Abstract
Background
Intensive glycaemic control can reduce the risk of microvascular complications in people with type 2 diabetes.
Aim
To examine the extent of monitoring and glycaemic control of patients with type 2 diabetes prescribed oral agents and/or insulin, and to investigate transition to insulin.
Design of study
Retrospective cohort study.
Setting
A total of 154 general practices in the UK contributing to the DIN-LINK database between 1995 and 2005.
Method
People with type 2 diabetes were identified using Read codes and prescribing data. Outcome measures were: glycaemic monitoring and control on multiple oral agents and/or insulin, and transition to insulin.
Results
A total of 14 824 people with type 2 diabetes were prescribed multiple oral agents concurrently, of whom 5064 (34.16%) had haemoglobin A1c (HbA1c) assessments 6 months before and following initiation of their last oral therapy. Mean HbA1c before therapy was 9.07%, which dropped to 8.16% following therapy (mean difference 0.91%, 95% confidence interval [CI] = 0.86 to 0.95, P<0.0001). Of the patients with HbA1c assessments, 3153 (62.26%) had evidence of poor glycaemic control following therapy. Median time to insulin for patients prescribed multiple oral agents was 7.7 years (95% CI = 7.4 to 8.5 years); 1513 people began insulin during the study and had HbA1c assessments 6 months before and following insulin. Mean HbA1c before insulin was 9.85% (standard deviation [SD] 1.96%) which decreased by 1.34%, (95% CI = 1.24% to 1.44%) following therapy, but 1110 people (73.36%) still had HbA1c ≥7.5%.
Conclusion
Many people with type 2 diabetes received inadequate monitoring and had poor glycaemic control. Intensive management is required to reduce the risk of microvascular complications.
Keywords: diabetes mellitus, type 2; drug therapy; primary health care; type 2
INTRODUCTION
Intensive glycaemic control has been shown to reduce the risk of complications in people with type 2 diabetes. In particular, it has been shown to reduce the risk of microvascular complications, including renal failure, and retinopathy. Intensive glycaemic control may also help to reduce the risk of macrovascular complications.1,2
The National Institute for Health and Clinical Excellence (NICE) has produced guidance on the management of people with type 2 diabetes including the management of blood glucose levels.3 These guidelines together with the targets set in the General Medical Services (GMS) contract provide a structured framework for the care of people with type 2 diabetes.4
Over the last decade, the prevalence of recognised diabetes has substantially increased, with much of the extra workload falling in primary care.5,6 In general practice, structured personal care is associated with better outcomes, such as glycaemic control, and can be sustained over time.6,7 Because of the progressive effect of the disease on insulin resistance and β-cell function, the efficacy of oral antidiabetic agents is diminished with time. Insulin therapy, alone or in combination with oral agents, may be required to achieve glycaemic control.8–10
How this fits in
Intensive treatment of people with type 2 diabetes to improve glycaemic control reduces the risk of subsequent complications. Despite this knowledge, there is evidence of suboptimal management in the community. Results from this study suggest that people with type 2 diabetes are not monitored adequately, have very poor blood sugar control before changes are made to treatment regimens, and are often inadequately controlled after such changes. Median delay from initiation of the first oral treatment to the introduction of insulin was 7.7 years. This may reflect the reluctance of professionals and patients to intensify treatment. Further research is required to identify appropriate interventions for improved glycaemic control.
This study evaluated the management of people with type 2 diabetes prescribed two or more oral agents, and/or insulin, according to NICE guidance. The study was based on retrospective analyses of UK primary care data from 1995 to 2005. The extent of monitoring and glycaemic control on oral agents and/or insulin, and transition to insulin in people with type 2 diabetes were assessed.
METHOD
Population
Data were obtained from 154 general practices contributing data to the DIN-LINK database over a 10-year period from 1 May 1995 to 30 April 2005. The DIN-LINK database contains anonymised computer records from representative general practices in the UK, including morbidity coding and prescribing data.11
Patients were identified with type 2 diabetes if they had a Read code for diabetes and/or one or more prescriptions for oral antidiabetic agents, insulin, or glucose testing kits (British National Formulary Chapter 6, sections 6.1.1, 6.1.2, 6.1.6), and if they did not have a Read code for gestational or type 1 diabetes.12 The point prevalence of type 2 diabetes in the study population was estimated on 30 April 2005, based on a reported total list size of 1285 316 patients. Insulin prescriptions were classified as short-acting, medium/long acting, or premixed insulin, based on information provided in the British National Formulary.13
Procedure and analyses
All patients with type 2 diabetes concurrently prescribed two or more types of oral agents (at initiation of their last oral agent) and/or insulin, during the study period were identified. NICE guidance currently recommends that haemoglobin A1c (HbA1c) should be measured at 2- to 6-monthly intervals, and that a target HbA1c should be set between 6.5 and 7.5%, based on the risk of macrovascular and microvascular complications.3 Therefore, an assessment was made of the proportion of patients with HbA1c measurements in the 6 months prior to and following initiation of their last prescribed oral agent (where patients received two or more concurrent oral agents), or their first insulin prescription. Mean HbA1c before and following therapy and the proportions of patients with an HbA1c ≥7.5% and ≥8.5% following therapy were assessed. For patients with multiple assessments of HbA1c the most recent values for each patient within the 6 months before and following therapy were evaluated. Glycaemic control early and late in the study period was assessed by dichotomising the dataset on 1 January 2003. This gave approximately the same numbers of patients in each group and also followed the release of NICE guidance3 (September 2002).
The time from initiation of the last oral agent until the date of the first prescription for any insulin was estimated for patients with type 2 diabetes with prescriptions for two or more types of oral agent using Kaplan–Meier survival analysis. For patients who did not receive insulin, the patient was censored on the date of deregistration or the end of study date (whichever came first).14
Mixed linear modelling was used to assess the change in pretreatment HbA1c (prior to initiation of patients' last oral agent or insulin) pre-2003 and from 1 January 2003 with practices as random effects (to account for any practice-related differences).15 Non-linear mixed models were used to assess the relationship between the proportion of patients with HbA1c assessments and study year, and the relationship between type of insulin regimen first prescribed with pretreatment HbA1c and the date of first prescription with practices as random effects.15 Analyses were performed using SAS version 9.1 (SAS Institute, Cary NC, US).
RESULTS
The 154 practices with available data employed a mean number of 4.6 GPs (standard deviation [SD] 2.1) and had a mean estimated list size on 30 April 2005 of 8346 patients (SD 3852). This resulted in a total estimated study population on this date of 1285 316 patients. During the 10-year study period, 62 533 patients with type 2 diabetes were identified. Of these, 40 267 remained registered on 30 April 2005, giving a point prevalence of 3.13%.
Glycaemic control and monitoring in patients prescribed two or more types of oral agent
Of patients from 154 practices, 14 824 people with type 2 diabetes were prescribed two or more oral agents concurrently at the time of initiating their last oral agent during the study period. Patients had a mean age of 64.2 years (SD 12.5 years), a mean body mass index of 30.1 kg/m2 (SD 6.8 kg/m2), and 6632 (44.74%) were female. Altogether, 5064 (34.16%) had HbA1c assessments in both the 6 months before and following initiation of their last oral agent (Table 1). A significant increase in the proportion of patients with an HbA1c assessment in the 6 months following initiation of oral therapy was observed during the study, rising from 16.77% in 1996–1997 to 52.80% in 2001–2002, and reaching a peak in 2003–2004 at around 57%.
Table 1.
Glycaemic control in people with type 2 diabetes prescribed two or more oral agents concurrently.
| Number of types of oral agents prescribed | n | Pre-therapy HbA1ca Mean % (SD) | Post-therapy HbA1cb Mean % (SD) | Mean difference % Pre-HbA1c – Post-HbA1c (95% CI) | P-value | Number (%) with HbA1c ≥7.5% post-therapy | Number (%) with HbA1c ≥8.5% post-therapy |
|---|---|---|---|---|---|---|---|
| ≥2 | 5064 | 9.07 (1.51) | 8.16 (1.57) | 0.91 (0.86 to 0.95) | <0.001 | 3153 (62.26) | 1712 (33.81) |
| 2 | 4174 | 9.04 (1.51) | 8.04 (1.51) | 1.00 (0.95 to 1.05) | <0.001 | 2468 (59.13) | 1280 (30.67) |
| 3 | 854 | 9.21 (1.49) | 8.73 (1.72) | 0.48 (0.37 to 0.59) | <0.001 | 658 (77.05) | 411 (48.13) |
| 4 | 36 | 9.16 (1.49) | 9.34 (2.18) | −0.19 (−0.79 to 0.42) | 0.54 | 27 (75.00) | 21 (58.33) |
| Pre-2003 | 2713 | 9.22 (1.56) | 8.28 (1.66) | 0.93 (0.87 to 0.99) | <0.001 | 1755 (64.69) | 1023 (37.71) |
| Post-2003 | 2351 | 8.90 (1.43) | 8.02 (1.46) | 0.87 (0.81 to 0.94) | <0.001 | 1398 (59.46) | 689 (29.31) |
Pre-therapy HbA1c (haemoglobin A1c) is the last available HbA1c assessment in the 6 months prior to initiation of a patient's last oral agent.
Post-therapy HbA1c is the last available HbA1c assessment in the 6 months following initiation of their last oral agent.
Mean HbA1c prior to patients' last additional oral therapy was 9.07%, which dropped to 8.16% (mean difference 0.91%, 95% confidence interval [CI] = 0.86 to 0.95%) following the additional therapy. However, 3153 (62.26%) patients had HbA1c ≥7.5% following this treatment intensification. The addition or substitution of a second or third type of oral agent led to significant reductions in HbA1c. However, no further benefit was observed when patients received more than three types of oral agent (Table 1). Comparing patients receiving their last treatment intensification post-2003 with those before 2003, pretreatment HbA1c was significantly lower post-2003 (9.22%, SD 1.56% pre-2003 versus 8.90%, SD 1.43% post-2003; P<0.001); the post-2003 group also had lower post-treatment HbA1c with higher proportions controlled below recommended targets.
Transition to insulin
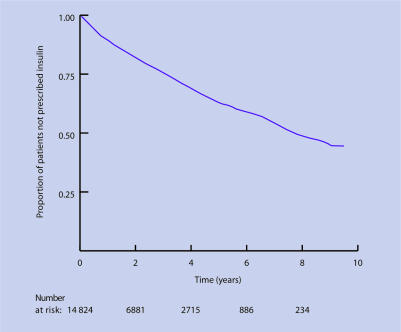
Median time from initiation of the last oral agent to insulin for patients prescribed two or more types of oral agents concurrently (n = 14824) was 7.7 years (95% CI = 7.4 to 8.5 years) as shown in Figure 1. Of the 3153 patients with poor glycaemic control following initiation of their last oral agent, 847 (26.86%) were prescribed insulin during the study.
Figure 1.
Kaplan–Meier estimates of time to first insulin from initiation of last oral agent for patients prescribed two or more oral agents.
Glycaemic control and monitoring on insulin
In total, 6442 patients were prescribed insulin for the first time during the 10-year study period, of whom 2698 (41.88%) had a HbA1c assessment in the 6 months before initiation of insulin and 1513 (23.49%) had HbA1c assessments both in the 6 months before and following therapy. A significant increase in the proportion of patients prescribed insulin with HbA1c assessments was observed over the study, rising from 13.10% in 1996–1997 to 51.21% in 2003–2004.
Mean HbA1c observed for patients prior to therapy was 9.85% (SD 1.96%). Although patients experienced significant reductions in HbA1c in response to insulin, with a mean decrease of 1.34% (95% CI = 1.24 to 1.44%), 1110 (73.36%) had HbA1c ≥7.5% post-therapy (Table 2). As with oral treatment, there was a significant decrease in pre-insulin HbA1c during the study pre-2003 (9.96% SD 1.98%) versus post-2003 (9.74% SD 1.92% P<0.02).
Table 2.
Control of people with type 2 diabetes receiving their first insulin therapy.
| n | Pre-therapy HbA1ca Mean % (SD) | Post-therapy HbA1cb, Mean % (SD) | Mean difference Pre-HbA1c – PostHbA1c (95% CI) | P-value | Number (%) with HbA1c ≥7.5% post-therapy | Number (%) with HbA1c ≥8.5% post-therapy | |
|---|---|---|---|---|---|---|---|
| All | 1513 | 9.85 (1.96) | 8.51 (1.58) | 8.51 (1.58) | <0.001 | 1110 (73.36%) | 703 (46.46) |
| Pre-2003 | 784 | 9.96 (1.98) | 8.55 (1.63) | 8.55 (1.63) | <0.001 | 581 (74.11) | 367 (46.81) |
| Post-2003 | 729 | 9.74 (1.92) | 8.48 (1.52) | 8.48 (1.52) | <0.001 | 529 (72.57) | 336 (46.09) |
Pre-therapy HbA1c (haemoglobin A1c) is the last available HbA1c assessment in the 6 months prior to initiation of insulin
Post-therapy HbA1c is the last available HbA1c assessment in the 6 months following initiation of insulin.
In total, 5941 of the 6442 patients initiating insulin were registered for at least 3 months following therapy, of whom 5647 (95.05%) had a prescription for an oral antidiabetic agent during the study period, and 3597 (60.55%) had a prescription for an oral agent following their first prescription for insulin.
Data quality
The recording of HbA1c varied by practice. For patients initiating their last oral agent, the median number with no HbA1c assessment in the 6 months before and following initiation of their last oral agent by practice was 66.67% (interquartile range = 50.60–80.92%). Eight practices (5%) recorded no HbA1c assessments in the 6 months before and following therapy, and three practices (1.95%) had less than 25% of patients without assessments both pre- and post-therapy. A total of 1629 patients (25.28%) had no HbA1c assessment following initiation of insulin and may have been monitored entirely in secondary care.
DISCUSSION
Summary of main findings
This large population-based study has examined the management and treatment progression of people with type 2 diabetes using data from practices throughout the UK over a 10-year period. Results indicate that, while HbA1c assessment and glycaemic control have improved during the study, many patients may benefit from improved monitoring, improved glycaemic control, and earlier transition from oral therapy to insulin.
Although recorded monitoring of HbA1c improved during the study, in 2003–2005 over 40% of patients had no evidence of monitoring before and following changes in oral medication as recommended by NICE guidance.3 Changes in oral medication and the addition of insulin did not occur until HbA1c levels were high (mean HbA1c 9% or more), and despite significant improvements in glycaemic control, most patients remained above target levels at least 6 months after intensification of treatment. Median time from first prescription of last oral agent to commencing insulin was nearly 8 years. However, some patients' conditions may have remained well controlled on oral agents during this time.
Patients who were prescribed insulin in the study and had HbA1c assessments available before and following therapy achieved a substantial benefit from initiation of insulin (mean decrease in HbA1c of 1.34%). Even though many patients did not meet target levels, this decrease is likely to reduce microvascular and macrovascular complications to a level that is clinically relevant.
Strengths and limitations of the study
This study assessed daily practice in primary care but relied on data input and transfer from practices.16 The definition of type 2 diabetes used in the study relies on accurate coding, particularly as this study included all practices with data available throughout the study period rather than applying data quality criteria.5 Data checks suggest that there were variations in data quality between practices. It is not clear whether these variations reflect differences in data recording or of the underlying management. If practices did not electronically code all results, actual performance may have been underestimated; however, results suggest a high level of completeness of diabetes coding.17 The time to initiation of insulin may have been overestimated if deregistration recording was inaccurate.
It is important to consider the generalisability of the results, that is, whether the prevalence of diabetes, achievement of targets, and treatments observed in this study are nationally representative. The age–sex structure of the DIN-LINK database has been shown to be similar to the UK average, but practices in the north of the UK (including Wales and the Midlands) and lower socioeconomic groups are under-represented.18 Despite this, prevalence rates for a wide range of diseases (including diabetes) have been shown to be equivalent to those in another national primary care database (General Practice Research Database) and are similar to those seen in the Quality and Outcomes Framework (QOF).5,19–21
Results of this suggest that glycaemic control has improved over time, but this may arise from reasons other than improvements in quality of care. Patients earlier in the study may have been managed based on blood glucose levels rather than HbA1c so a reduced HbA1c recording earlier in the study may not reflect poor management. There has been an increase in the prevalence of type 2 diabetes, with an almost 50% increase in diagnosis.5 At the same time there has been a shift away from specialist management with increased workload in primary care.22 The present study estimated that approximately 25% of patients continued to receive the majority of treatment in secondary care, which is consistent with other studies, and may explain why patients do not appear to have routine HbA1c assessment.22 At the same time, increased linkage of laboratory results to practice computer systems may have improved capturing of data which appeared to be improved monitoring. Finally, there are clinical and patient effects that should be noted. GPs may be more likely to request or report HbA1c where control is perceived to be poor, leading to unduly pessimistic results. The analyses were unable to account for patient adherence to prescribed therapies or to account for change in type of oral agent or insulin dose.
Comparison with existing literature
The point prevalence of type 2 diabetes observed in this study was 3.1%, similar to that observed in another large community-based survey and in the unadjusted national prevalence for all diabetes in the 2004–2005 NHS QOF results of 3.3%, but is more than double that reported by the Office of National Statistics in 1998 using similar practice-based data.23–25 This increase in prevalence has been noted by others.5
Observed improvements in clinical practice may reflect changes in response to important trial data, particularly from the UK Prospective Diabetes Study, which appeared in the first half of the study period showing the benefit from improved glycaemic control.2 National guidelines from NICE (2002) and the Scottish Intercollegiate Guidelines Network (2001) may also have led to change in practice, although evidence suggests implementation of NICE guidance in clinical practice is variable.3,26,27
The evidence observed of poor control despite apparent intensification of therapy (with both oral treatment and/or insulin) is consistent with findings from earlier studies, but contrasts with results achieved in trial conditions, and suggests that barriers remain towards optimum diabetes management in primary care.2,5,28,29 These differences between trial- and practice-based results may arise from patient, professional, or system characteristics. A recent international study found that patients had low levels of belief in the potential efficacy of insulin, and high self-blame for having to take insulin.30 Professionals may be guilty of clinical inertia leading to suboptimal dosing and lack of treatment intensification; however, it is difficult to determine clinical behaviour with the current data.31 Finally, the complex care required for the intensive management of poorly controlled type 2 diabetes, particularly with insulin, may not be available in all practices due to lack of resources.32,33
Implications for future research and clinical practice
Routine monitoring of glycaemic control is an important part of diabetes management and features prominently in NICE guidance and in the QOF detailed in the GMS contract.3,34 Only the last year of the study period included data collected under the QOF regime, and it could be expected that this would be a driver for change in subsequent years.
This paper has considered the management of glycaemic control in a large population of people with type 2 diabetes. Future work should include the assessment of management of other risk factors in diabetes, particularly hypertension and obesity, along with the impact of the QOF on outcomes.34
This study confirms that most patients with type 2 diabetes in the UK who are prescribed multiple oral agents and/or insulin do not achieve target levels of glycaemic control in routine practice, although the results show some improvement over time. The increasing prevalence of type 2 diabetes and the aging population are likely to compound these problems. While it may be unrealistic for some patients to meet current targets, many patients appear to require more routine monitoring and intensive treatment.
Effective management of type 2 diabetes requires changes in professional practice and interventions for patients to themselves understand that diabetes is a progressive disease, to reduce self-blame and to understand the potential benefits of improved glycaemic control.35–38
Funding body
This study was supported by an unrestricted research grant from Pfizer and Aventis, part of the Sanofi-Aventis group. Analyses and interpretation were performed independently of the sponsor
Ethics committee
Not applicable
Competing interests
Nick Freemantle has received funding for research, travel, fees for speaking and consulting from a number of companies that manufacture drugs and devices for the treatment of diabetes. Melanie Calvert and Richard McManus have received funding for research from Pfizer and Sanofi-Aventis.
REFERENCES
- 1.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macro Vascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.National Institute for Clinical Excellence. Management of type 2 diabetes. Management of blood glucose. London: National Institute for Clinical Excellence; 2002. Guideline G1-27. [Google Scholar]
- 4.Department of Health. GMS contract. www.dh.gov.uk/PolicyAndGuidance/OrganisationPolicy/PrimaryCare/PrimaryCareContracting/GMS/fs/en (accessed 28 Mar 2007)
- 5.De Lusignan S, Sismanidis C, Carey I, et al. Trends in the prevalence and management of diagnosed type 2 diabetes 1994–2001 in England and Wales. BMC Fam Pract. 2005;6:13. doi: 10.1186/1471-2296-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitford DL, Roberts SH, Griffin S. Sustainability and effectiveness of comprehensive diabetes care to a district population. Diabet Med. 2004;21:1221–1228. doi: 10.1111/j.1464-5491.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- 7.Olivarius NdF, Beck-Nielsen H, Andreasen AH, et al. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ. 2001;323:970. doi: 10.1136/bmj.323.7319.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook MN, Girman CJ, Stein PP, et al. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 9.Turner RC, Cull CA, Frighi V, Holman RR, for the UK Prospective Diabetes Study Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 10.Brunton S, Carmichael B, Funnell M, et al. Type 2 diabetes: the role of insulin. J Fam Pract. 2005;54:445–452. [PubMed] [Google Scholar]
- 11.London School of Hygiene and Tropical Medicine. Directory of clinical databases. www.lshtm.ac.uk/docdat/records.php?t=records&id=DIN-LINK (accessed 28 Mar 2007)
- 12.NHS Connecting for Health. Read codes. www.nhsia.nhs.uk/terms/pages/default.asp (accessed 28 Mar 2007)
- 13.British National Formulary. www.bnf.org/bnf/ (accessed 28 Mar 2007)
- 14.Bland JM, Altman DG. Statistics notes: survival probabilities (the Kaplan–Meier method) BMJ. 1998;317:1572–1580. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCulloch CE, Searle SR. Generalized, linear and mixed models. New York: John Wiley; 2001. [Google Scholar]
- 16.Hippisley-Cox J, Pringle M, Cater R, et al. The electronic patient record in primary care-regression or progression? A cross sectional study. BMJ. 2003;326:1439–1443. doi: 10.1136/bmj.326.7404.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan K, Porcheret M, Croft P. Quality of morbidity coding in general practice computerized medical records: a systematic review. Fam Pract. 2004;21:396–412. doi: 10.1093/fampra/cmh409. [DOI] [PubMed] [Google Scholar]
- 18.Carey IM, Cook DG, De Wilde S, et al. Developing a large electronic primary care database (Doctors' Independent Network) for research. Int J Med Inform. 2004;73:443–453. doi: 10.1016/j.ijmedinf.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 19.DeWilde S, Carey IM, Bremner SA, et al. Evolution of statin prescribing 1994–2001: a case of ageism but not of sexism? Heart. 2003;89:417–421. doi: 10.1136/heart.89.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeWilde S, Carey IM, Bremner S, et al. A comparison of the recording of 30 common childhood conditions in the Doctors' Independent Network and General Practice Research Databases. Health Stat Q. 2004;22:21–30. [PubMed] [Google Scholar]
- 21.QOF database. GP contract. www.gpcontract.co.uk/browse.php?year=5 (accessed 28 Mar 2007)
- 22.Whitford DL, Roberts SH. Changes in prevalence and site of care of diabetes in a health district 1991–2001. Diabet Med. 2004;21:640–643. doi: 10.1111/j.1464-5491.2004.01164.x. [DOI] [PubMed] [Google Scholar]
- 23.Hippisley-Cox J, Pringle M. Prevalence, care, and outcomes for patients with diet-controlled diabetes in general practice: cross sectional survey. Lancet. 2004;364:423–428. doi: 10.1016/S0140-6736(04)16765-2. [DOI] [PubMed] [Google Scholar]
- 24.NHS Health and Social Care Information Centre. Quality and outcomes framework information. www.ic.nhs.uk/services/qof (accessed 28 Mar 2007)
- 25.National Statistics. Key health statistics from general practice 1998. www.statistics.gov.uk/downloads/theme_health/Key_Health_Stats_1998.pdf (accessed 28 Mar 2007)
- 26.Scottish Intercollegiate Guidelines Network. Management of diabetes. A national clinical guideline. Glasgow: Scottish Intercollegiate Guidelines Network; 2001. [Google Scholar]
- 27.Sheldon TA, Cullum N, Dawson D, et al. What's the evidence that NICE guidance has been implemented? Results from a national evaluation using time series analysis, audit of patients' notes, and interviews. BMJ. 2004;329:999. doi: 10.1136/bmj.329.7473.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayward RA, Manning WG, Kaplan SH, et al. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA. 1997;278:1663–1669. [PubMed] [Google Scholar]
- 29.Fox KM, Gerber Pharmd RA, Bolinder B, et al. Prevalence of inadequate glycemic control among patients with type 2 diabetes in the United Kingdom general practice research database: a series of retrospective analyses of data from 1998 through 2002. Clin Ther. 2006;28:388–395. doi: 10.1016/j.clinthera.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Peyrot M, Rubin RR, Lauritzen T, et al. on behalf of the International DAWN Advisory Panel Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 31.Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28:600–606. doi: 10.2337/diacare.28.3.600. [DOI] [PubMed] [Google Scholar]
- 32.Greaves CJ, Brown P, Terry RT, et al. Converting to insulin in primary care: an exploration of the needs of practice nurses. J Adv Nurs. 2003;42:487–496. doi: 10.1046/j.1365-2648.2003.02648.x. [DOI] [PubMed] [Google Scholar]
- 33.Tanenberg R. Transitioning pharmacologic therapy from oral agents to insulin for type 2 diabetes. Curr Med Res Opin. 2004;20:541–553. doi: 10.1185/030079903125003134. [DOI] [PubMed] [Google Scholar]
- 34.Department of Health. Quality and outcomes framework. www.dh.gov.uk/assetRoot/04/08/86/93/04088693.pdf (accessed 28 Mar 2007)
- 35.Ellis SE, Speroff T, Dittus RS, et al. Diabetes patient education: a meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97–105. doi: 10.1016/s0738-3991(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 36.Wolpert HA, Anderson BJ. Management of diabetes: are doctors framing the benefits from the wrong perspective? BMJ. 2001;323:994–996. doi: 10.1136/bmj.323.7319.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox C, Kilvert A. Intensive education for lifestyle change in diabetes. BMJ. 2003;327:1120–1121. doi: 10.1136/bmj.327.7424.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gary TL, Genkinger JM, Guallar E, et al. Meta-analysis of randomized educational and behavioral interventions in type 2 diabetes. Diabetes Educ. 2003;29:488–501. doi: 10.1177/014572170302900313. [DOI] [PubMed] [Google Scholar]