Abstract
Objectives
To study inhalation and dermal exposure to hexamethylene diisocyanate (HDI) and its oligomers as well as personal protection equipment (PPE) use during task performance in conjunction with urinary hexamethylene diamine (HDA) in car body repair shop workers and industrial spray painters.
Methods
Personal task based inhalation samples (n = 95) were collected from six car body repair shops and five industrial painting companies using impingers with di‐n‐butylamine (DBA) in toluene. In parallel, dermal exposure was assessed using nitril rubber gloves. Gloves were submerged into DBA in toluene after sampling. Analysis for HDI and its oligomers was performed by LC‐MS/MS. Urine samples were collected from 55 workers (n = 291) and analysed for HDA by GC‐MS.
Results
Inhalation exposure was strongly associated with tasks during which aerosolisation occurs. Dermal exposure occurred during tasks that involve direct handling of paint. In car body repair shops associations were found between detectable dermal exposure and glove use (odds ratio (OR) 0.22, 95% confidence interval (CI) 0.09 to 0.57) and inhalation exposure level (OR 1.34, 95% CI 0.97 to 1.84 for a 10‐fold increase). HDA in urine could be demonstrated in 36% and 10% of car body repair shop workers and industrial painting company workers respectively. In car body repair shops, the frequency of detectable HDA was significantly elevated at the end of the working day (OR 2.13, 95% CI 1.07 to 4.22 for 3–6 pm v 0–8 am). In both branches HDA was detected in urine of ∼25% of the spray painters. In addition HDA was detected in urine of a large proportion of non‐spray painters in car body repair shops.
Conclusion
Although (spray) painting with lacquers containing isocyanate hardeners results in the highest external exposures to HDI and oligomers, workers that do not perform paint related tasks may also receive a considerable internal dose.
Keywords: isocyanate, oligomers, dermal, biomonitoring, spray painting
In industrialised countries isocyanates are one of the most common causes of occupational asthma.1,2,3 As part of an epidemiological study on isocyanate exposure and related health effects in the Netherlands, we recently assessed inhalation exposure to a range of isocyanates for end users of polyurethane (PU) lacquers in car body repair shops and industrial painting companies.4 Although inhalation exposure is probably the most important route through which allergic sensitisation is achieved, there is limited evidence that dermal contact may result in respiratory sensitisation5 as well as disease aggravation in humans.6 Animal studies have shown that topical exposure to isocyanates can result in respiratory sensitisation7,8,9 and vice versa.10 During the process of spray painting, direct contact with lacquers is likely to occur.11,12 The presence of contaminated surfaces and skin exposure to isocyanates in car body repair shops was confirmed by a qualitative study.13 However, no established methods exist for quantitative assessment of dermal exposure to isocyanates.
The field of external isocyanate sampling and analysis is complex because isocyanates are highly reactive and may be present as mono‐, di‐, and poly‐isocyanates, both in vapour and particulate form.14,15 In the present paper all polymeric isocyanates, which are indicated with different terms (polyisocyanates, oligomers, adducts) in the literature, will be indicated as oligomers. In addition, large variability in exposure levels and the widespread use of control measures like filtering respirators during high risk tasks complicate the interpretation of external concentrations with respect to a total internal dose.4 Therefore biomonitoring of corresponding amines in hydrolysed urine has been suggested as a measure of total internal diisocyanate exposure received by all routes of exposure.
Few, mostly small, studies on urinary hexamethylene diamine (HDA) in relation to inhalation exposure data have been conducted. Elevated HDA levels were found in hexamethylene diisocyanate (HDI) monomer production and manufacturing workers16 as well as subjects exposed by inhalation to HDI monomer in a test chamber.17,18 In addition, several studies have been conducted on toluene diisocyanate (TDI) and methylenebisphenyl diisocyanate (MDI) exposure in relation to corresponding amines.19,20,21,22,23,24 Although in most product formulations monomeric diisocyanates have been replaced by their oligomers, knowledge on bio‐transformation and metabolites reflecting isocyanate oligomer exposure is lacking. Apart from one test chamber study on HDI biuret and urinary HDA, no (field) studies on exposure to isocyanate oligomers in relation to corresponding amines in urine have been performed.25
In the present study a method for the quantitative short term, task based assessment of dermal exposure to isocyanates was developed. Determinants of dermal exposure were studied. In addition, task based inhalation and dermal exposure measurements, taken in parallel, were used to identify tasks with elevated exposure. Furthermore, urine samples were taken during the measurement day and analysed for HDA. These samples were compared to tasks performed on the measurement day to gain insight in HDA in relation to tasks and exposure. This study is the first to quantitatively assess dermal exposure and combine dermal and inhalation exposure measurements with biomonitoring.
Methods
Population
Exposure assessment was carried out as part of an epidemiological study on isocyanate exposure and health effects in car body repair shop workers and industrial spray painters in the Netherlands. The study currently involves 520 workers from 98 companies. Car body repair shops were enrolled using databases from the branch organisation (written information to 763 companies; positive response rate: 11%) and the Chamber of Commerce (written information to 93 companies; positive response rate: 9%). Industrial painting companies were contacted through shipyards; companies were therefore specialised in painting of mainly ships and harbour equipment (telephone contact with 16 companies; positive response rate: 69%). Measurements were performed in a random selection of these companies.
Sampling strategy
Within the epidemiological study, large scale task based inhalation exposure assessment has been carried out. Preliminary data from this study showed that paint related tasks result in highest exposures.4 As dermal exposure is only expected during manual handling of paint, we chose to include mainly paint related tasks in the present study. Welding was also included since heating of PU containing materials may give rise to emission of the original monomers and other isocyanate species. Because the inhalation study demonstrated that HDI and its oligomers are the most dominant compounds, these were used as exposure measures.
In six car body repair shops, 68 task based inhalation and dermal samples were taken in parallel during the following tasks: mixing PU lacquer, spraying PU lacquer, cleaning spray guns, and welding. Additionally, in five industrial painting companies, 27 task based inhalation and dermal samples were taken in parallel during the following tasks: mixing PU lacquer, spraying PU lacquer, rolling/brushing PU lacquer, and assisting a spray painter. Together with the personal exposure measurements, data on work circumstances and PPE use were collected.
In addition to exposure measurements, all workers in the company were asked to collect urine samples during 24 hours, starting from pre‐shift on the measurement day until pre‐shift on the next day. Only workers of whom at least three urine samples were collected were included in the biomonitoring study. Coinciding with urine collection, workers were asked to complete a short questionnaire on activities and PPE use during the measurement day. In total, 45 car body repair shop workers and 10 industrial paint shop workers participated in the urine collection, from which 239 and 52 urine samples were collected, respectively. Workers on whom external exposure measurements were performed did not always participate in the biomonitoring study.
Sampling was carried out during July–November 2004.
Inhalation exposure measurements
Task based personal air samples were collected at 1 l/min using midget impingers, containing 10 g (11.5 ml) 0.01 M di‐n‐butylamine (DBA) in toluene, attached to the lapel.26 Gillian personal sampling pumps were calibrated before and after sampling with a rotameter; average flows were used for calculations. When spray painting involved several layers, the pump was stopped during intermitting times. The use of impingers in combination with the volatile toluene results in limited sampling times and therefore eliminates the possibility of taking eight hour samples.
Dermal sampling method
A dermal sampling procedure was set up using nitril rubber gloves (Romed powder free NT 810) without a reagent for sampling. After sampling, gloves are immediately submerged into 0.01 M DBA in toluene and removed after an extraction time. Prior to field sampling, two laboratory experiments were conducted to determine extraction time and the effect of measurement time.
Toluene causes decomposition or leaching of compounds from the gloves, resulting in interference with the liquid chromatography and tandem mass spectrometry (LC‐MS/MS) analysis. Suitable extraction times were determined as follows. A drop of hardener without solvent was applied to four nitril rubber gloves that were immediately submerged into flasks containing 200 ml 0.01 M DBA in toluene. Gloves were removed after 8, 24, 72, and 168 hours and analysed.
Since gloves do not contain a reagent to capture isocyanates during sampling, the effect of measurement time on recovery of different masses of isocyanates was studied. A dilution series of HDI (Sigma‐Aldrich, Zwijndrecht, Netherlands) and of a mixture of HDI oligomers (poly(hexamethylene diisocyanate); Sigma‐Aldrich, Zwijndrecht, Netherlands) in toluene was applied separately on nitril rubber gloves. The following masses were used: 2.5, 10, and 40 µg HDI (1.25, 5, and 20 µg NCO) or oligomer mixture (NCO content unknown) in 20 µl toluene. Gloves were submerged into 200 ml 0.01 M DBA in toluene after 0, 10, and 30 minutes and removed after 24 hours. In addition, both dilution series were directly added to 200 ml 0.01 M DBA in toluene. The whole experiment was conducted in duplicate.
Dermal exposure measurements
During field sampling, task based dermal samples were collected in parallel with inhalation samples using nitril rubber gloves for sampling. Workers wore the gloves underneath normal PPE during the task. Gloves from both hands were submerged into one flask containing 200 ml 0.01 M DBA in toluene immediately after the task was finished. Measurement times were equal to air sampling times, except for when air sampling was stopped in between separate layers of spray painting.
Urine sampling
Urine samples were collected by the workers during 24 hours, starting from pre‐shift on the measurement day until the morning of the next day. Printed instructions on the procedure and hygiene of urine collection and name labelled urine containers (500 ml) for each worker were delivered at the company on the day before the measurement day. A sticker was adhered to each container on which the worker registered date and time of urine collection. Urine samples were stored at room temperature and collected on the day following the measurement day.
Analysis and quantification of air and dermal samples
Immediately after sampling, isocyanate groups derivatise with DBA in the impingers for inhalation samples, or in the sampling flasks for dermal samples. After sampling, samples were stored at 4°C.
Compounds were separated by reversed phase high performance liquid chromatography (RP‐HPLC) ionised with electrospray in the positive ionisation mode and detected with tandem mass spectrometric detection (MS‐MS). All compounds were quantified in a single analytical run. Analysed compounds in the field samples were HDI and oligomers of HDI (uretidone, isocyanurate, biuret, diisocyanurate, unknown oligomer of HDI).
A deuterated internal standard (derivatised with DBA) and derivatised external standard were used for calibration of HDI.26 D9‐DBA derivatised external standards were available for the quantification of biuret, isocyanurate, and diisocyanurate. For uretidone and an unknown oligomer of HDI, no standards were available. Based on the structure of the molecule it was decided to use the biuret calibration for uretidone and consequently express uretidone in biuret equivalents. The unknown oligomer of HDI was expressed in diisocyanurate equivalents.
To be able to interpret the contribution of individual compounds, all concentrations are expressed in μg/m3 isocyanate groups (N = C = O group: NCO group) in air or total mass (μg) NCO group on two gloves (irrespective of sampling time), calculated as the concentration of the compound divided by its molecular weight times the number of NCO groups times the molecular weight of NCO (42): (Ccompound/MWcompound)×NNCO×MWNCO.
The limit of detection (LOD) for inhalation samples depends on compound and measurement time. The maximum LOD (calculated with the minimum measurement time of 1 min, standard volume of 11.5 ml, and standard flow of 1 l/min) in this study is roughly 0.1 μg/m3 NCO for HDI and 1.4–37.7 μg/m3 for oligomers of HDI. These LODs decrease linearly when measurement time increases.
The LOD for dermal samples depends merely on the compound and is 0.3 μg on two gloves for HDI and 0.3–5.3 μg on two gloves for oligomers of HDI (based on 200 ml derivatisation solution).
In the samples from the laboratory experiments for the dermal sampling method, HDI and isocyanurate were analysed. Isocyanurate was selected because this is the most common oligomer found in the inhalation study.4 Setting the dilution series that was directly added to DBA in toluene at 100%, the results of these samples are expressed as mean percentage recovery for each duplicate.
Analysis and quantification of urine samples
Urine samples were frozen until sample preparation was carried out. Aliquots of 2 ml of each sample were at first mixed with 3 ml 6 M hydrochloric acid (HCl) (37%, Merck, Darmstadt, Germany) and then spiked with 50 µl internal standard solution (1,7–diaminoheptane (HpDA) (purity 98%, Aldrich, Taufkirchen, Germany, at 1.0 mg/l in water). Samples were heated for 16 hours (overnight) at 100°C. After cooling to room temperature, the acid hydrolysate was basified by the addition of 4 ml fresh saturated solution of sodium hydroxide (NaOH) (purity 99%, Merck, Darmstadt, Germany). The samples were then extracted with 3 ml toluene (purity 99.5%, Merck, Darmstadt, Germany). Two ml dried organic phase by Na2SO4 (p.a. Merck, Darmstadt, Germany) were used in derivatisation by adding 25 µl pentafluoropropionic anhydride (purity 99%, Aldrich, Taufkirchen, Germany). The vials were closed tightly, and shaken for 1 min. The derivatisation was stopped by adding 3 ml 1 M phosphate buffer (tri‐potassium phosphate p.a., Riedel‐de‐Haen, Taufkirchen, Germany, pH 7.5). After centrifugation, the organic phase was supplemented with 100 µl n‐decane (purity 98%, Fluka, Taufkirchen, Germany) as keeper and then evaporated with nitrogen to a residual volume of about 100 µl.
Two µl of this solution containing HDA amide derivative were analysed by gas chromatography/mass spectrometry in selected ion‐monitoring mode on an Agilent mass spectrometry detector MSD HP 5973 connected to a gas chromatograph HP 6890, equipped with an autosampler (GC/MS: Agilent, Waldbronn, Germany). The separation was performed on a capillary column HP‐5MS (30 m×0.25 mm) with a film thickness of 0.25 µm. The column was held at 100°C for 2 min, ramped at 10°C/min to 280°C. Injections were performed in the splitless mode under helium at a flow rate of 1.2 ml/min. Under these conditions, the retention times for 1,6‐diaminohexane (HDA) (purity 98%, Aldrich, Taufkirchen, Germany) and HpDA were 9.63 min and 10.68 min, respectively. The specific ions, i.e. m/z 176 and 232 for HDA and m/z 176 and 303 for HpDA, were selected as quantifier and qualifier, respectively. The quantification was achieved by comparison with a calibration curve in the range 5–100 µg/l. The limit of detection for HDA calculated from a signal‐to‐noise ration of 3:1 was found to be 3.0 µg/l. Urinary creatinine in each sample was determined in g/l using HPLC (Merck‐Hitachi, Darmstadt, Germany).27 HDA concentration was then expressed in µg/g creatinine.
Each urine sample was analysed twice. The mean was reported when the difference relative to the arithmetic mean was less than 5% (>90% of samples). If the difference was more than 5%, the analysis was repeated at least twice.
To compare our method with another described method measuring HDA diurethane derivative, part of the samples was assayed with both procedures.28 The resulting regression between data from both sources was linear and the correlation coefficient was greater than 0.95.
Statistical analysis
SAS statistical software (SAS System for Windows, version 8.02; SAS Institute, Cary, NC) was used for data analysis. Due to the large proportion of samples with non‐detectable concentrations, exposure distributions were (severely) truncated to the left for the majority of the individual compounds. Therefore external as well as internal exposure is described by the frequency of detects and the minimum, median, and maximum concentration for samples above LOD.
The association between both dermal exposure and urinary HDA and covariates was studied by means of logistic regression (PROC GENMOD). To account for correlation between repeated measurements on the same worker, the generalised estimating equations (GEE) method was used.29 Consequently, all presented odds ratios (ORs) are corrected for correlation among repeated measurements. The binary response variable for dermal exposure (detected/not detected, diisocyanates or oligomers) was modelled as a function of glove use (yes/no) and inhalation exposure level (10‐fold increase, µg/m3). For this analysis inhalation concentrations below LOD were replaced by LOD/2. The binary response variable for HDA in urine (detected/not detected) was modelled as a function of a categorised time interval variable (8–12 am, 0–3 pm, 3–6 pm, 6–12 pm, and 0–8 am on the next day versus 0–8 am on the measurement day).
Results
Dermal sampling method
Isocyanates could be determined in the samples from which gloves were removed after 8 and 24 hours. Longer removal periods resulted in samples in which too many glove compounds were present that interfered with the LC‐MS/MS analysis. The extraction time was therefore set to a minimum of 8 and maximum of 24 hours.
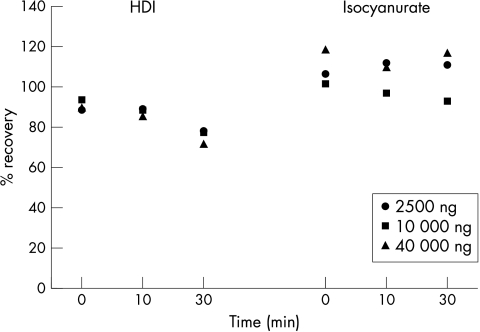
Figure 1 shows the mean percentage recovery for different masses of HDI and isocyanurate after 0, 10, and 30 minutes on the glove before submersion into DBA in toluene (isocyanates directly added to DBA in toluene = 100%). The recovery of HDI ranged form 75 to 95% and a slightly declining time trend could be observed. For isocyanurate the recovery was around 100% without a clear time trend. The percentage difference relative to the arithmetic mean was below 17% for the HDI and isocyanurate duplicates.
Figure 1 Percentage recovery (mean) for HDI and isocyanurate (2500, 10 000, and 40 000 ng) after 0, 10, and 30 minutes on a nitril rubber glove before submersion in DBA solution (dilution series directly added to DBA solution = 100%).
Inhalation and dermal exposure
Table 1 gives an overview of the inhalation exposure levels to HDI and the sum of its oligomers separately. Oligomers of HDI dominated over the monomer during all tasks. In both branches inhalation exposure was highest during tasks where paint is aerosolised: spraying and assisting the spray painter. During other tasks the frequencies of detectable samples as well as exposure levels were lower.
Table 1 Task based inhalation exposures in car body repair shops and industrial painting companies; percentage of samples above the limit of detection (LOD) and exposure median and range (µg/m3 NCO) for samples above LOD.
| n | Sampling time (min) | 1,6‐HDI | Oligomers | |||
|---|---|---|---|---|---|---|
| Median (range) | % >LOD | Median (range) | % >LOD | Median (range) | ||
| Car body repair shops | ||||||
| Mixing PU lacquer | 15 | 4 (2–7) | 20 | 1.0 (0.2–2.7) | 27 | 1.4 (0.3–33.1) |
| Spraying PU lacquer | 31 | 8 (3–40) | 65 | 2.1 (0.2–6.5) | 87 | 116.3 (2.5–728.4) |
| Cleaning spray gun | 19 | 3 (1–8) | 0 | – | 32 | 11.1 (1.6–45.3) |
| Welding | 3 | 22 (5–29) | 33 | 0.04 | 33 | 0.1 |
| Industrial painting company | ||||||
| Spraying PU lacquer | 10 | 25 (7–33) | 100 | 3.7 (0.03–28.8) | 100 | 199.6 (6.4–2613.8) |
| Rolling/brushing PU lacquer | 11 | 25 (7–41) | 100 | 0.02 (0.01–0.1) | 46 | 0.7 (0.1–5.3) |
| Mixing PU lacquer | 3 | 8 (4–10) | 67 | 0.5 (0.01–1.0) | 67 | 10.8 (1.6–20.0) |
| Assisting spray painting | 3 | 31 (29–40) | 100 | 0.3 (0.09–4.4) | 100 | 14.2 (6.3–347.7) |
Table 2 shows dermal exposure mass to HDI and the sum of its oligomers separately. As in the case of inhalation exposure, oligomers of HDI dominated over the monomer during all tasks. In both car body repair shops and industrial painting companies, dermal exposure was found most frequently during tasks where direct exposure with lacquers might occur: mixing, applying, and cleaning. Detectable oligomer exposure was found more frequently in industrial painting companies but exposure ranges were larger in car body repair shops. Less contrast in dermal exposure masses was observed between the tasks than in inhalation exposure levels.
Table 2 Task based dermal exposure mass on two gloves in car body repair shops and industrial painting companies; percentage of samples above the limit of detection (LOD) and exposure median and range (µg NCO) for samples above LOD.
| n | Sampling time (min) | 1,6‐HDI | Oligomers | |||
|---|---|---|---|---|---|---|
| Median (range) | % >LOD | Median (range) | % >LOD | Median (range) | ||
| Car body repair shops | ||||||
| Mixing PU lacquer | 15 | 4 (2–7) | 47 | 2.2 (0.3–20.1) | 53 | 207.3 (19.5–2848.5) |
| Spraying PU lacquer | 31 | 8 (3–40) | 39 | 1.3 (0.3–10.2) | 42 | 133.2 (6.5–1507.0) |
| Cleaning spray gun | 19 | 3 (1–8) | 42 | 1.3 (0.3–2.0) | 32 | 33.8 (15.5–315.8) |
| Welding | 3 | 22 (5–29) | 0 | – | 0 | – |
| Industrial painting company | ||||||
| Spraying PU lacquer | 10 | 25 (7–33) | 10 | 0.5 | 90 | 43.7 (3.8–209.8) |
| Rolling/brushing PU lacquer | 11 | 25 (7–41) | 0 | – | 91 | 15.7 (3.5–153.9) |
| Mixing PU lacquer | 3 | 8 (4–10) | 0 | – | 100 | 63.0 (3.5–95.3) |
| Helper spray painting | 3 | 31 (29–40) | 0 | – | 33 | 0.7 |
ORs for the association between detectable isocyanate masses on the sampling gloves and glove use (observed by a field worker) as well as inhalation exposure level separately for both branches are given in table 3. In car body repair shops the association for glove use was highly significant. When including both covariates in the same model, the effect of glove use and inhalation exposure remained similar. In industrial painting companies no positive association could be found with inhalation levels and no OR for glove use could be computed since all workers wore gloves.
Table 3 Odds ratios for the univariate association between detectable task based dermal exposure and glove use and inhalation exposure level separately during all measured tasks.
| Car body repair shops | Industrial painting companies | |
|---|---|---|
| Gloves v no gloves | 0.22 (0.09–0.57) | –* |
| Inhalation exposure level (µg/m3 NCO)† | 1.34 (0.97–1.84) | 0.97 (0.68–1.38) |
*No OR calculated since all workers used gloves.
†OR for a 10‐fold increase in inhalation exposure levels.
Urinary HDA
On the sampling days, 239 urine samples were collected from 45 car body repair shop workers and 52 urine samples were collected from 10 industrial painting company workers (table 4). On average, five urine samples per worker were collected (standard deviation: 1.5) in both branches.
Table 4 Overview of urine samples taken in car body repair shops and industrial painting companies.
| Car body repair shop | Industrial painting companies | |||||||
|---|---|---|---|---|---|---|---|---|
| No. workers | % workers pos. HDA | No. urine samples | % samples pos. HDA | No. workers | % workers pos. HDA | No. urine samples | % samples pos. HDA | |
| Spray painting | 15 | 27% | 74 | 19% | 4 | 25% | 18 | 22% |
| Paint handling | 1 | 100% | 6 | 67% | 2 | 0% | 12 | 0% |
| Welding | 8 | 50% | 44 | 27% | – | – | – | – |
| Other tasks—bystander | 4 | 50% | 22 | 41% | 3 | 0% | 19 | 0% |
| Other tasks—office | 1 | 0% | 5 | 0% | 1 | 0% | 3 | 0% |
| Other tasks—no byst./office | 5 | 20% | 25 | 4% | – | – | – | – |
| Other tasks—unspecified | 11 | 36% | 63 | 14% | – | – | – | – |
| Total | 45 | 36% | 239 | 21% | 10 | 10% | 52 | 8% |
Questionnaires were used to categorise workers into mutually exclusive categories based on tasks performed: spray painting, paint handling (no spraying), and welding. Workers who did not perform any of these tasks were classified as bystanders if they had been near a spray painting job, as office if they had spent a full day in the office, and as no bystander/office when the worker had neither been near a spray painting job nor spent a full day in the office. Eleven car body repair shop workers who neither worked with paint nor welded and of whom no specific information on bystanding or office work was available were indicated as unspecified.
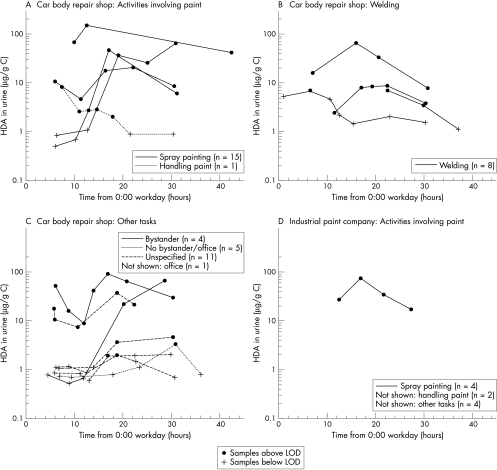
Figure 2 shows the HDA curves for workers with at least one positive HDA sample for different groups of workers. In car body repair shops HDA could be detected in urine of ∼25% of the spray painters, ∼50% of the welders, ∼50% of the bystanders, and ∼25% of other workers. Additionally, no obvious differences in urine levels could be observed between the different groups of workers. For the industrial spray company workers, HDA could be detected in urine of ∼25% of the spray painters and none of the bystanders or office workers.
Figure 2 HDA in urine from car body repair shop workers (A, B, and C) and industrial spray painters (D) per activity during the working day (0:00 = midnight before working day). Car body repair shop workers: (A) activities involving paint; (B) welding; (C) other tasks. Industrial painting companies: (D) activities involving paint. Only workers with at least one positive urine sample are shown. The total number of workers in each category is given in the boxes.
Table 5 shows the fraction of detectable urine samples, the OR for having a positive sample (reference = 0–8 am), as well as the HDA concentration for different time intervals of the measurement day in car body repair shops. Four urine samples (four workers) were excluded since they were collected after 8 am on the next day. The frequency of detectable urine samples was significantly elevated in the late afternoon (3–6 pm) when compared to early morning (0–8 am).
Table 5 Characteristics of urine samples from car body repair shop workers per time interval (0 = midnight before working day); fraction of samples above LOD, OR of being above the LOD versus 0–8 am working day (corrected for repeated measurements), and concentration range and median in samples above LOD.
| Time interval | Fraction above LOD | OR (95% CI) detectable samples | Median level (range) detects (μg HDA/g C) |
|---|---|---|---|
| 0–8 am | 6/38 | – | 13.1 (6.8–50.2) |
| 8–12 am | 7/41 | 1.10 (0.85–1.44) | 7.3 (2.3–67.8) |
| 0–3 pm | 4/35 | 1.06 (0.59–1.91) | 21.5 (2.7–150.2) |
| 3–6 pm | 7/30 | 2.13 (1.07–4.22) | 18.0 (1.9–89.9) |
| 6–12 pm | 12/45 | 2.00 (0.91–4.40) | 20.6 (1.9–61.7) |
| 0–8 am next day | 12/46 | 2.04 (0.91–4.59) | 8.1 (3.2–65.6) |
From 37 workers, urine was collected before 8 am on the measurement day (one worker collected two samples). Four of 29 workers that did not spray on the preceding day had detectable HDA levels before 8 am. In contrast, 2 of 8 workers that did spray on the day before started with detectable HDA levels (OR 2.0, CI 0.3 to 14.2).
Information on task based use of PPE during the measurement day was collected through the questionnaires. During spray painting respiratory protection was widespread, but gloves were used by 40% and 75% of the workers in car body repair shops and industrial painting companies respectively. Less than 50% of workers in both industries reported having used respiratory protection during mixing of paint. The same applies for glove use during mixing of paint.
Discussion
In this study we focused on task based inhalation and dermal exposure measurements of HDI and its oligomers in conjunction with urinary HDA levels in car body repair shop workers and industrial spray painters. This is the first field study to quantitatively assess dermal exposure as well as to combine dermal and inhalation exposure measurements of HDI and oligomers with biomonitoring.
In the literature no existing methods on quantitative assessment of dermal isocyanate exposure could be found. Due to the high reactivity of isocyanates, a reagent is necessary to capture the isocyanates. However, the reagent used for inhalation sampling (DBA) is not suitable for direct application on patches, sampling gloves, or wipes because of toxicity and volatility. Moreover, the importance of a good sense of touch during spray painting prevents the use of cotton gloves or patches on the fingers. The present study shows that it is possible to quantitatively assess task based dermal exposure using nitril rubber gloves, which are submerged into DBA immediately after sampling, as a sampling matrix. The different masses of HDI and isocyanurate were efficiently collected from the gloves. In addition, recovery after 30 minutes on the glove before submersion into DBA in toluene was above 75% for both compounds.
The field samples show that dermal exposure occurs during a substantial fraction of all tasks that involve direct handling of paint. In car body repair shops, detectable dermal exposure was negatively associated with glove use and positively associated with inhalation exposure level. Differences in frequency of detects as well as exposure levels between paint related tasks are small compared to inhalation exposure, which is mainly found during tasks where aerosol formation occurs. Several mass transport processes as described in the conceptual model for assessment of dermal exposure may be involved.30 The association between inhalation exposure level and the occurrence of dermal exposure in car body repair shops suggests aerosol deposition. In addition, emission (splashing or spilling of exposure source) and transfer of isocyanates (from surface contaminant) may occur during all paint related tasks.
To date information on the role of dermal exposure in respiratory sensitisation and disease aggravation is lacking. Yet, allergic contact dermatitis as well as skin irritation as a result of dermal exposure have been reported by isocyanate workers.31,32,33,34,35,36
To summarise, task based external exposure estimates indicate that in car body repair shops and industrial painting companies, mainly (spray) painters are exposed to HDI and its oligomers through inhalation as well as dermal contact. Interpretation of these short term exposure levels with respect to a total internal dose is complicated by large variability in levels within tasks, PPE use, and the lack of knowledge on the relevance of dermal exposure.4
In this context biomonitoring of HDA may give better insight into total HDI uptake. The half‐life of HDA after inhalation is a few hours, which makes it possible to study the effect of recent exposure.17,18 Knowledge on the metabolism of oligomers of HDI is lacking. Biomonitoring indeed demonstrates HDA in urine of 36% and 10% of all car body repair shop and industrial painting company workers, respectively. Detectable HDA is found in urine of only about 25% of the spray painters in both branches. Similar results were found in an earlier study.39 No obvious differences were found between groups of workers performing different tasks in car body repair shops. The highest external exposures were found during spray painting tasks, which indicates that PPE use and/or performance may be better and even sufficient among spray painters. However, spray painters perform multiple tasks on a working day during which both respiratory and dermal exposure may occur and task based PPE use may be variable. Therefore this study is not suitable for analysing the efficacy of PPE. An experimental design would be more appropriate for this purpose.
Surprisingly, in car body repair shops urinary HDA is not confined to spray painters but is also detected in a large proportion of the workers that neither handled paint nor reported bystander exposure. A possible explanation is a different source of HDA, which is, for example, also used as a raw material for the production of Nylon 66.37 Another explanation is a longer half‐life of HDI oligomers than of the monomer, resulting in detectable HDA levels on days following HDI oligomer exposure. Although not significant, the OR of 2.0 for a worker being HDA positive before 8 am when having sprayed on the preceding day may be indicative of an effect from earlier exposures. Yet, in car body repair shops the frequency of detectable samples is significantly elevated in the late afternoon, indicating that HDA in urine was at least partly a result of exposures during the measurement day. More likely, non‐spray painters without personal protection may receive unprotected “bystander” exposure on the general work floor that is not picked up by task based measurements. Possibly exposure peaks are generated when spray painting takes place outside the spray booth or when the spray booth is opened after the painted object has been cured. Long clearing times of air isocyanate levels after spraying have been described.39 Furthermore, dermal exposure may occur from contaminated surfaces. Another explanation is inhalation exposure through contaminated dust. Although the levels of analysed compounds in welding samples were very low, possibly other HDI based components may be released as a result of thermal degradation during welding or grinding.
Differences between car body repair shops and industrial painting companies responsible for the smaller proportion of positive bystanders and other workers remain unclear.
Like external exposure measurements, the use of HDA for internal exposure assessment of HDI based compounds is subject to difficulties. Large interindividual variability exists, possibly as a result of acetylator status.37 A test chamber study found net increases of HDA ranging from 0.4 to 101 μg/g creatinine (GM 16.2, GSD 3.5) four hours after exposure to a HDI biuret aerosol of 58.2 μg/m3 NCO (GM, GSD 1.6).25 In addition the short half‐life of HDI means that the urine samples only represent exposure over the past few hours and HDA levels will vary greatly within a person over a working day.17,25 Moreover the LOD for aliphatic amines is relatively high, resulting in low sensitivity.38 Besides large variability between persons, the test chamber study by Liu et al showed that although urinary HDA was present after exposure to a biuret aerosol, the correlation between external exposure and the biomarker was weak.25 Thus, HDA might be more indicative of monomer exposure. However, knowledge on metabolites that better reflect isocyanate oligomer exposure is lacking and to date HDA is the only usable biomarker of HDI based compounds.
In conclusion, in this study we quantitatively assessed dermal exposure using nitril rubber gloves. External exposure measurements indicate that mostly workers performing (spray) painting tasks are inhalatory and dermally exposed to mainly oligomers of HDI. Biomonitoring indeed demonstrates HDA in urine of a proportion of the workers. Due to large inter‐ and intra‐person variability, urinary HDA as an exposure measure for isocyanates is not easily interpretable. However, the results demonstrate that although (spray) painting results in highest external exposures, workers that do not perform paint related tasks may receive considerable doses of isocyanates. These findings need to be taken into account when using external exposure assessment data for risk assessment or epidemiological purposes.
Acknowledgements
The authors would like to acknowledge the following individuals for their contribution to this work: all company owners and workers who cooperated and made this study possible; Sjaak de Vreede and Marc Lurvink for conducting the field work; Heike Laudehr, Kim Hue Tieu, Susann Finger, Marie‐Helene Hein, John Gonsalves, and Dick van de Lagemaat for their technical assistance in the chemical analyses. Additionally we would like to thank the Dutch branch organisation for car body repair shops (FOCWA) for their contribution to the enrolment of companies. This research was granted by the Ministry of Social Affairs and Employment, the Netherlands.
Abbreviations
DBA - di‐n‐butylamine
HDA - hexamethylene diamine
HDI - hexamethylene diisocyanate
MDI - methylenebisphenyl diisocyanate
PPE - personal protection equipment
PU - polyurethane
TDI - toluene diisocyanate
Footnotes
Competing interests: none declared
References
- 1.Latza U, Baur X. Occupational obstructive airway diseases in Germany: frequency and causes in an international comparison. Am J Ind Med 200548144–152. [DOI] [PubMed] [Google Scholar]
- 2.Redlich C A, Karol M H. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol 20022213–224. [DOI] [PubMed] [Google Scholar]
- 3.Wisnewski A V, Redlich C A. Recent developments in diisocyanate asthma. Curr Opin Allergy Clin Immunol 20011169–175. [DOI] [PubMed] [Google Scholar]
- 4.Pronk A, Tielemans E, Skarping G.et al Inhalation exposure to isocyanates of car body repair shop workers and industrial spray painters. Ann Occup Hyg 2006501–14. [DOI] [PubMed] [Google Scholar]
- 5.Kimber I, Dearman R J. Chemical respiratory allergy: role of IgE antibody and relevance of route of exposure. Toxicology 2002181–2311–315. [DOI] [PubMed] [Google Scholar]
- 6.Petsonk E L, Wang M L, Lewis D M.et al Asthma‐like symptoms in wood product plant workers exposed to methylene diphenyl diisocyanate. Chest 20001181183–1193. [DOI] [PubMed] [Google Scholar]
- 7.Scheerens H, Buckley T L, Muis T L.et al Long‐term topical exposure to toluene diisocyanate in mice leads to antibody production and in vivo airway hyperresponsiveness three hours after intranasal challenge. Am J Respir Crit Care Med 19991591074–1080. [DOI] [PubMed] [Google Scholar]
- 8.Rattray N J, Botham P A, Hext P M.et al Induction of respiratory hypersensitivity to diphenylmethane‐4,4′‐diisocyanate (MDI) in guinea pigs. Influence of route of exposure. Toxicology 19948815–30. [DOI] [PubMed] [Google Scholar]
- 9.Karol M H, Hauth B A, Riley E J.et al Dermal contact with toluene diisocyanate (TDI) produces respiratory tract hypersensitivity in guinea pigs. Toxicol Appl Pharmacol 198158221–230. [DOI] [PubMed] [Google Scholar]
- 10.Ebino K, Ueda H, Kawakatsu H.et al Isolated airway exposure to toluene diisocyanate results in skin sensitization. Toxicol Lett 200112179–85. [DOI] [PubMed] [Google Scholar]
- 11.Delgado P, Porcel J, Abril I.et al Potential dermal exposure during the painting process in car body repair shops. Ann Occup Hyg 200448229–236. [DOI] [PubMed] [Google Scholar]
- 12.Hughson G W, Aitken R J. Determination of dermal exposures during mixing, spraying and wiping activities. Ann Occup Hyg 200448245–255. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Sparer J, Woskie S R.et al Qualitative assessment of isocyanate skin exposure in auto body shops: a pilot study. Am J Ind Med 200037265–274. [DOI] [PubMed] [Google Scholar]
- 14.Streicher R P, Kennedy E R, Lorberau C D. Strategies for the simultaneous collection of vapours and aerosols with emphasis on isocyanate sampling. Analyst 199411989–97. [DOI] [PubMed] [Google Scholar]
- 15.Streicher R P, Reh C M, Key‐Schwartz R.et al Selecting isocyanate sampling and analytical methods. Appl Occup Environ Hyg 200217157–162. [DOI] [PubMed] [Google Scholar]
- 16.Maitre A, Berode M, Perdrix A.et al Urinary hexane diamine as an indicator of occupational exposure to hexamethylene diisocyanate. Int Arch Occup Environ Health 19966965–68. [DOI] [PubMed] [Google Scholar]
- 17.Brorson T, Skarping G, Nielsen J. Biological monitoring of isocyanates and related amines. II. Test chamber exposure of humans to 1,6‐hexamethylene diisocyanate (HDI). Int Arch Occup Environ Health 199062385–389. [DOI] [PubMed] [Google Scholar]
- 18.Tinnerberg H, Skarping G, Dalene M.et al Test chamber exposure of humans to 1,6‐hexamethylene diisocyanate and isophorone diisocyanate. Int Arch Occup Environ Health 199567367–374. [DOI] [PubMed] [Google Scholar]
- 19.Kaaria K, Hirvonen A, Norppa H.et al Exposure to 4,4′‐methylenediphenyl diisocyanate (MDI) during moulding of rigid polyurethane foam: determination of airborne MDI and urinary 4,4′‐methylenedianiline (MDA). Analyst 2001126476–479. [DOI] [PubMed] [Google Scholar]
- 20.Lind P, Dalene M, Skarping G.et al Toxicokinetics of 2,4‐ and 2,6‐toluenediamine in hydrolysed urine and plasma after occupational exposure to 2,4‐ and 2,6‐toluene diisocyanate. Occup Environ Med 19965394–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lind P, Dalene M, Tinnerberg H.et al Biomarkers in hydrolysed urine, plasma and erythrocytes among workers exposed to thermal degradation products from toluene diisocyanate foam. Analyst 199712251–56. [DOI] [PubMed] [Google Scholar]
- 22.Tinnerberg H, Dalene M, Skarping G. Air and biological monitoring of toluene diisocyanate in a flexible foam plant. Am Ind Hyg Assoc J 199758229–235. [DOI] [PubMed] [Google Scholar]
- 23.Kaaria K, Hirvonen A, Norppa H.et al Exposure to 2,4‐ and 2,6‐toluene diisocyanate (TDI) during production of flexible foam: determination of airborne TDI and urinary 2,4‐ and 2,6‐toluenediamine (TDA). Analyst 20011261025–1031. [DOI] [PubMed] [Google Scholar]
- 24.Sakai T, Morita Y, Roh J.et al Improvement in the GC‐MS method for determining urinary toluene‐diamine and its application to the biological monitoring of workers exposed to toluene‐diisocyanate. Int Arch Occup Environ Health 200578459–466. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Berode M, Stowe M H.et al Urinary hexane diamine to assess respiratory exposure to hexamethylene diisocyanate aerosol: a human inhalation study. Int J Occup Environ Health 200410262–271. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson D, Dahlin J, Skarping G.et al Determination of isocyanates, aminoisocyanates and amines in air formed during the thermal degradation of polyurethane. J Environ Monit 20024216–222. [DOI] [PubMed] [Google Scholar]
- 27.Muller H. HPLC‐Methode zur simultanen bestimmung von harnstoff, kreatinin und harnsäure in serum und urin. Fresenius Z Anal Chem 1988332464–467. [Google Scholar]
- 28.Lewalter J, Skarping G, Ellrich D.et al Hexamethylene diisocyanate (HDI) and hexamethylenediamine (HDA). In: Angerer J, Schaller K, eds. Analyses of hazardous substances in biological materials. Weinheim: Wiley‐VCH, 20038119–131. [Google Scholar]
- 29.Zeger S L, Liang K Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 198642121–130. [PubMed] [Google Scholar]
- 30.Schneider T, Vermeulen R, Brouwer D H.et al Conceptual model for assessment of dermal exposure. Occup Environ Med 199956765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goossens A, Detienne T, Bruze M. Occupational allergic contact dermatitis caused by isocyanates. Contact Dermatitis 200247304–308. [DOI] [PubMed] [Google Scholar]
- 32.Estlander T, Keskinen H, Jolanki R.et al Occupational dermatitis from exposure to polyurethane chemicals. Contact Dermatitis 199227161–165. [DOI] [PubMed] [Google Scholar]
- 33.Daftarian H S, Lushniak B D, Reh C M.et al Evaluation of self‐reported skin problems among workers exposed to toluene diisocyanate (TDI) at a foam manufacturing facility. J Occup Environ Med 2002441197–1202. [DOI] [PubMed] [Google Scholar]
- 34.Larsen T H, Gregersen P, Jemec G B. Skin irritation and exposure to diisocyanates in orthopedic nurses working with soft casts. Am J Contact Dermat 200112211–214. [DOI] [PubMed] [Google Scholar]
- 35.Frick M, Bjorkner B, Hamnerius N.et al Allergic contact dermatitis from dicyclohexylmethane‐4,4′‐diisocyanate. Contact Dermatitis 200348305–309. [DOI] [PubMed] [Google Scholar]
- 36.Frick M, Isaksson M, Bjorkner B.et al Occupational allergic contact dermatitis in a company manufacturing boards coated with isocyanate lacquer. Contact Dermatitis 200348255–260. [DOI] [PubMed] [Google Scholar]
- 37.Brorson T, Skarping G, Sandstrom J F.et al Biological monitoring of isocyanates and related amines. I. Determination of 1,6‐hexamethylene diamine (HDA) in hydrolysed human urine after oral administration of HDA. Int Arch Occup Environ Health 19906279–84. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg C, Nikkila K, Henriks‐Eckerman M L.et al Biological monitoring of aromatic diisocyanates in workers exposed to thermal degradation products of polyurethanes. J Environ Monit 20024711–716. [DOI] [PubMed] [Google Scholar]
- 39.Williams N R, Jones K, Cocker J. Biological monitoring to assess exposure from use of isocyanates in motor vehicle repair. Occup Environ Med 199956598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]