Abstract
Environmental cues (e.g., the sight of a cigarette) have long been recognized as important triggers for craving in smokers. Available imaging technologies (e.g., fMRI) allow investigation of the neural mechanisms for cue-induced craving, but there stands a need for a cue-delivery system compatible with an MRI environment. We developed a standardized set of 24 high-resolution videos, 12 containing cigarette smoking scenes (e.g., lighting up), and 12 containing neutral scenes (e.g., reading a book), each 30 seconds long, with comparable lighting, visual complexity, and background filmed by a professional cinematographer. Study participants were 20 smokers (mean age=37.7 years, 50% female). Each was exposed to the 24 videos in a random order under laboratory conditions. Dependent measures included heart rate, blood pressure, skin conductance, skin temperature, and self-reported craving (0–100) following each video. Overall findings indicated that smokers had greater reactivity to the smoking videos than to neutral videos (p<.01). Follow-up univariate analyses revealed significant cue effects on self-reported craving, galvanic skin response, and skin temperature. Interestingly, exploratory examination of gender revealed that men had higher blood pressure and skin temperature responses than women, and that women had higher responses when viewing videos of women smoking than when viewing men smoking. Results support this set of videos as an effective tool for investigation of cue-elicited craving, and raise the possibility of unique gender effects in cue reactivity.
Keywords: Cue-reactivity, Craving, Nicotine, Smoking, Video
Cigarette smoking continues to pose a major worldwide public health threat (American Cancer Society [ACS], 2006). While some gains have been made in reducing the initiation of smoking in various targeted groups, cessation rates have increased little in the past decade in spite of numerous widely available intervention strategies (e.g., nicotine replacement) to smokers (ACS, 2006). Indeed, estimates indicate that across the United States, of those who attempt to quit, only 4.7% remain abstinent at one year post-cessation (ACS, 2006).
In considering the factors that make successful cessation so difficult, studies over the past decade have pointed to a preeminent role of environmental cues (e.g., sight or smell of cigarettes) that can trigger strong cravings or urges, long after the acute effects of nicotine withdrawal have subsided (Carter & Tiffany, 1999). These cue-induced craving reactions have been modeled reliably under laboratory conditions with smokers experimentally exposed to smoking paraphernalia. Theorists (Wikler, 1948; Stewart et al., 1984) have argued that the phenomenon operates by the principles of classical conditioning, such that exposure to a cue (the conditioned stimulus) after repeatedly being paired with the effects of the cigarette (the unconditioned stimulus) elicits conditioned craving responses that are manifested in both biological reactions (e.g., increased heart rate and blood pressure) and self-report measures (i.e., craving). Empirical data have largely supported this conceptualization, as a number of experimental studies (Lazev et al., 1999) have demonstrated that smokers can be conditioned to display craving reactions to novel stimuli after repeated pairing with cigarettes. Effects of cue exposure on cigarette cravings tend to be robust across a variety of modalities, including in vivo exposures (e.g., handling one’s cigarette) and imaginal exposures (e.g., imaging a scenario involving smoking) (Burton & Tiffany, 1997; Carter & Tiffany, 1999; Drobes & Tiffany, 1997; Erblich & Bovbjerg, 2004; Shadel et al., 1998; Tiffany, 1990).
Accumulating evidence has now suggested that exposure to cues associated with addictive agents can trigger increased fMRI activity in key areas of the brain, in particular, the richly dopaminergic regions. Studies, primarily among cocaine addicts (e.g. Kosten et al., 2006), but also among alcoholics (e.g. Hermann et al., 2006) and smokers (McClernon, Hiott, Huettel, & Rose, 2005; Smolka et al., 2006; Lee, Lim, Wiederhold, & Graham, 2005; McBride, Barrett, Kelly, Aw, & Dagher, 2006; Lim et al., 2005), have revealed increased activity in the anterior cingulate gyrus, the orbitofrontal cortex, the temporal lobe, nucleus accumbens, the amygdala, and the ventral striatum in response to drug cues presented under laboratory conditions. The rapid development of this literature has engendered an acute need for alternative sets of cues that are compatible for presentation in an MRI environment. Progress, however, is hampered by methodological difficulties with traditional cue exposure in the MRI setting. Indeed, there is a need for the development of multiple cues to be delivered in rapid succession to avoid habituation effects as observed by other fMRI studies investigating the auditory cortex (Pfleiderer, Ostermann, Michael, & Heindel, 2002; Michael, Ostermann, Sörös, Schwindt, & Pfleiderer, 2004; Rabe, Michael, Kugel, Heindel, & Pfleiderer, 2006; Straube, Weiss, Mentzel, & Miltner, 2007), as well as several studies of visual processing (Breiter et al., 1996; Fischer et al., 2000; Wright et al., 2001).
To date, the few fMRI studies that have employed smoking cues, have used photographs as cues (McClernon, Hiott, Huettel, & Rose, 2005; Smolka et al., 2006) while others have used virtual reality (Lee et al., 2005) and a limited set of video clips (McBride et al., 2006; Lim et al., 2005). While still photographs may be useful in eliciting craving, videos containing smoking stimuli may provide contextually richer and more realistic cues that may more accurately represent real world cue-exposures. Indeed, a number of studies have demonstrated that the use of videos is associated with better attention to stimuli (Huston & Wright, 1983; Reeves et al., 1985; Singer, 1980), and that exposure to videos produces neurophysiological changes that mimic the effects of actually engaging in that behavior. Thus, video cue presentations are preferable.
To our knowledge, though, only three studies have reported on the use of video cues to elicit cigarette craving (McBride et al., 2006; Lim et al., 2005; Shadel, Niaura, & Abrams, 2001). As expected, in these studies of smokers, exposure to smoking-related videos elicited craving reactions that were similar in magnitude to previously reported reactions using in vivo cues. These studies do not, however, report the development of multiple brief videos that would be necessary for the repeated contrasts of an fMRI study without risking habituation to the same video. In addition to fMRI research, the availability of multiple video cues might well be useful in other types of studies that involve repeated-measures designs susceptible to habituation effects. The purpose of the research described here was to develop multiple smoking video cues that would be useful for the conduct of fMRI studies, as well as other studies in which cue habituation effects are a concern.
Accumulating evidence also points to the importance of examining the unique challenges that men and women face at all stages of smoking behavior (e.g., US Department of Health and Human Services, 2004; Perkins, 2001). For example, evidence has suggested that women demonstrate craving and withdrawal symptoms that are generally more refractory to nicotine replacement therapies (e.g., Hatsukami et al., 1995). Other studies have found that women are less likely to successfully quit than men are (Wetter et al., 1999). In a recent meta-analysis, Cepeda-Benito et al. (2004) found that women benefited less from nicotine replacement therapy, but benefited more from non-pharmacological treatments, than did men. Hence, gender is an important factor to consider when evaluating smoking behaviors, cue-induced craving, and cessation-related variables. Few studies, however, have examined the effects of gender on cue-elicited craving. In this study, we recruited an equal number of men and women to explore possible gender effects.
Based on the considerations, we hypothesized that compared to neutral video cues, smoking video cues would elicit stronger: 1) self-reported cravings; and, 2) psychophysiological reactions. We also explored the possibility that men and women may differ in the magnitude of these cue-induced effects.
Method
Overview
Working with a professional cinematographer, we developed a set of 30-second smoking video cues and a set of neutral cues. These video cues were tested for their ability to elicit an urge to smoke in a sample of 20 current smokers under laboratory conditions. Participants completed standard self-report measures of cigarette craving before the first and after each subsequent video cue presentation, and were monitored continuously for psychophysiological variables (e.g., skin conductance) throughout the session to provide an objective indicator of reactivity.
Participants
Participants (n=10 men and 10 women) were regular daily smokers recruited by advertisement in New York City to participate voluntarily in a research study about smoking. In order to qualify for study inclusion, participants had to have smoked an average of at least 10 cigarettes per day over the previous 5 years. In addition, participants were required to have no previous or current cancer, cardiovascular disease, emphysema, or other smoking-related illnesses, not be currently attempting to quit smoking, and report no history of hospitalization or treatment for other substance abuse. All participants provided informed consent prior to participation in the study; all study procedures were approved by the Mount Sinai Medical Center’s Institutional Review Board. Twenty percent of the sample reported being Caucasian, 20% reported being African American, 50% reported being Hispanic, and 10% reported other ethnic backgrounds. The mean age of the sample was 37.7 years of age (SD = 13.9). Forty percent of the participants reported having household incomes of $40,000 or greater. Forty percent of participants had a high school education or below, 40% had completed 8 some college, and 20% had completed college. As a group, participants had smoked for an average of 18.5 years (SD = 13.3). They reported smoking a mean of 17.5 cigarettes per day (SD = 8.3) and had attempted to quit smoking an average of 2 times (SD = 1.5). Their mean score on the Fagerstrom Test for Nicotine Dependence (FTND) was 4.7 (SD = 1.6).
Materials and Measures
Participants completed demographic (age, gender, education level, income level) and smoking history (cigarettes per day, years of smoking) questionnaires. In addition, participants completed two standardized and psychometrically validated indices, the Fagerstrom Test of Nicotine Dependence (FTND) (Heatherton, Kozlowski, Freck, & Fagerstrom, 1991), which assesses habit strength and the severity of dependence, and the Minnesota Nicotine Withdrawal Questionnaire (MNWQ) (Hughes & Hatsukami, 1986), which assesses strength of withdrawal symptoms experienced over the past 24 hours. Both instruments have been used extensively in the literature and have strong psychometric properties (Heatherton, Kozlowski, Freck, & Fagerstrom, 1991; Hughes & Hatsukami, 1986).
A five item 0–100 craving questionnaire was used to assess craving after each of the videos (described below). The instrument assessed craving using a variety of descriptors, including ‘urge,’ ‘desire,’ and ‘craving,’ has been employed in a number of laboratory craving-induction studies by our group and others (Hutchison, Niaura, & Swift, 1999; Erblich, Lerman, Self, Diaz, & Bovbjerg, 2005), and evidenced high levels of internal consistency in the current sample (alpha = 0.94).
Psychophysiological monitoring consisted of heart rate (HR), systolic and diastolic blood pressure (SBP and DBP), galvanic skin response (GSR), and skin temperature (SKT). These indices were recorded using the Biopac MP150 System (Goleta, CA) and analyzed using AcqKnowledge 3.7.3 Lab Assistant software (Biopac Systems, Goleta, CA).
Twenty-four 30-second silent video clips (available from the authors on request) 12 with actors performing cigarette smoking-related behaviors (e.g., lighting up) and 12 with neutral behaviors (e.g., reading a book) were staged and filmed by a professional cinematographer. Filming was performed so that the neutral and smoking related videos had comparable lighting and visual complexity. In five of the smoking videos it was clear that female smokers were depicted, six clearly had male actors, and one was ambiguous with respect to actor’s gender. Of the 12 neutral videos 6 had male actors, 3 had female actors, and 3 were ambiguous.
Procedures
All smokers participated in a 45-min session during which they were exposed to the 24 video clips. Upon arrival for the study session, participants smoked one cigarette to standardize deprivation levels and to prevent ceiling effects in craving, as previously described (e.g., Erblich & Bovbjerg, 2004). They then completed the questionnaires described above (FTND, MNWQ). Subjects were then seated comfortably and psychophysiological measurement devices were connected. Surface recording electrodes were attached bilaterally in the subclavicular region for HR (from ECG wave) recording, on the palmar aspect of the thumb and index fingers of the non-dominant hand for GSR recording, and on the non-dominant middle finger for SKT. A wrist cuff placed over the radial artery of the non-dominant hand measured SBP and DBP. Biopotentials were amplified using the Biopac MP150 system, and digitized and acquired using Acknowledge 3.7.3 software (Biopac, Goleta, CA). Following equipment placement, one pre-test craving assessment was taken. Participants then sat quietly for 10 minutes to establish a stable psychophysiological baseline. Participants were then presented with the 24 video clips in random order, followed each time by the craving questionnaire. The video clips were displayed in full screen mode using Quicktime media player software on a Dell desktop PC with a 24″ monitor that was placed immediately in front of participants. After each craving assessment, there was a 60-second rest before proceeding to the next video. Participants were offered $50.00 for their travel expenses, time and effort in the study.
Data Analysis
Psychophysiological data points in the 30-second epochs corresponding to each video were averaged to form composite HR, SBP, DBP, GSR and SKT values for each of the 24 videos. To provide an indication of internal consistency across the cue responses, Cronbach’s alphas were calculated for each outcome for each of the two sets of videos. Initial analyses indicated very high levels of internal consistency (alpha’s all > 0.92) for all outcomes (self-reported craving, HR, SBP, DBP, GSR and SKT) in both sets of videos (neutral and smoking). In addition, initial repeated measures ANOVAs conducted separately on the smoking and neutral videos revealed no differences between individual videos and no order of presentation effects across time. Similarly, there was no order of presentation effect when all 24 videos were analyzed together (all ps > 0.15). As a result, no individual comparisons of videos were warranted in the main analyses below. A multivariate ANOVA including all six outcomes, followed by repeated measures ANOVAs for each of the outcomes (five psychophysiological and one self-report), were computed, comparing neutral video responses to smoking video responses (“Cue Type”). Background variables (e.g., demographics and smoking characteristics) were considered as covariates in preliminary analyses.
Results
Background Variables
Preliminary analyses revealed some non-systematic correlations between Background variables and outcomes (e.g., education was related to self-reported craving, and Fagerstrom score was related to HR and DBP). To take a conservative approach, we included those potentially confounding Background variables as covariates in the respective analyses (hence the small variations in degrees of freedom reported below). Finally, because 50% of the sample reported Hispanic ethnicity, we included a dichotomous ethnicity covariate (Hispanic vs. other) to explore possible effects. To reduce Type II error probability in this small sample, only those covariates found to be significant in the preliminary analyses were included in the respective analyses.
Effects of exposure to smoking videos (Table 1)
Table 1.
Covariate-adjusted mean self-report and psychophysiological responses to neutral and smoking Videos: Univariate models.
| Dependent Variable | Neutral Videos (Mean ± SE) | Smoking Videos (Mean ± SE) |
|---|---|---|
| Self-reported Craving (0–100)* | 39.2 (7.6) | 49.5 (7.4) |
| Heart Rate (beats/min) | 77.8 (2.6) | 77.9 (2.7) |
| Systolic Blood Pressure (mmHg) | 125.1 (2.5) | 125.5 (2.7) |
| Diastolic Blood Pressure (mmHg) | 70.5 (1.7) | 70.7 (1.7) |
| Galvanic Skin Response (mmho)* | 5.2 (0.7) | 5.3 (0.7) |
| Skin Temperature (degrees F)** | 87.8 (1.1) | 87.9 (1.1) |
p < 0.05,
p < 0.01
To compare responses to the smoking videos and neutral videos (Cue Type) across all domains (psychophysiological and self-report) a multivariate ANOVA was performed. As hypothesized, findings indicated a significant multivariate effect of Cue Type; F (1, 18)=8.54, p < 0.01. To further explore this significant effect, univariate ANOVAs were then conducted for each of the six outcomes. Examination of results for self-reported craving (including ethnicity and education as covariates) revealed a significant effect of Cue Type, F (1, 16)=5.46, p < 0.05; cravings were significantly higher following smoking cues than following neutral cues. Similarly, the smoking videos induced higher skin conductance levels than the neutral videos (including ethnicity as a covariate); F (1, 17)=6.52, p < 0.05. Finally, there was a very small but significant effect of Cue Type on skin temperature (including cigarettes per day as a covariate); F (1, 16)=9.81, p < 0.01, which was slightly higher during smoking videos than during neutral videos. While HR, SBP, and DBP all tended to be slightly higher during the smoking videos than during the neutral videos, these small increases were not statistically significant in the univariate models (F’s < 3, p’s > 0.15). There were no effects of ethnicity on any of the outcomes.
Effects of participant’s gender on reactions to videos (Table 2)
Table 2.
Effects of neutral and smoking videos by gender
| Dependent Variable | Men (Mean ± SE) | Women (Mean ± SE) | ||
|---|---|---|---|---|
| Neutral Videos | Smoking Videos | Neutral Videos | Smoking Videos | |
| Self-reported Craving (0–100) | 44.8 (10.3) | 57.4 (9.5) | 39.8 (10.7) | 48.5 (9.8) |
| Heart Rate (beats/min) | 78.8 (2.8) | 78.1 (2.9) | 76.9 (2.8) | 77.8 (3.1) |
| Systolic Blood Pressure (mmHg)* | 129.7 (2.8) | 132.4 (2.6) | 120.5 (2.8) | 118.6 (2.6) |
| Diastolic Blood Pressure (mmHg)** | 73.3 (2.3) | 74.6 (2.3) | 67.8 (2.3) | 66.7 (2.3) |
| Galvanic Skin Response (mmho) | 4.7 (1.1) | 4.8 (1.1) | 5.8 (1.1) | 5.8 (1.1) |
| Skin Temperature (degrees F)** | 89.2 (1.6) | 89.4 (1.6) | 86.5 (1.6) | 86.4 (1.6) |
p < 0.05
0.05 < p < 0.10
To examine possible effects of gender, participant’s gender was added as a between-subjects factor. The omnibus MANOVA across all outcomes failed to yield a significant Cue Type x Gender effect; F (1, 18)=1.98, p > 0.10. Interestingly, though, exploratory examination of univariate analyses revealed a significant Cue Type x Gender interaction for the SBP outcome. Specifically, smoking videos induced slight increases in SBP among men, but slight decreases among women; F (1, 16)=7.2, p < 0.05. Similar patterns of results were observed with regard to DBP and skin temperature, although effects were only marginally significant; F ‘s = 3.90 & 3.29, 0.05 < p’s < 0.10.
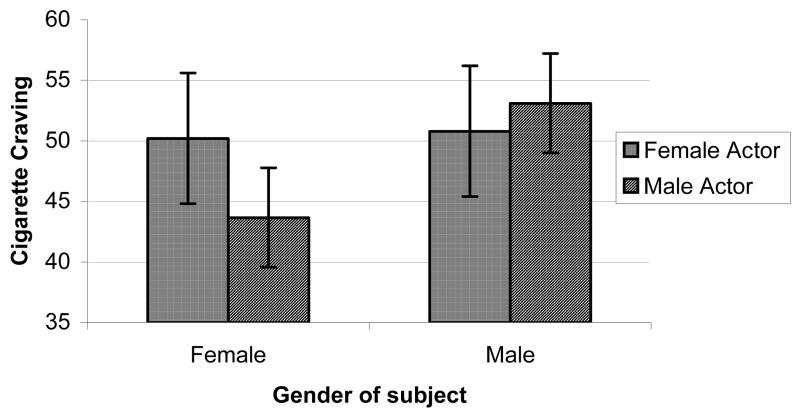
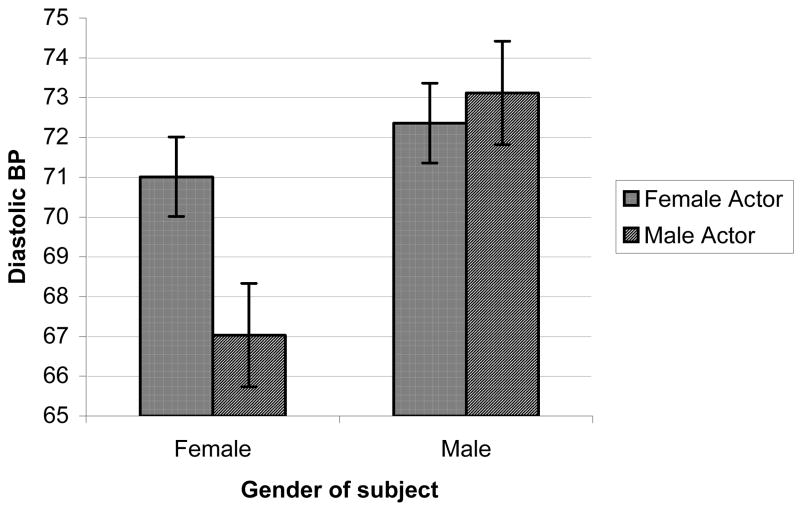
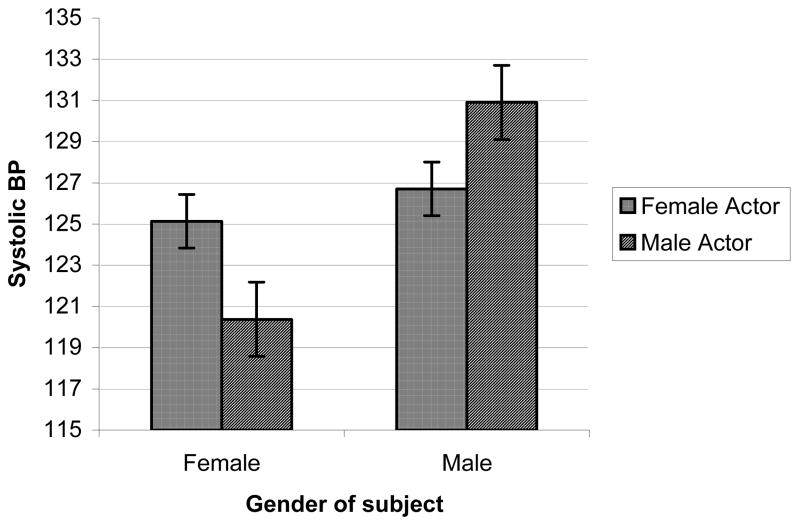
Effects of actor’s gender on reactions to smoking videos (Figures 1a–1c)
Figure 1.
Figure 1a. Video cue-induced craving by genders of subject and actor.
Figure 1b. Video cue-induced DBP by genders of subject and actor
Figure 1c. Video cue-induced SBP by genders of subject and actor
We also explored possible effects of actor’s gender on craving reactions by adding this within-subjects factor (responses to the 6 male-acted smoking videos vs. the 5 female-acted smoking videos, excluding data from the 1 ambiguous smoking video) into a follow-up ANOVA of reactions to the smoking videos, controlling for responses to the neutral videos1. Perhaps most interesting, were findings regarding the effects of the actor’s gender on reactions to the smoking videos. While there were no main effects of Actor’s Gender on outcomes in a MANOVA or in univariate models, there were significant Participant’s Gender x Actor’s Gender interaction effects for self-reported craving, DBP and SBP; F ‘s = 8.45, 4.94, & 5.56, p’s < 0.01, 0.05, & 0.05, respectively. As depicted in Figures 1a–1c, women displayed substantially higher self-reported craving reactions, as well as diastolic blood pressure and systolic blood pressure responses, when viewing smoking videos with female actors, compared to responses when viewing male actors. Men, on the hand displayed comparable levels of self-reported craving, DBP, and SBP, regardless of the actor’s gender.
Discussion
Results of the present study are consistent with our goal of developing a set of videotaped smoking cues. The smoking videos, as hypothesized, elicited significantly greater self-reported craving reactions and psychophysiological responses compared to neutral cues. In particular, exposure to the smoking videos resulted in elevated skin conductance and skin temperature, as well as self-reported craving. Interestingly, there were no differences in cardiovascular responses to the smoking and neutral videos, although upon separate examination of gender, it was found that men, but not women exhibited elevations in blood pressure during the smoking videos, compared to the neutral videos.
The significant reactivity results observed here are partially consistent with the broader literature on smoking cue-reactivity. For example, Burton and Tiffany (1997) found that although neither imaginal nor in vivo smoking cues elicited changes in skin temperature, in vivo (but not imaginal) cues induced higher levels of skin conductance. Other studies found that imaginal cues also elicited elevations in skin conductance (e.g., Cepeda-Benito & Tiffany, 1996). Finally, Drobes & Tiffany (1997) found that skin conductance was elevated during both imaginal and in vivo cue exposures, but skin temperature was lowered. Results are also partially consistent with a meta-analysis by Carter and Tiffany (1999), in which self-reported cigarette cravings among smokers showed the largest effects of cue exposure and skin temperature the smallest.
Variability in results from study to study continues to be an issue in this research area. Results of the present study suggest one possible source of this variability —gender. Indeed, across all videos, cardiovascular effects were only observed among men. Moreover, these effects were found to be dependent on the gender of the actor in the videos, suggesting that such modifying factors may need to be taken into account when analyzing cue-reactivity data. In addition, the results raise the possibility of important gender differences in cue-reactivity that may have implications in understanding gender-specific motivational processes. Specifically, it is possible that compared to men, women may either perceive, or respond to, more detailed contextual cues when confronted with smoking stimuli. While this supposition has broader support from the cognitive psychology literature (Witkin, 1967; Kimura, 1999), additional research to confirm the role of these processes in smoking cue-reactivity is warranted.
The results of this study provide support for the use of video cues to elicit cigarette craving. Findings are consistent with Shadel, Niaura, & Abrams, (2001), who found significant reactivity effects with a single video. In this study, we found that multiple video cue administration was feasible. There were no significant order effects, ruling out effects of habituation to multiple cues, nor any trends of increasing craving across the passage of time. In addition, all of the videos within each category (smoking, neutral) elicited comparable levels of reactivity. These characteristics may be especially useful in neuroimaging studies, in which exposures to more traditional cues are not feasible, and multiple exposures are desirable. Moreover, in light of research from the cognitive neurosciences (Huston & Wright 1983; Reeves et al., 1985; Singer, 1980), the use of videos is likely to be a more powerful approach than photographs. Studies directly comparing the two modalities, though, would be needed to confirm the superiority of video cues in this context.
Strengths of the study include the professional-quality development of the stimulus videos, and the use of multiple videos, which is highly desirable for many typical neuroimaging paradigms (Amaro & Barker, 2006). In addition, the study employed multi-method assessments of reactivity (psychophysiological, as well as self-report), and included both men and women, which allowed for analyses of gender effects, often not addressed in the literature. The primary limitation of the study is the small sample size. In addition, given the single time point assessment; formal test-retest reliability of these cue-exposures has yet to be established. Gender effects were found in the univariate analyses, but not in the omnibus MANOVA model, so results should be interpreted with caution. Also, because the neutral videos had more male than female actors and more gender-ambiguous scenes, it was not feasible to directly compare actor-gender effects across the smoking and neutral videos. Therefore, we cannot definitely rule out the possibility that the actor-gender effects would have been observed even in response to the neutral videos. Future research might productively focus on replication of current findings in a larger independent sample, and ultimately, on the application of these video cues in neuroimaging and other studies using repeated exposure designs, as well on developing videos for other substance use paradigms (e.g., alcohol).
Acknowledgments
This work was supported in part by NIH grant no. K07CA93387 and American Cancer Society grant no. CRTG-01-153-04-CCE. We would also like to acknowledge Francine Fernandez and Lauralea Colamussi, who assisted in data collection.
Footnotes
Because three of the neutral videos were ambiguous with regard to the actor’s gender (and would have to be dropped from these analyses), direct comparisons of actor’s gender between the smoking and neutral videos would have been confounded by number of exposures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro E, Barker G. Study design in fMRI: basic principles. Brain and Cognition. 2006;60:220–32. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2005. Atlanta, GA: 2005. [Google Scholar]
- Breiter H, Etcoff N, Whalen P, Kennedy W, Rauch S, Buckner R, Strauss M, Hyman S, Rosen B. Response and habituation of the human amygdale during visual processing of facial information. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Burton S, Tiffany S. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Centers for Disease Control Prevention, Cigarette smoking among adults – United States. MMWR Morb Mortal Wkly Rep 2005. 2005:551145–68. [Google Scholar]
- Carter B, Tiffany S. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Tiffany S. The use of a dual-task procedure for the assessment of cognitive effort associated with cigarette craving. Psychopharmacology. 1996;127:155–63. doi: 10.1007/BF02805989. [DOI] [PubMed] [Google Scholar]
- Drobes D, Tiffany S. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg D. In Vivo Versus Imaginal Smoking Cue Exposures: Is Seeing Believing? Experimental and Clinical Psychopharmacology. 2004;12:208–215. doi: 10.1037/1064-1297.12.3.208. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African-American smokers. Molecular Psychiatry. 2005;10:407–14. doi: 10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Fischer H, Furmark T, Wik G, Fredrikson M. Brain representation of habituation to repeated complex visual stimulation studied with PET. NeuroReport. 2000;11:123–126. doi: 10.1097/00001756-200001170-00024. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Skoog K, Allen S, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Experimental & Clinical Psychopharmacology. 1995;3:163–173. [Google Scholar]
- Heatherton T, Kozlowski L, Freck T, Fagerstrom K. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulous J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;8:1349–54. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Huston AC, Wright JC. Children’s processing of television: the informative functions of formal features. In: Bryant J, Anderson DR, editors. Children’s understanding of television. New York: Academic Press; 1983. pp. 35–68. [Google Scholar]
- Hutchison K, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp Clin Psychopharmacol. 1999;7:250–6. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex and Cognition. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler B. Cue-Induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;3:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Lazev AB, Herzog T, Brandon TH. Classical Conditioning of Environmental Cues to Cigarette Smoking. Exp and Clin Psychopharmacology. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim Y, Wiederhold BK, Graham SJ. A Functional Magnetic Resonance Imaging (fMRI) Study of Cue-Induced Smoking Craving in Virtual Environments. Applied Psychophysiology and Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Lim HK, Pae CU, Joo RH, Yoo SS, Choi BG, Kim DJ, Lee C, Lee CU. fMRI investigation on cue-induced smoking craving. J Psychiatr Res. 2005;3:333–5. doi: 10.1016/j.jpsychires.2004.08.004. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of Expectancy and Abstinence on the Neural Response to Smoking Cues in Cigarette Smokers: an fMRI Study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-Induced Changes in Self-Report Craving Correlate with Event-Related fMRI Responses to Smoking Cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N, Ostermann J, Sörös P, Schwindt Wolfram, Pfleiderer B. Altered habituation in the auditory cortex in a subgroup of depressed patients by functional magnetic resonance imaging. Neuropsychobiology. 2004;49:5–9. doi: 10.1159/000075331. [DOI] [PubMed] [Google Scholar]
- Perkins K. Smoking cessation in women, special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Ostermann J, Michael N, Heindel W. Visualization of Auditory Habituation by fMRI. NeuroImage. 2002;17:1705–10. doi: 10.1006/nimg.2002.1308. [DOI] [PubMed] [Google Scholar]
- Rabe K, Michael N, Kugel H, Heindel W, Pfleiderer B. fMRI studies of sensitivity and habituation effects within the auditory cortex at 1.5 T and 3T. Journal of Magnetic Resonance Imaging. 2006;23:454–58. doi: 10.1002/jmri.20547. [DOI] [PubMed] [Google Scholar]
- Reeves BE, Thorson E, Rothschild M, McDonald D, Hirsch J, Goldstein R. Attention to television: Intrastimulus effects of movement and scene changes on alpha variation over time. International Journal of Neuroscience. 1985;25:241–255. doi: 10.3109/00207458509149770. [DOI] [PubMed] [Google Scholar]
- Shadel W, Niaura R, Abrams D. Effect of different cue stimulus delivery channels on carving reactivity: comparing in vivo and video cues in regular cigarette smokers. Journal of Behavior Therapy and Experimental Psychiatry. 2001;32:203–9. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Shadel W, Niaura R, Abrams D, Goldstein M, Rohsenow D, Sirota A, Monti P. Scripted imagery manipulations and smoking cue reactivity in a clinical sample of self-quitters. Experimental and Clinical Psychopharmacology. 1998;6:179–186. doi: 10.1037//1064-1297.6.2.179. [DOI] [PubMed] [Google Scholar]
- Singer J. The power and limitations of television: Cognitive-affective analysis. In: Tannenbaum P, editor. The entertainment functions of television. Hillsdale, NJ: Erlbaum; 1980. pp. 31–65. [Google Scholar]
- Smolka MN, Bühler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Stewart J, deWit H, Eikelboom R. The role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:51–268. [PubMed] [Google Scholar]
- Straube T, Weiss T, Mentzel H, Miltner W. Time course of amygdale activation during aversive conditioning depends on attention. NeuroImage. 2007;34:462–69. doi: 10.1016/j.neuroimage.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Tiffany S. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Women, Tobacco, and Cancer. An agenda for the 21st century; July 2004; National Cancer Institute monograph. [Google Scholar]
- Wetter D, Kenford S, Smith S, Fiore M, Jorenby DE, Baker T. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiological basis of morphine addiction. American Journal of Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Witkin HA. A cognitive style approach to cross-cultural research. International Journal of Psychology. 1967;2:233–250. [Google Scholar]
- Wright C, Fischer H, Whalen P, McInerney S, Shin L, Rauch S. Differential prefrontal cortex and amygdale habituation to repeatedly presented emotional stimuli. NeuroReport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]