Abstract
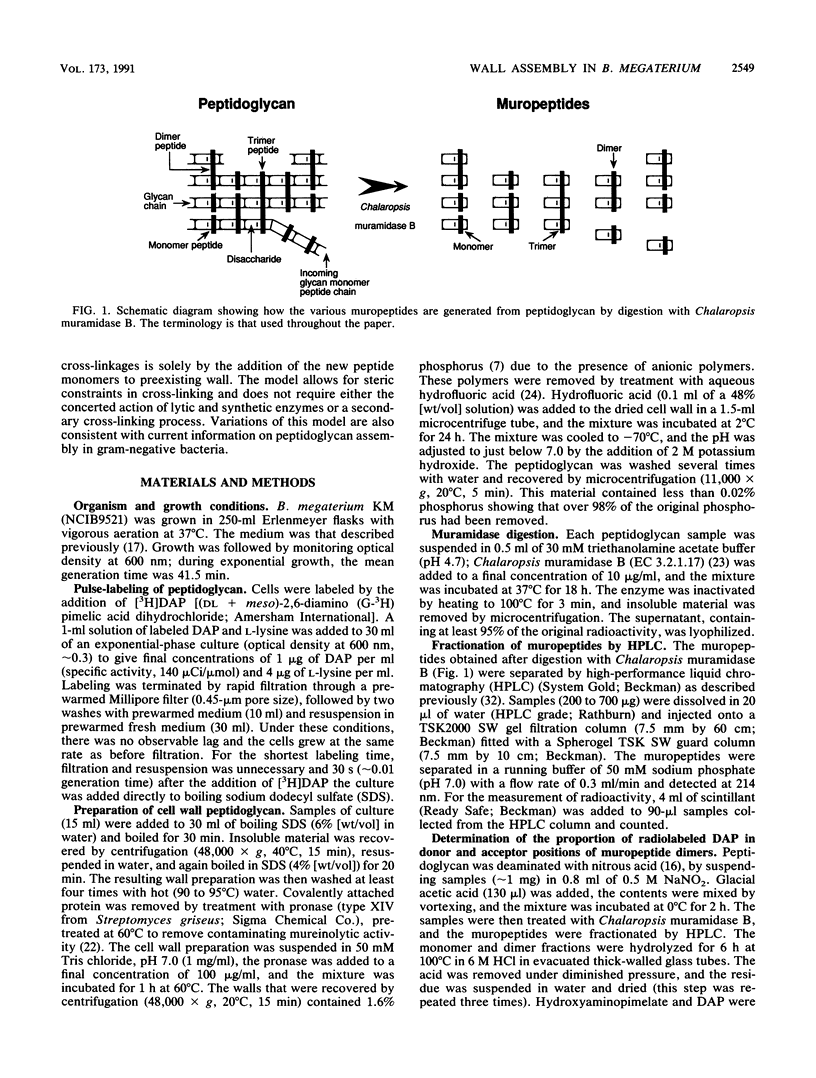
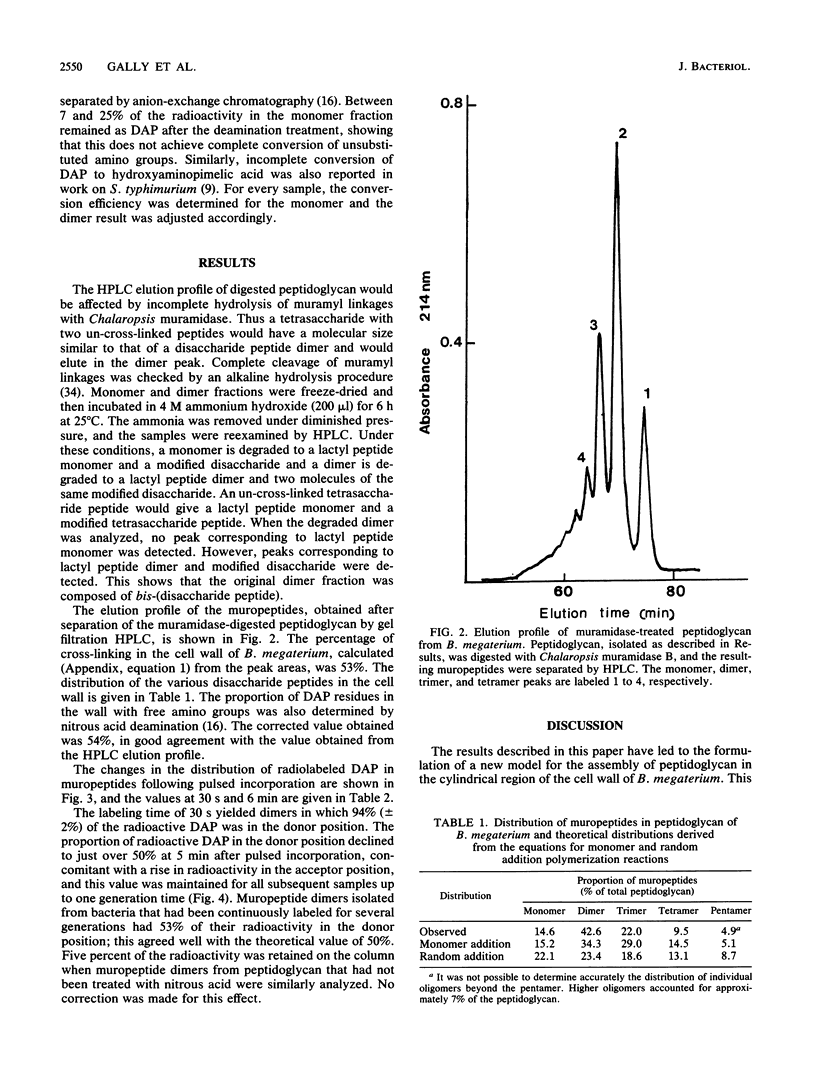
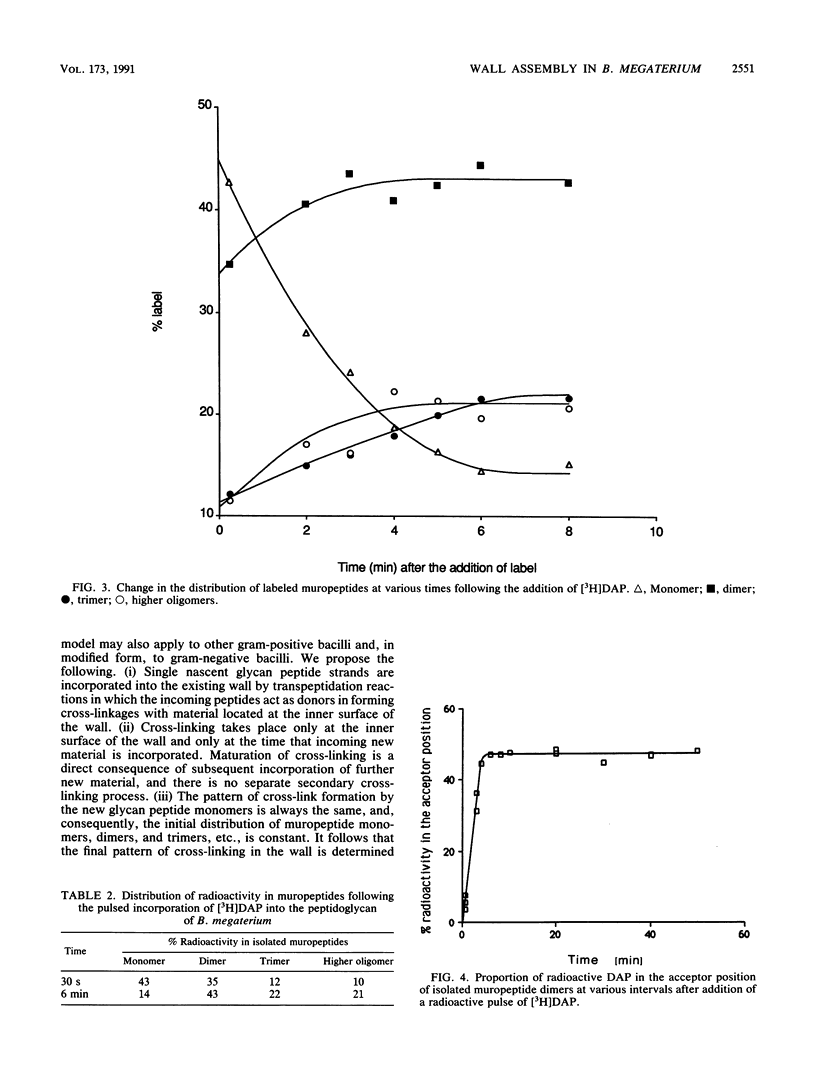
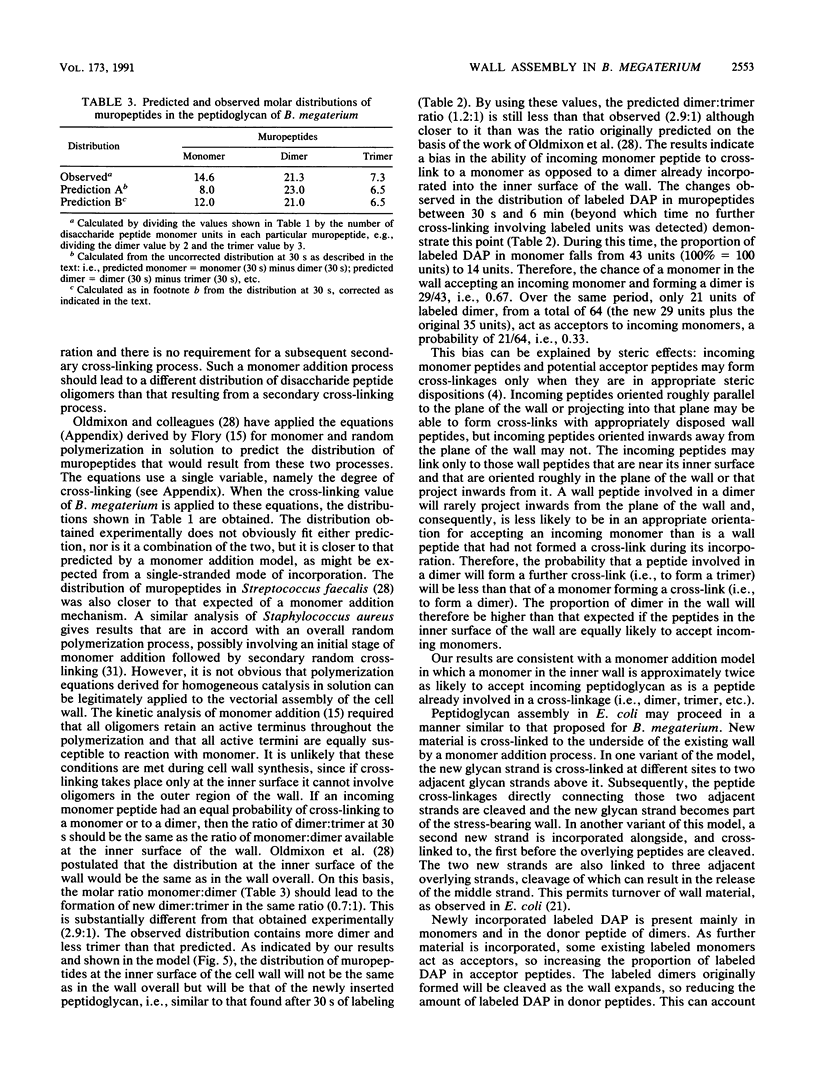
The pattern of cross-linking in the peptidoglycan of Bacillus megaterium has been studied by the pulsed addition of radiolabeled diaminopimelic acid. The distribution of label in muropeptides, generated by digestion with Chalaropsis muramidase and separated by high-performance liquid chromatography, stabilized after 0.15 of a generation time. The proportion of label in the acceptor and donor positions of isolated muropeptide dimers stabilized over the same period of time. The results have led to the formulation a new model for the assembly of peptidoglycan into the cylindrical wall of B. megaterium by a monomer addition process. Single nascent glycan peptide strands form cross-linkages only with material at the inner surface of the wall. Maturation is a direct consequence of subsequent incorporation of further new glycan peptide strands, and there is no secondary cross-linking process. The initial distribution of muropeptides is constant. It follows that the final pattern of cross-linking in the wall is determined solely by, and can be forecast from, this repetitive pattern of incorporation. In a modified form, this model can also be applied to assembly of cell walls in rod-shaped gram-negative bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burman L. G., Park J. T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Reichler J., Park J. T. Evidence for multisite growth of Escherichia coli murein involving concomitant endopeptidase and transpeptidase activities. J Bacteriol. 1983 Oct;156(1):386–392. doi: 10.1128/jb.156.1.386-392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke-Sturman A. J., Archibald A. R., Hancock I. C., Harwood C. R., Merad T., Hobot J. A. Cell wall assembly in Bacillus subtilis: partial conservation of polar wall material and the effect of growth conditions on the pattern of incorporation of new material at the polar caps. J Gen Microbiol. 1989 Mar;135(3):657–665. doi: 10.1099/00221287-135-3-657. [DOI] [PubMed] [Google Scholar]
- Cooper S., Hsieh M. L., Guenther B. Mode of peptidoglycan synthesis in Salmonella typhimurium: single-strand insertion. J Bacteriol. 1988 Aug;170(8):3509–3512. doi: 10.1128/jb.170.8.3509-3512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E., Gardner-Eckstrom H. L. Mode of cell wall synthesis in gram-positive bacilli. J Bacteriol. 1975 Sep;123(3):1157–1162. doi: 10.1128/jb.123.3.1157-1162.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Pelvit M. C., Cunningham W. P. Structural difference between walls from ends and sides of the rod-shaped bacterium Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1266–1272. doi: 10.1128/jb.109.3.1266-1272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham W. D., Gilvarg C. Kinetics of cross-linking of peptidoglycan in Bacillus megaterium. J Biol Chem. 1974 Apr 25;249(8):2478–2482. [PubMed] [Google Scholar]
- Giles A. F., Reynolds P. E. The direction of transpeptidation during cell wall peptidoglycan biosynthesis in Bacillus megaterium. FEBS Lett. 1979 May 15;101(2):244–248. doi: 10.1016/0014-5793(79)81017-0. [DOI] [PubMed] [Google Scholar]
- Goodell E. W. Recycling of murein by Escherichia coli. J Bacteriol. 1985 Jul;163(1):305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Cleavage and resynthesis of peptide cross bridges in Escherichia coli murein. J Bacteriol. 1983 Oct;156(1):136–140. doi: 10.1128/jb.156.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J Bacteriol. 1985 Apr;162(1):391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hash J. H., Rothlauf M. V. The N,O-diacetylmuramidase of Chalaropsis species. I. Purification and crystallization. J Biol Chem. 1967 Dec 10;242(23):5586–5590. [PubMed] [Google Scholar]
- Jayatissa P. M., Rose A. H. Role of wall phosphomannan in flocculation of Saccharomyces cerevisiae. J Gen Microbiol. 1976 Sep;96(1):165–174. doi: 10.1099/00221287-96-1-165. [DOI] [PubMed] [Google Scholar]
- Martin H. H., Gmeiner J. Modification of peptidoglycan structure by penicillin action in cell walls of Proteus mirabilis. Eur J Biochem. 1979 Apr;95(3):487–495. doi: 10.1111/j.1432-1033.1979.tb12988.x. [DOI] [PubMed] [Google Scholar]
- Merad T., Archibald A. R., Hancock I. C., Harwood C. R., Hobot J. A. Cell wall assembly in Bacillus subtilis: visualization of old and new wall material by electron microscopic examination of samples stained selectively for teichoic acid and teichuronic acid. J Gen Microbiol. 1989 Mar;135(3):645–655. doi: 10.1099/00221287-135-3-645. [DOI] [PubMed] [Google Scholar]
- Oldmixon E. H., Dezélée P., Ziskin M. C., Shockman G. D. Monomer addition as a mechanism of forming peptide cross-links in the cell-wall peptidoglycan of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1976 Sep;68(1):271–280. doi: 10.1111/j.1432-1033.1976.tb10786.x. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden M. A., Perkins H. R. Peptidoglycan cross-linking in Staphylococcus aureus. An apparent random polymerisation process. Eur J Biochem. 1990 Jul 31;191(2):373–377. doi: 10.1111/j.1432-1033.1990.tb19132.x. [DOI] [PubMed] [Google Scholar]
- Snowden M. A., Perkins H. R., Wyke A. W., Hayes M. V., Ward J. B. Cross-linking and O-acetylation of newly synthesized peptidoglycan in Staphylococcus aureus H. J Gen Microbiol. 1989 Nov;135(11):3015–3022. doi: 10.1099/00221287-135-11-3015. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. Peptidoglycan biosynthesis by preparations from Bacillus licheniformis: cross-linking of newly synthesized chains to preformed cell wall. Biochem J. 1974 Jun;139(3):781–784. doi: 10.1042/bj1390781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. The direction of glycan synthesis in a bacterial peptidoglycan. Biochem J. 1973 Dec;135(4):721–728. doi: 10.1042/bj1350721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge B. L., Wientjes F. B., Jurida I., Driehuis F., Wouters J. T., Nanninga N. Peptidoglycan synthesis during the cell cycle of Escherichia coli: composition and mode of insertion. J Bacteriol. 1989 Nov;171(11):5783–5794. doi: 10.1128/jb.171.11.5783-5794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]



