
- 15.Cowie R L. The epidemiology of tuberculosis in gold miners with silicosis. Am J Respir Crit Care Med 19941501460–1462. [DOI] [PubMed] [Google Scholar]
- 16.Hnizdo E, Murray J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occup Environ Med 199855 pp 496-502 erratum Occup Environ Med 199956215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balmes J. Silica exposure and tuberculosis: an old problem with some new twists [editorial]. J Occup Med 199032114–115. [PubMed] [Google Scholar]
- 18.Sherson D, Lander F. Morbidity of pulmonary tuberculosis among silicotic and nonsilicotic foundry workers in Denmark. J Occup Med 199032110–113. [PubMed] [Google Scholar]
- 19.Calvert G M, Rice F L, Boiano J M.et al Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 200360122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page‐Shipp R J, Harris E. A study of the dust exposure of white gold miners. J S Afr Inst Miner Met 19727310–24. [Google Scholar]
- 21.Sonnenberg P, Glynn J R, Fielding K.et al HIV and pulmonary tuberculosis: the impact goes beyond those infected with HIV. AIDS 200418657–662. [DOI] [PubMed] [Google Scholar]
- 22.Churchyard G J, Ehrlich R, teWaterNaude J M.et al Silicosis prevalence and exposure‐response relations in South African gold miners. Occup Environ Med 200461811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Labour Office Guidelines for the use of ILO international classification of radiographs of pneumoconioses. Occupational Safety and Health Series No. 22. Geneva: ILO, 1980
- 24.Stata Intercooled Stata 8.0 for Windows. USA: Stata Corpororation, 2003
- 25.Crewson P E. Reader agreement studies. Am J Roentgenol 2005184 pp 1391-7 (quoting Landis JR, Koch GG, A one‐way components of variance model for categorical data. Biometrics 197733671–679. [DOI] [PubMed] [Google Scholar]
- 26.White N W, Steen T W, Trapido A S.et al Occupational lung diseases among former gold miners in two labour sending areas. S Afr Med J 200191599–604. [PubMed] [Google Scholar]
- 27.Beeson W L, Abbey D E, Knutsen S F. Long‐term concentrations of ambient air pollutants and incident lung cancer in California adults: results from the AHSMOG study. Adventist Health Study on Smog. Environ Health Perspect 1998106813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kielblock A J, Franz R M, Unsted A D.et alQuantitation of occupational health risks in the South African mining industry and assessment of sources of uncertainty in the estimates. Johannesburg: Council for Scientific and Industrial Research Division of Mining Technology, 1997
- 29.Sherson D. Silicosis in the twenty first century. Occup Environ Med 200259721–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greaves I A. Not‐so‐simple silicosis: a case for public health action. Am J Ind Med 200037245–251. [DOI] [PubMed] [Google Scholar]
- 31.ACGIH Annual reports for the year 2004: Committees on Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs). Threshold Limit Values for chemical substances (TLV‐CS) Committee. Notice of Intended Changes: TLV NICs for 2005, American Conference of Governmental Industrial Hygienists, Cincinnati, OH 2005. (NIC)