Abstract
Dopamine has undergone extensive investigation due to its known involvement in a number of neurological and psychiatric disorders. In particular, studies into pathological conditions have focused on the roles of high amplitude, phasically evoked dopamine release in regions such as the prefrontal cortex and striatum. However, research has shown that dopamine release can be more complex than just phasic release; thus, there is also a tonic, background dopamine release, with alterations in tonic dopamine release likely having unique and important functional roles. Unfortunately, however, tonic dopamine release has received relatively little attention. In this review, we summarize our recent studies and discuss how modulation of the dopamine system, both in terms of phasic activation and attenuation of tonic dopamine are important for the functions of brain regions receiving this dopamine innervation, and that imbalances in these dopamine release mechanisms may play a significant role in psychiatric disorders such as schizophrenia.
Keywords: Limbic System, Prefrontal Cortex, Nucleus Accumbens, Cognitive Functions, Animal Model, Schizophrenia
1. Introduction
Since its description in the brain by Carlsson in 1957 (Carlsson et al., 1957), the roles of dopamine (DA) have been extensively studied because of the demonstrated involvement of this transmitter system in multidimensional brain functions such as learning and memory (Grecksch and Matties, 1981), motivation (Everitt and Robbins, 2005), and emotional behaviors (Nader and LeDoux, 1999). Moreover, disruption of DA systems have been implicated in major neurological and psychiatric disorders including Parkinson’s disease and schizophrenia (Hornykiewicz, 1966). In our recent studies, we provide a unique perspective on the functional relevance of DA system regulation, in which we suggest that a “decrease” of DA release may be as important as an “increase” of DA release in modulating behavior.
2. Dopamine spike firing and dopamine release
DA neurons exhibit two distinct modes of spike firing: tonic single spike activity and burst spike firing (Grace and Bunney, 1984a; Grace and Bunney, 1984b). Tonic firing refers to spontaneously occurring baseline spike activity and is driven by pacemaker-like membrane currents of DA neurons (Grace and Bunney, 1984b; Grace and Onn, 1989). However, these DA neurons are under the influence of a very potent GABAergic inhibition, preventing some DA neurons from firing spontaneously in the basal condition (Grace and Bunney, 1979). Tonic firing of DA neurons has been shown to underlie the baseline tonic level of DA concentration within the striatum (e.g. 10-20 nM within the striatal region (Keef et al., 1993)). Studies suggest that this is mediated by an escape of DA from the synapse into the extrasyanptic space (Floresco et al., 2003; Grace, 1991). Therefore, the concentration of tonic extracellular DA is dependent on the number of DA neurons demonstrating spontaneous tonic spike activity (Floresco et al., 2003; Grace, 1991).
In contract, phasic activation of the DA system represented by the burst spike firing pattern is dependent on glutamatergic excitatory synaptic drive onto DA neurons from a number of areas, including the pedunculopontine tegmentum (PPTg) (Floresco et al., 2003; Futami et al., 1995) and the subthalamic nucleus (Smith and Grace, 1992). Burst spike firing triggers a high amplitude (e.g. hundreds of μM to mM levels), transient, phasic DA release intrasynaptically within the targeted areas (Floresco et al., 2003; Grace, 1991). This high amplitude DA release is nonetheless suggested to be subject to powerful, immediate reuptake into pre-synaptic terminals via DA transporters (Chergui et al., 1994; Suaud-Chagny et al., 1995), and therefore, phasic DA release would act transiently within the synaptic cleft and in very close proximity to the synapse (Floresco, et al., 2003; Grace, 1991; Chergui et al., 1994; Venton et al., 2003).
A series of electrophysiological studies by Schultz (Schultz et al., 1993; Tobler et al., 2003; Waelti et al., 2001) have shown behavioral correlates of tonic and bust spike firing of DA neurons. Thus, DA neurons exhibit burst spike firing that is triggered by presentation of unexpected rewards or sensory signals predicting such rewards (Schultz et al., 1993). In contract, studies have also revealed that a transient suppression of tonic spike firing in DA neurons occurs in response to the omission of expected rewards (Tobler et al., 2003) or aversive stimuli (Grace and Bunney, 1979; Ungless et al., 2004). Schultz suggests that these patterns of DA spike firing could be used as learning signals in the targeted brain structures (Waelti et al., 2001). Nevertheless, the distinct functional impact of DA release that occurs in response to burst spike firing versus suppression of tonic spike activity of DA neurons in the targeted area was unclear.
3. Dopamine modulation of afferent input into the nucleus accumbens
To elucidate the functional relevance of DA system transmission in terms of the messages conveyed by burst firing versus suppression of tonic firing of DA neurons into the targeted regions, we investigated the influences of tonic and phasic DA release on the modulation of afferent inputs into the nucleus accumbens (NAcc), where a dense DA innervation from the ventral tegmental area (VTA) is present (Voorn et al., 1986). The NAcc is believed to regulate goal-directed behaviors (Mogenson et al., 1980) as it receives convergent synaptic inputs from limbic structures and the PFC (Finch, 1966; French and Totterdell, 2002). Thus, the NAcc is located where contextual and emotional information processed in limbic structures and motor planning processed in the PFC could be integrated (Grace, 2000).
Using in vivo electrophysiology combined with pharmacological manipulations of the DA system in the NAcc, we found that selective modulation of limbic and PFC inputs is mediated by DA D1 and D2 receptors, respectively (Goto and Grace, 2005). Thus, activation of D1 receptors facilitated limbic inputs into the NAcc without affecting PFC inputs, although blockade of D1 receptors with a D1 antagonist did not yield significant effects on either limbic or PFC inputs. In contrast, we found that activation and inactivation of D2 receptors attenuated and facilitated, respectively, responses mediated by PFC inputs without affecting limbic inputs. This suggests that, unlike D1 receptor stimulation, striatal D2 receptors are under the influence of DA at the baseline condition, and can be modulated up or down from this state. Moreover, we also manipulated phasic and tonic DA release in the NAcc with activation and inactivation of the basal ganglia nuclei that regulate these distinct activity patterns as we recently reported (Floresco et al., 2003). Selective facilitation of limbic inputs was observed when phasic DA release (mediated by DA neuron burst firing) is increased, whereas, increases and decreases in tonic DA release selectively attenuated and facilitated, respectively, the PFC inputs. Taken together, these observations suggest that phasic DA release activates D1 receptors to facilitate limbic inputs, whereas tonic DA release has bi-directional effects on PFC inputs via D2 receptors, with increasing tonic D2 stimulation attenuating PFC afferent inputs and decreases in tonic D2 stimulation facilitating PFC inputs.
In addition to the physiological consequences of tonic and phasic DA system modulation, these distinct DA activity states were also found to exhibit behaviorally selective effects. Thus, using a behavioral cue discrimination task, we found that facilitation of limbic inputs into the NAcc by phasic DA release activating D1 receptors is required for learning of a response strategy in reinforcement learning, whereas a reduction of tonic DA stimulation of D2 receptors is essential to allow switching to a new response strategy once the criteria to achieve the goals is changed (Goto and Grace, 2005). Therefore, suppression of tonic spike firing of DA neurons by omission of expected rewards, which should result in a reduction of tonic DA release in the NAcc, may be used to selectively facilitate cortico-striatal information processing that mediates behavioral flexibility (Meck and Benson, 2002).
4. Impact of stress on dopamine-dependent synaptic plasticity
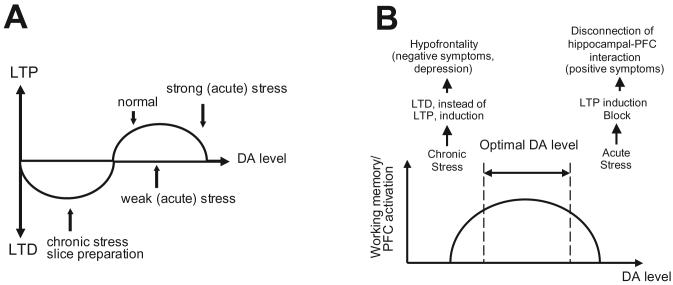
The PFC is another region that receives DA innervation from the VTA (Thierry et al., 1973). Unlike the striatum, this mesocortical DA innervation into the PFC is comparatively sparse; nonetheless, owing to the lower number of uptake sites and the high DA turnover in this region, DA still exerts prominent electrophysiological and behavioral effects in this brain region. DA release in the PFC has been shown to be critical for cognitive functions such as working memory (Goldman-Rakic, 1995). Moreover, alterations in DA release in the PFC are reported to occur upon exposure to stress. Thus, studies have shown that DA release in the PFC is increased under acute stress exposure (Gresch et al., 1994; Morrow et al., 2000), whereas when stress becomes chronic (e.g. over 2 weeks of stressful condition), a decrease of baseline DA release in the PFC is observed (Gresch et al., 1994). The impact of such increases and decreases in DA release on the induction of synaptic plasticity in PFC networks was examined as synaptic plasticity such as long-term potentiation (LTP) and depression (LTD) in the PFC: a process known to be DA-dependent (Otani et al., 2003). We found that LTP induction in hippocampal afferents into the PFC, which depends on D1 activation (Gurden et al., 2000), was facilitated with a short period of acute stress exposure, whereas when the exposure to stress is prolonged, LTP induction is impaired (Goto and Grace, 2006). As a result, there is an inverted U-shaped relation between the induction of synaptic plasticity in the hippocampal-PFC pathway and the duration of stress exposure, which is correlated with the amount of DA release during the stress exposure. While it is unclear whether the increase in DA release persists during the time of the LTP induction, the DA-induced changes in the phosphorylation of second messenger molecules such as CREB and DARPP-32 (Greengard, 1999), which are required for induction of LTP in this pathway (Hotte et al., 2007), are known to have effects that far outlast the period of DA receptor stimulation (Fig. 1A and 2B).
Figure 1.
Based on findings from animal studies, several models can be derived to account for some of the observations made regarding possible underlying biological mechanisms of psychiatric disorders such as schizophrenia. (A) In the normal condition at a moderate level of DA tone, long-term potentiation (LTP) is induced with high frequency activation of PFC afferent fibers such as those arising from the hippocampus and participating in memory-guided behavior. A brief exposure to stress would increase DA release in the PFC, and thereby facilitate DA-dependent induction of LTP, whereas if the stress exposure is severe, an excess in DA release could occur, leading to impairment of LTP (e.g. via stimulation of extrasynaptic DA receptors). In contrast, in the case of chronic stress, where a significant decrease in DA tone is produced, PFC networks would preferentially shown LTD. Such a condition is likely to be present in the in vitro slice preparation, in which the DA tone would be expected to be low. (B) Acute stress induces excessive DA release, which in turn over-stimulates D1 receptors in the PFC. As a result, information processing mediated by hippocampal-PFC interactions, which depends on LTP, would be disrupted. In contrast, attenuated DA tone, which could be produced by chronic stress exposure, would also interfere with proper information processing in the hippocampal-PFC pathway due to the abnormal induction of LTD. Indeed, this LTD may contribute to suppression of PFC activity (i.e. hypofrontality).
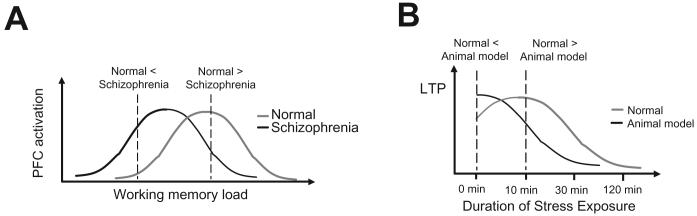
Figure 2.
Alterations in the inverted U-shaped relationships could contribute to the pathophysiology of schizophrenia. (A) Studies suggest that the relationship between working memory and PFC activation may also present as an inverted U-shape. In this example, schizophrenia patients would exhibit normal PFC activation only with a low working memory load. However, as the working memory load increases, PFC activation fails to compensate and becomes lower than that of normal subjects. Therefore, the hypofrontal condition reported in schizophrenia may be a function of working memory capacity of the PFC. Adapted from Manoach, 2003. (B) Our studies revealed that, in a developmental disruption model of schizophrenia, a similar inverted U-shaped relationship may exist with respect to LTP in PFC networks and stress exposure, with the schizophrenia model showing a leftward shift in the debilitating aspects of stressful stimuli. Adapted from Goto and Grace, 2006.
Using the in vitro slice preparation, we have provided data that has important implications with respect to the functional impact produced by a reduction in tonic, background DA release in the PFC (Matsuda et al., 2006). Thus, in the slice preparation where DA afferents are transected from cell bodies and a significant amount of residual DA is washed out during incubation, the background DA concentration would be expected to be significantly lower than that present in the intact, in vivo condition. We found that under such conditions, high frequency tetanic stimulation that is normally sufficient to induce LTP in vivo instead resulted in the induction of LTD. However, when a low concentration of DA was applied into the bath solution to mimic tonic background DA release present in vivo, high frequency stimulation now results in the induction of LTP, suggesting that the level of background tonic DA tone could determine the polarity of the synaptic plasticity that can be induced in PFC networks (Fig. 1A). A similar reduction in background DA tone is reported to occur within the PFC following chronic stress exposure (Gresch et al., 1994). Indeed, our preliminary evidence suggests that high frequency stimulation that normally induces LTP at hippocampal afferents into the PFC in the in vivo condition will instead result in the induction of LTD when animals are exposed to 2 weeks of chronic cold or restrain stress exposure (Goto et al., 2007).
5. Implications of tonic and phasic dopamine release in psychiatric disorders
Hypofrontality and attenuated DA release in the PFC have been proposed as pathophysiological factors in schizophrenia (Andreasen et al., 1992; Yang and Chen, 2005), with a particular association with the negative symptoms of this disorder (e.g. anhedonia, social withdrawal) (Andreasen et al., 1992). A similar hypofrontal condition is also reported in individuals with mood disorders such as depression (Galynker et al., 1998). Given that chronic stress is known to induce a depressive state and, therefore, has been employed as an animal model of depression (Katz et al., 1981), abnormal induction of LTD with attenuation of background tonic DA release in the PFC may be involved in negative symptoms of schizophrenia and depression (Fig. 1B).
Although hypofrontality has been proposed to be present in schizophrenia patients, there are some reports suggesting that PFC activity could be even higher in schizophrenia patients when compared to normal subjects in certain condition such as in performing comparatively easy working memory tasks (Callicott et al., 2003; Manoach, 2003). Thus, these studies suggest that an inverted U-shaped relation exists between working memory and activation of the PFC, and that schizophrenia patients may exhibit a lower working memory capacity compared to controls, leading to higher activation with simpler tasks (Fig 2A) (Manoach, 2003). Indeed, we have found a similar inverted U-shaped relation between LTP induction in the PFC and the effects of acute stress (Goto and Grace, 2006). In particular, we have also observed a shift of this inverted U-shaped relation toward greater acute stress vulnerability in an animal model of schizophrenia (Fig 2B) (Goto and Grace, 2006). In fact, it is known that schizophrenia patients exhibit a characteristic of greater vulnerability to stress, which has been correlated with susceptibility to relapse (Rabkin, 1980).
6. Conclusion
Increases and decreases in DA release can have markedly different effects on brain function, which can be both “Yin” and “Yang” depending on the state of the organism. Therefore, consideration of the bi-directional nature of DA changes is important for the normal functions of brain regions receiving DA innervation including the NAcc and PFC. An abnormal balance of DA release, especially in the PFC, may play a significant role in the pathophysiology of psychiatric disorders such as schizophrenia and depression.
Acknowledgements
This work was supported by NARSAD Young Investigator Award, HFSP Short Term Fellowship (Y.G.), French Minister of Research, Centre National de la Recherche Scientifique (S.O.), and USPHS MH57440 (A.A.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen NC, Rezai K, Alliger R, Swayze VW, 2nd, Flaum M, Kirchner P, et al. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49(12):943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62(3):641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6(5):495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat neurosci. 6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446(2):151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. 1995;21(4):331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39(4):608–612. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry. 2006;60(11):1259–1267. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Goto Y, Williams G, Otani S, Radley J. Dopamine, stress, and plasticity in the prefrontal cortex; 40th Winter Conference on Brain Reserach; Snowmass, CO. 2007.pp. 58–59. [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31(23):330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol. 1979;59(34):211–218. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4(11):2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9(10):3463–81. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecksch G, Matties H. The role of dopaminergic mechanisms in the rat hippocampus for the consolidation in a brightness discrimination. Psychopharmacology (Berl) 1981;75(2):165–168. doi: 10.1007/BF00432180. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23(3):435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63(2):575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor- dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18(2):925–64. [PubMed] [Google Scholar]
- Hotte M, Thuault S, Dineley KT, Hemmings HC, Jr, Nairn AC, Jay TM. Phosphorylation of CREB and DARPP-32 during late LTP at hippocampal to prefrontal cortex synapses in vivo. Synapse. 2007;61(1):24–28. doi: 10.1002/syn.20339. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2):247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Zigmond MJ, Abercrombie ED. In vivo regulation of extracellular dopamine in the neostriatum: influence of impulse activity and local excitatory amino acids. J Neural Transm Gen Sect. 1993;91(23):223–240. doi: 10.1007/BF01245233. [DOI] [PubMed] [Google Scholar]
- Lloyd K, Hornykiewicz O. Parkinson’s disease: activity of L-dopa decarboxylase in discrete brain regions. Science. 1970;170(963):1212–1213. doi: 10.1126/science.170.3963.1212. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(23):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Marzo A, Otani S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci. 2006;26(18):4803–4810. doi: 10.1523/JNEUROSCI.5312-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(23):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864(1):146–151. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux J. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav Neurosci. 1999;113(1):152–165. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- Otani S, Daniel H, Roisin MP, Crepel F. Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex. 2003;13(11):1251–1256. doi: 10.1093/cercor/bhg092. [DOI] [PubMed] [Google Scholar]
- Rabkin JG. Stressful life events and schizophrenia: a review of the research literature. Psychol Bull. 1980;87(2):408–425. [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12(4):287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Dugast C, Chergui K, Msghina M, Gonon F. Uptake of dopamine released by impulse flow in the rat mesolimbic and striatal systems in vivo. J Neurochem. 1995;65(6):2603–2611. doi: 10.1046/j.1471-4159.1995.65062603.x. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Blanc G, Sobel A, Stinus L, Golwinski J. Dopaminergic terminals in the rat cortex. Science. 1973;182(4111):499–501. doi: 10.1126/science.182.4111.499. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23(32):10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Philips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87(5):1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Voorn P, Jorritsma-Byham B, Van Dijk C, Buijs RM. The dopaminergic innervation of the ventral striatum in the rat: a light- and electron-microscopical study with antibodies against dopamine. J Comp Neurol. 1986;251(1):84–99. doi: 10.1002/cne.902510106. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412(6842):43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Yang CR, Chen L. Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11(5):452–470. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]