Abstract
The hydroperoxo-ferric complex, or Compound 0 (Cpd 0), is an unstable transient intermediate common for oxygen activating heme enzymes such as the cytochromes P450, nitric oxide synthases and heme oxygenases, as well as the peroxidases and catalases which utilize hydrogen peroxide as a source of oxygen and reducing equivalents. Detailed understanding of the mechanism of oxygen activation and formation of the higher valent catalytically active intermediates in heme enzyme catalysis requires the structural and spectroscopic characterization of this immediate precursor, Cpd 0. Using the method of cryoradiolytic reduction of the oxy-ferrous heme complex, we have prepared and characterized hydroperoxo-ferric complex in chloroperoxidase (CPO) and compared this to the same intermediate generated in cytochrome P450 CYP101. Optical absorption spectrum of Cpd 0 in CPO has a Soret band at 449 nm and poorly resolved α, β bands at 576 and 546 nm.
Keywords: Chloroperoxidase, Cytochrome P450, Peroxo-ferric intermediate, Cryogenic spectroscopy, Radiolytic reduction, Heme enzyme
Introduction
The hydroperoxo-ferric intermediate, or “Compound 0” is a common intermediate in heme enzyme catalysis [1]. It appears as a distinct transient species on the path to the higher valent ferryl-oxo states in the enzymatic cycles of the cytochromes P450, nitric oxide synthases and heme oxygenases, all of which which reduce dioxygen, as well as in heme peroxidases and catalases in their reactions with hydrogen peroxide (Scheme 1). Compound 0 (Cpd 0) is intrinsically unstable and usually decomposes at ambient conditions faster than it forms, and hence is extremely difficult to observe and characterize in the native reactions [2, 3]. However, Cpd 0 can be obtained via cryogenic radiolytic reduction of the one electron precursor oxy-ferrous complex in the frozen solutions or crystals [4–6]. Using this method, hydroperoxo-ferric complexes were characterized by electron paramagnetic resonance (EPR) [7–17], optical absorption [15, 18–20], and Raman spectroscopy [20, 21] in cytochrome P450 [12, 18, 21], myoglobin and hemoglobin [7–9, 20], heme oxygenase [14, 17, 19], and nitric oxide synthase [13].
Scheme I.
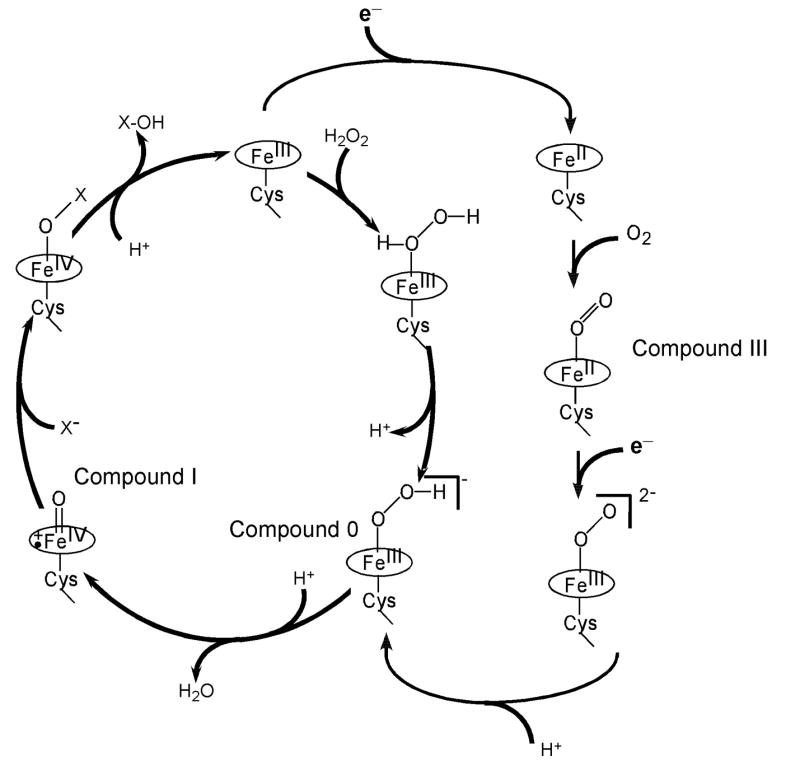
The reaction cycles of chloroperoxidase (shown in the left cycle with the pathway from the resting state to Cpd0 utilizing hydrogen peroxide as a source of electrons and oxygen) and cytochrome P450 (the right pathway, utilizing dioxygen and two external electrons from redox partners). Compound 0 and Compound I are common intermediates for peroxidases and cytochromes P450.
The various features of hydroperoxo-ferric intermediates in the heme enzymes and model compounds have been reviewed [1, 22–25]. Specifically, in all heme enzymes the EPR spectra of the invariably ferric low-spin peroxo-/hydroperoxo- complexes show typical narrow span of g values regardless of the axial ligand [23]. The optical absorption spectra are in turn sensitive to the nature of the proximal ligand trans to coordinated hydroperoxide. In myoglobin, heme oxygenase and horseradish peroxidase, which all have His-ligated heme iron, the Soret band is red-shifted by 3 – 8 nm as a result of the one-electron reduction of the oxy-ferrous precursor, while in cytochromes P450 and CPO this shift is larger, 20 – 22 nm [1]. Based on these spectral signatures, EPR and UV-Vis absorption spectroscopy are becoming the methods of choice for the assignment of the transient intermediates observed in the reactions of hydrogen peroxide with the heme enzymes [2, 3, 25–29].
Recently the first high resolution X-ray structure of Cpd 0 in chloroperoxidase was published [6]. In these experiments, Cpd 0 was obtained via in situ radiolytic reduction of Compound III (Cpd III), the ferrous oxygenated state [30, 31], which was prepared by soaking crystals of the ferric CPO in peracetic acid. The redox state of the enzyme during X-ray irradiation and data collection was controlled using absorption spectroscopy, and the assignment of Cpd 0 was based on the pronounced red shift of Soret band as compared to the spectrum of oxy-ferrous complex (Cpd III) in CPO single crystals before irradiation [6]. However, the anisotropic optical properties of protein crystals and the variability of the spectra with respect to the position of the sample make it necessary to compare the results obtained by single crystal microspectroscopy to the spectra measured using isotropic frozen solutions. For instance, the position of the Soret band of the Cpd III in CPO was identified between 430 and 445 nm in the crystal [6], while in solution it was reported at 427 nm [30, 31]. The red shift and apparently low amplitudes of Soret bands in the single crystal spectra [6] may be attributed to the high background and/or scattering in single crystal cryogenic microspectroscopy, complicating the precise assignment of the Soret maximum. In order to provide an absolute spectral assignment of oxy-ferrous and hydroperoxo-ferric intermediates of CPO, we document in this communication the high resolution optical spectra of these unstable intermediates measured at 77 K in comparison with the spectra of the same complexes in cytochrome P450.
Methods
Chloroperoxidase isolated from the fungi Caldariomyces fumago (CPO) was prepared in the laboratory of Dr. L. Hager as described [32]. Samples for cryogenic spectroscopy were prepared in 0.1 M phosphate buffer pH 6.0 containing 65% v/v glycerol. The substrate thioanisole (C6H5SCH3) was added as 0.5 M stock solutions in ethanol. Ferrous CPO was prepared by addition of minimal amounts of dry sodium dithionite and subsequent anaerobic buffer exchange using small G25 sepharose (Pharmacia) to remove unreacted reductant and unwanted by-products. Oxy-ferrous CPO was obtained by gentle bubbling of oxygen gas into the solution of reduced enzyme at 255 K followed by stirring as described [15, 18]. The samples were placed into the pre-cooled optical cryostat (details of construction to be found in reference [4]), and the spectra were acquired while cooling to 80 K using liquid nitrogen.
Radiolytic reduction was accomplished by γ-irradiation of the samples immersed in liquid nitrogen using a 60Co-source for 210 min at the dose rate 16.8 krad/min with a total dose 3.5 Mrad. The yield of Cpd 0 in CPO was estimated based on the optical absorption of the oxy-ferrous CPO before and after irradiation [4, 33].
Results and Discussion
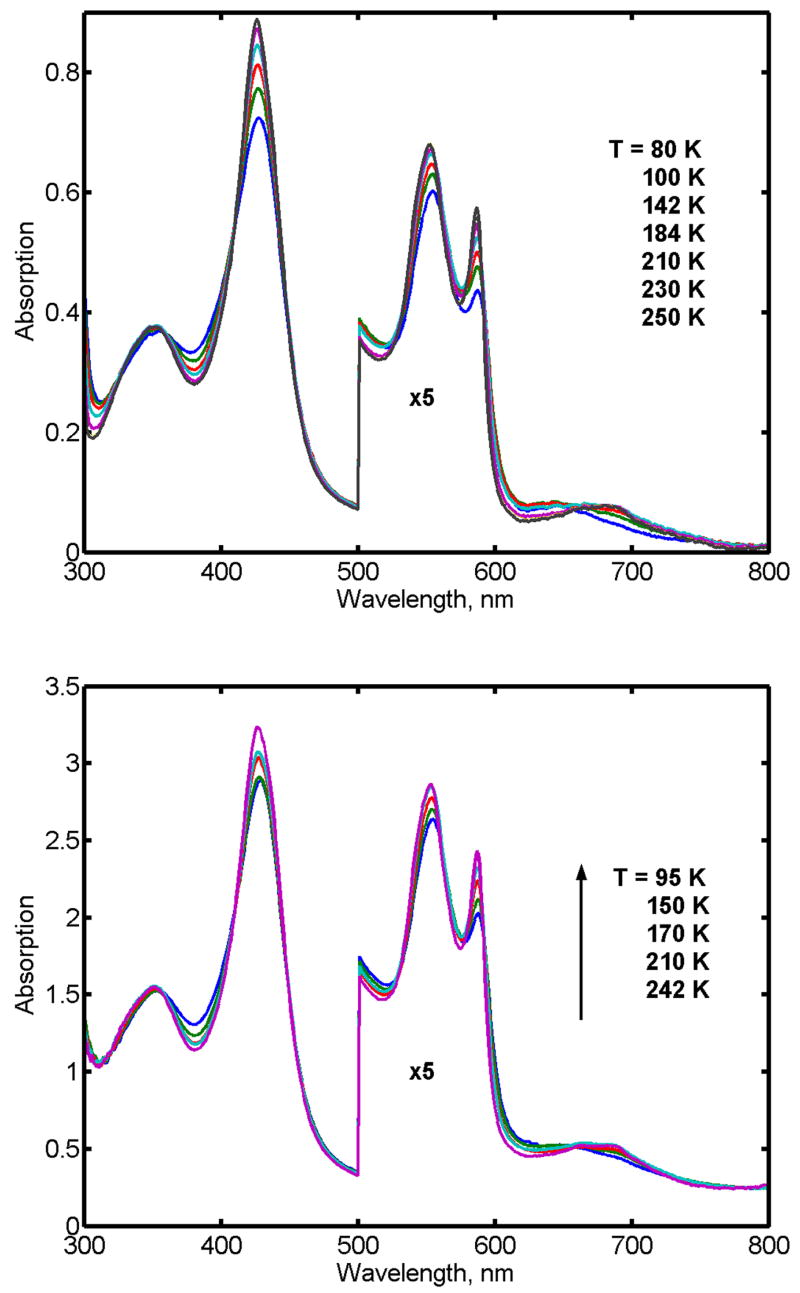
The observed temperature dependent spin equilibrium in ferric CPO at pH 6.0 in the absence and in the presence of the substrate thioanisole is shown in Figure 1 as the sample of the ferrous-oxy complex is cooled following oxygenation. In agreement with earlier observations [34, 35] at pH 6.0, CPO undergoes a transition from predominantly high-spin state at the ambient conditions to the low-spin state at 77 K, Figure 1. This spin shift is caused by coordination of the oxygen atom from the water molecule at the sixth position of the heme iron, resolved in the X-ray structure of the substrate free CPO [36]. The presence of thioanisole did not change the apparent spin state of CPO as monitored by optical spectroscopy. At room temperature, a broad Soret maximum is observed at 404 nm, while below 170 K the spectrum in the Soret region is represented by the typically sharp low-spin band at 423 nm with a broad band at 355 nm. In the visible region, the temperature decrease results in the gradual replacement of the high spin bands at 515 nm and 650 nm by the low-spin spectra with two well resolved peaks at 541 and 582 nm and several weak broad bands at 650 – 750 nm. Following reduction at the ambient conditions, the main maximum of the Soret band is observed at 409 nm with the shoulders at 421 nm and 446 nm.
Figure 1.
Absorption spectra of ferric CPO at varying temperatures in the absence (A) and in the presence (B) of the substrate thioanisole.
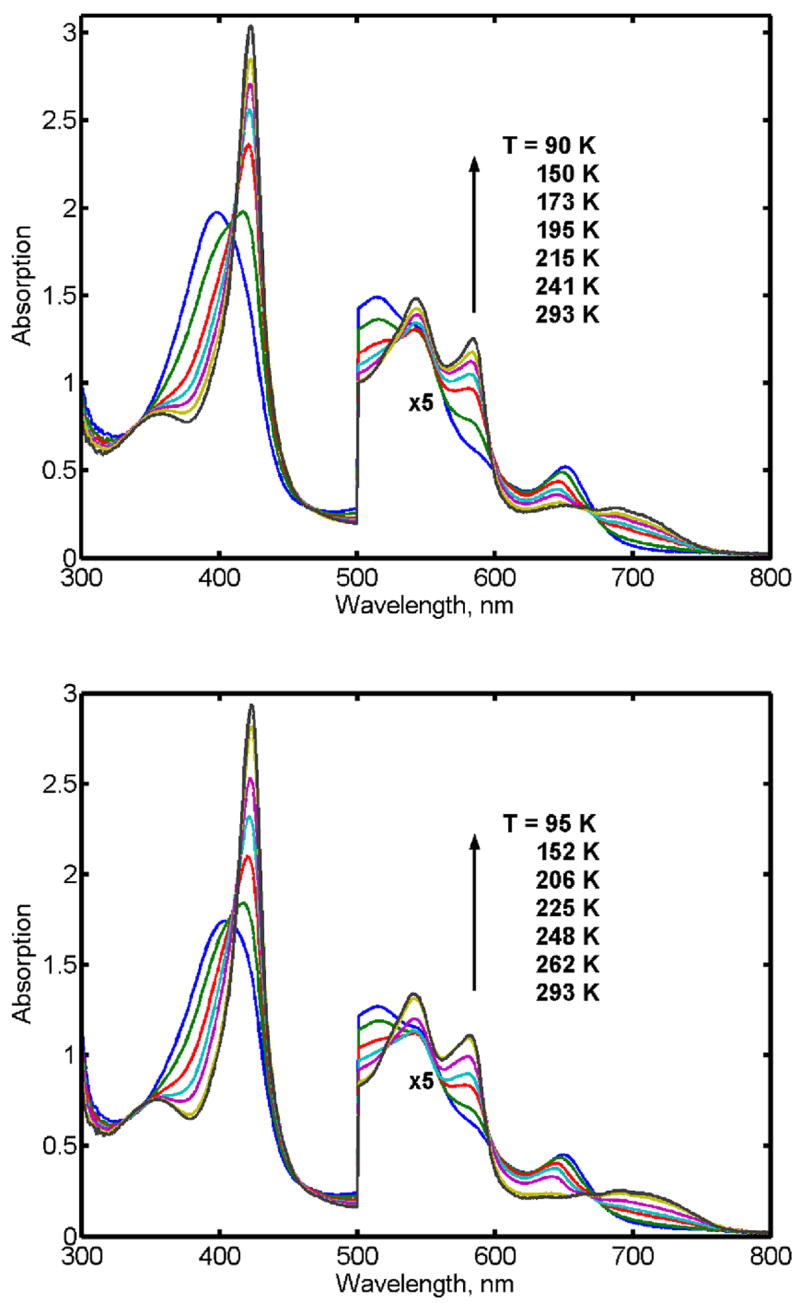
Spectra of the oxyferrous CPO are illustrated in Figure 2. As reported earlier [31], the Soret band clearly reveals features similar to those observed for the oxy-complexes of all thiolate ligated heme protein, including the distinctive so-called “split Soret” [37–41]. In the low temperature spectra of the oxy-complex of CPO, the main Soret band is observed at 428 nm, and the broad secondary band at 354 nm. In the visible region, the α and β bands are well resolved at T < 170 K with the maxima at 553 nm and 587 nm.
Figure 2.
Absorption spectra of oxyferrous CPO at different temperatures in the absence (A) and in the presence (B) of thioanisole
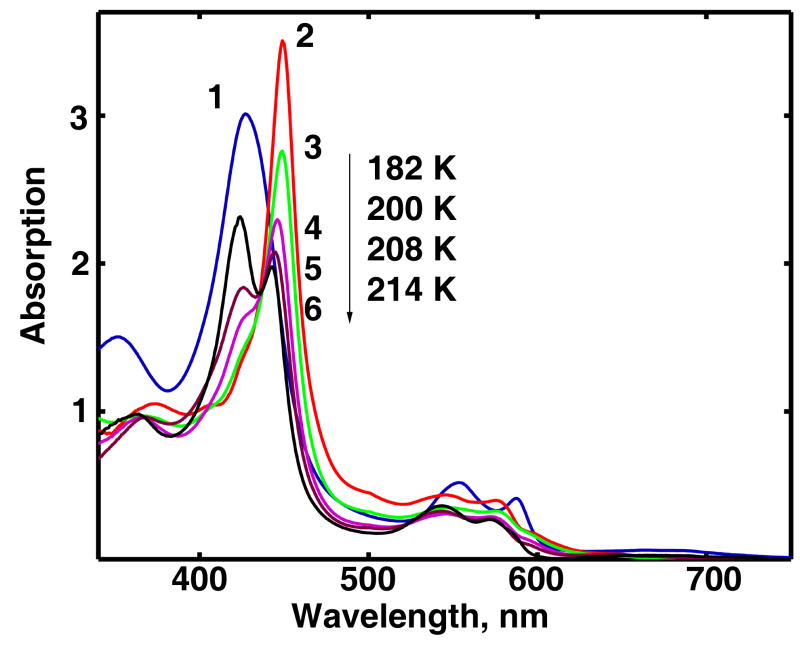
Irradiation at 77 K results in partial reduction of oxy-ferrous CPO and formation of hydroperoxo-ferric intermediate with approximately 50% yield as indicated by the appearance of the new Soret band at 449 nm at the expense of the band at 428 nm. The absorption spectrum corresponding to the pure hydroperoxo-ferric CPO at 77 K calculated from the experimental spectra is shown in Figure 3 together with the spectrum of oxy-ferrous CPO before irradiation and the spectra measured after gradual annealing of the irradiated sample at 182 K, 200 K, 208 K and 214 K. In addition to the red shifted Soret band two poorly resolved bands characteristic for the low-spin complexes of the ferric heme proteins are observed at 576 and 546 nm.
Figure 3.
Annealing of hydroperoxo-ferric intermediate (Compound 0) in CPO at different temperatures. Shown are absorption spectra of oxy-ferrous CPO (Compound III) at 80 K (1), and hydroperoxo-ferric CPO (Compound 0) at 80 K (2), 182 K (3), 200 K (4), 208 K (5), and 214 K (6). Compound 0 is stable in the frozen solution below 170 K, and decomposes at higher temperatures. Spectra of Compound 0 are calculated based on 50% yield as described in the text. .
Comparison of the spectral features of the hydroperoxo-ferric CPO with the earlier results obtained for the same intermediate in CYP101 and CYP119 [18, 42] reveals an interesting pattern common for these enzymes having thiolate as a fifth ligand to the heme iron. While in both cytochromes P450 the maximum of Soret band of Cpd 0 is obtained at 439 – 440 nm, in CPO it is shifted to 449 nm. However, a similar red shift is also observed for the position of Soret band of the oxy-ferrous CPO, 428 nm, as compared to 417 – 419 nm for the corresponding oxy-ferrous complex in cytochromes P450. This means that one-electron reduction of the oxy-complex results in the same 21 –22 nm (or 1150 – 1300 cm−1) red shift of the Soret band in both P450 and CPO. In contrast, the red shift of Soret band is only 3 – 8 nm (200 – 500 cm−1) in myoglobin [20], peroxidase [15] and heme oxygenase [19], all of which have histidine as a proximal ligand.
The 10 nm shift in Soret peak position for the oxyferrous states of CPO vs. CYP101 vs. CPO may be attributed to the difference in H-bonding to the proximal Cys, two H-bonds for CPO and three for CYP101 [43]. The similar differences in absorption spectra documented for the complexes of heme proteins in the ferric state [44, 45] can be explained by more electron-rich thiolate sulfur atom ligating heme iron in CPO than in CYP101. The red-shifted Soret maximum for the hydroperoxo-ferric intermediate in CPO as compared to cytochromes P450 is in agreement with these observations.
In conclusion, we document, for the first time, the formation and decay of hydroperoxo-ferric intermediate in CPO via an oxygenase/oxidase pathway. The oxy-ferrous complex of the enzyme is used as a reference point. This precursor has been created by direct oxygenation of the ferrous enzyme at low temperature (255 K) in aqueous – glycerol buffered solution. Although unstable at ambient conditions due to fast autoxidation, oxy- CPO is stable at low temperatures, as shown by UV-Vis spectroscopy. After formation, the samples of oxy-CPO in the presence or absence of the substrate (thioanisole) have been cooled and irradiated at 77 K by γ-rays. Cryoradiolysis results in partial reduction of the heme complexes, forming the peroxo-ferric CPO complex. The identity of this complex has been confirmed by low-temperature spectroscopy (see Figure 3). The peroxo-ferric complex of CPO revealed the same features as the same complex in cytochromes P450, namely the split Soret band (max at 449 nm and 374 nm), and poorly resolved α, β bands at 576 and 546 nm. Gradual annealing at elevated temperatures resulted in a decrease of the amplitude and broadening of the peaks characteristic for peroxo-/hydroperoxo- ferric complex. No new intermediate was detected, while the hydroperoxo- ferric CPO returned to the resting ferric state at 210 – 220 K
The new results reported herein support our earlier suggestions in analogous studies using cytochromes P450 CYP101 and CYP119 [1, 18, 42]. Cryoradiolytic reduction provides a useful tool to form the key intermediates of oxygen activation in heme enzymes, which could not be obtained for direct spectroscopic studies by other approaches. We obtained well resolved absorption spectra of peroxo-/hydroperoxo-ferric CPO and followed the direct decay of this intermediate to the resting state of the enzyme. Together with EPR results from B.M. Hoffman’s group [12–14, 16, 17, 46], our studies provide further insight into the nature and properties of active heme – oxygen intermediates in heme enzymes.
Acknowledgments
This work was supported by grants from the National Institutes of Health GM 31756 (SGS), and GM 26730 (JHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sligar SG, Makris TM, Denisov IG. Thirty years of microbial P450 monooxygenase research: peroxo-heme intermediates--the central bus station in heme oxygenase catalysis. Biochem Biophys Res Commun. 2005;338:346–354. doi: 10.1016/j.bbrc.2005.08.094. [DOI] [PubMed] [Google Scholar]
- 2.Egawa T, Yoshioka S, Takahashi S, Hori H, Nagano S, Shimada H, Ishimori K, Morishima I, Suematsu M, Ishimura Y. Kinetic and spectroscopic characterization of a hydroperoxy compound in the reaction of native myoglobin with hydrogen peroxide. J Biol Chem. 2003;278:41597–41606. doi: 10.1074/jbc.M210383200. [DOI] [PubMed] [Google Scholar]
- 3.Shintaku M, Matsuura K, Yoshioka S, Takahashi S, Ishimori K, Morishima I. Absence of a detectable intermediate in the compound I formation of horseradish peroxidase at ambient temperature. J Biol Chem. 2005;280:40934–40938. doi: 10.1074/jbc.M503472200. [DOI] [PubMed] [Google Scholar]
- 4.Denisov IG, Makris TM, Sligar SG. Cryoradiolysis for the study of P450 reaction intermediates. Methods Enzymol. 2002;357:103–115. doi: 10.1016/s0076-6879(02)57670-9. [DOI] [PubMed] [Google Scholar]
- 5.Makris TM, Davydov R, Denisov IG, Hoffman BM, Sligar SG. Mechanistic enzymology of oxygen activation by the cytochromes P450. Drug Metab Rev. 2002;34:691–708. doi: 10.1081/dmr-120015691. [DOI] [PubMed] [Google Scholar]
- 6.Kuhnel K, Derat E, Terner J, Shaik S, Schlichting I. Structure and quantum chemical characterization of chloroperoxidase compound 0, a common reaction intermediate of diverse heme enzymes. Proc Natl Acad Sci USA. 2007;104:99–104. doi: 10.1073/pnas.0606285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Symons MCR, Petersen RL. Electron capture at the iron-oxygen centre in single crystals of oxymyoglobin studied by electron spin resonance spectroscopy. Biochim Biophys Acta. 1978;535:241–247. doi: 10.1016/0005-2795(78)90090-9. [DOI] [PubMed] [Google Scholar]
- 8.Kappl R, Hoehn-Berlage M, Huettermann J, Bartlett N, Symons MCR. Electron spin and electron nuclear double resonance of the FeO2- (ferrite) center from irradiated oxyhemo- and oxymyoglobin. Biochim Biophys Acta. 1985;827:327–343. [Google Scholar]
- 9.Davydov RM. Optical and ESR-spectroscopic study of electronic adducts of oxymyoglobin and oxyhemoglobin. Biofizika. 1980;25:203–207. [PubMed] [Google Scholar]
- 10.Davydov RM, Ledenev AN. Activation mechanism of molecular oxygen with cytochrome P-450. Biofizika. 1981;26:1096. [PubMed] [Google Scholar]
- 11.Davydov R, Kappl R, Huttermann J, Peterson JA. EPR-spectroscopy of reduced oxyferrous-P450cam. FEBS Lett. 1991;295:113–115. doi: 10.1016/0014-5793(91)81398-r. [DOI] [PubMed] [Google Scholar]
- 12.Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM. Hydroxylation of camphor by reduced oxy-cytochrome P450cam: mechanistic implications of EPR and ENDOR studies of catalytic intermediates in native and mutant enzymes. J Am Chem Soc. 2001;123:1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- 13.Davydov R, Ledbetter-Rogers A, Martasek P, Larukhin M, Sono M, Dawson JH, Masters BS, Hoffman BM. EPR and ENDOR characterization of intermediates in the cryoreduced oxy-nitric oxide synthase heme domain with bound L-arginine or N(G)-hydroxyarginine. Biochemistry. 2002;41:10375–10381. doi: 10.1021/bi0260637. [DOI] [PubMed] [Google Scholar]
- 14.Davydov R, Kofman V, Fujii H, Yoshida T, Ikeda-Saito M, Hoffman BM. Catalytic mechanism of heme oxygenase through EPR and ENDOR of cryoreduced oxy-heme oxygenase and its Asp 140 mutants. J Am Chem Soc. 2002;124:1798–1808. doi: 10.1021/ja0122391. [DOI] [PubMed] [Google Scholar]
- 15.Denisov IG, Makris TM, Sligar SG. Formation and decay of hydroperoxo-ferric heme complex in horseradish peroxidase studied by cryoradiolysis. J Biol Chem. 2002;277:42706–42710. doi: 10.1074/jbc.M207949200. [DOI] [PubMed] [Google Scholar]
- 16.Davydov R, Kofman V, Nocek JM, Noble RW, Hui H, Hoffman BM. Conformational substates of the oxyheme centers in alpha and beta subunits of hemoglobin as disclosed by EPR and ENDOR studies of cryoreduced protein. Biochemistry. 2004;43:6330–6338. doi: 10.1021/bi036273z. [DOI] [PubMed] [Google Scholar]
- 17.Davydov R, Chemerisov S, Werst DE, Rajh T, Matsui T, Ikeda-Saito M, Hoffman BM. Proton transfer at helium temperatures during dioxygen activation by heme monooxygenases. J Am Chem Soc. 2004;126:15960–15961. doi: 10.1021/ja044646t. [DOI] [PubMed] [Google Scholar]
- 18.Denisov IG, Makris TM, Sligar SG. Cryotrapped reaction intermediates of cytochrome p450 studied by radiolytic reduction with phosphorus-32. J Biol Chem. 2001;276:11648–11652. doi: 10.1074/jbc.M010219200. [DOI] [PubMed] [Google Scholar]
- 19.Denisov IG, Ikeda-Saito M, Yoshida T, Sligar SG. Cryogenic absorption spectra of hydroperoxo-ferric heme oxygenase, the active intermediate of enzymatic heme oxygenation. FEBS Lett. 2002;532:203–206. doi: 10.1016/s0014-5793(02)03674-8. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim M, Denisov IG, Makris TM, Kincaid JR, Sligar SG. Resonance Raman spectroscopic studies of hydroperoxo-myoglobin at cryogenic temperatures. J Am Chem Soc. 2003;125:13714–13718. doi: 10.1021/ja036949d. [DOI] [PubMed] [Google Scholar]
- 21.Mak PJ, Denisov IG, Victoria D, Makris TM, Deng T, Sligar SG, Kincaid JR. Resonance Raman detection of the hydroperoxo intermediate in the cytochrome P450 enzymatic cycle. J Am Chem Soc. 2007;129:6382–6383. doi: 10.1021/ja071426h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makris TM, Denisov IG, Sligar SG. Haem-oxygen reactive intermediates: catalysis by the two-step. Biochem Soc Trans. 2003;31:516–519. doi: 10.1042/bst0310516. [DOI] [PubMed] [Google Scholar]
- 23.Makris TM, Denisov IG, Schlichting I, Sligar SG. Activation of molecular oxygen by cytochrome P450. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Function, Genetics. Kluwer Academic/Plenum Publishers; New York: 2005. pp. 149–182. [Google Scholar]
- 24.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim M, Kincaid JR. Spectroscopic studies of peroxo/hydroperoxo derivatives of heme proteins and model compounds. J Porph Phthalocyanines. 2004;8:215–225. [Google Scholar]
- 26.Cooper CE, Jurd M, Nicholls P, Wankasi MM, Svistunenko DA, Reeder BJ, Wilson MT. On the formation, nature, stability and biological relevance of the primary reaction intermediates of myoglobins with hydrogen peroxide. Dalton Trans. 2005:3483–3488. doi: 10.1039/b505786h. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa Y, Nakajima H, Watanabe Y. Characterization of peroxide-bound heme species generated in the reaction of thermally tolerant cytochrome c552 with hydrogen peroxide. ChemBioChem. 2006;7:1582–1589. doi: 10.1002/cbic.200600135. [DOI] [PubMed] [Google Scholar]
- 28.Svistunenko DA, Reeder BJ, Wankasi MM, Silaghi-Dumitrescu RL, Cooper CE, Rinaldo S, Cutruzzola F, Wilson MT. Reaction of Aplysia limacina metmyoglobin with hydrogen peroxide. Dalton Trans. 2007:840–850. doi: 10.1039/b615770j. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe Y, Nakajima H, Ueno T. Reactivities of oxo and peroxo intermediates studied by hemoprotein mutants. Acc Chem Res. 2007;40:554–562. doi: 10.1021/ar600046a. [DOI] [PubMed] [Google Scholar]
- 30.Lambeir AM, Dunford HB. Oxygen binding to dithionite-reduced chloroperoxidase. Eur J Biochem. 1985;147:93–96. doi: 10.1111/j.1432-1033.1985.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 31.Sono M, Eble KS, Dawson JH, Hager LP. Preparation and properties of ferrous chloroperoxidase complexes with dioxygen, nitric oxide, and an alkyl isocyanide. Spectroscopic dissimilarities between the oxygenated forms of chloroperoxidase and cytochrome P-450. J Biol Chem. 1985;260:15530–15535. [PubMed] [Google Scholar]
- 32.Hollenberg PF, Hager LP. Purification of chloroperoxidase from Caldariomyces fumago. Methods Enzymol. 1978;52:521–529. doi: 10.1016/s0076-6879(78)52057-0. [DOI] [PubMed] [Google Scholar]
- 33.Denisov IG, Victoria DC, Sligar SG. Cryoradiolytic reduction of heme proteins: Maximizing dose-dependent yield. Radiat Phys Chem. 2007;76:714–721. doi: 10.1016/j.radphyschem.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollenberg PF, Hager LP, Blumberg WE, Peisach J. An electron paramagnetic resonance study of the high and low spin forms of chloroperoxidase. J Biol Chem. 1980;255:4801–4807. [PubMed] [Google Scholar]
- 35.Lambeir AM, Dunford HB. A kinetic and spectral study of the alkaline transitions of chloroperoxidase. Arch Biochem Biophys. 1983;220:549–556. doi: 10.1016/0003-9861(83)90446-0. [DOI] [PubMed] [Google Scholar]
- 36.Kuhnel K, Blankenfeldt W, Terner J, Schlichting I. Crystal structures of chloroperoxidase with its bound substrates and complexed with formate, acetate, and nitrate. J Biol Chem. 2006;281:23990–23998. doi: 10.1074/jbc.M603166200. [DOI] [PubMed] [Google Scholar]
- 37.Hanson LK, Sligar SG, Gunsalus IC. Electronic structure of cytochrome P450. Croat Chem Acta. 1977;49:237–250. [Google Scholar]
- 38.Hanson LK, Eaton WA, Sligar SG, Gunsalus IC, Gouterman M, Connell CR. Origin of the anomalous Soret spectra of carboxycytochrome P-450. J Am Chem Soc. 1976;98:2672–2674. doi: 10.1021/ja00425a050. [DOI] [PubMed] [Google Scholar]
- 39.Harris D, Loew G, Waskell L. Structure and spectra of ferrous dioxygen and reduced ferrous dioxygen model cytochrome P450. J Am Chem Soc. 1998;120:4308–4318. [Google Scholar]
- 40.Loew G. Electronic structure of heme sites. In: Solomon EI, Lever ABP, editors. Inorganic Electronic Structure and Spectroscopy. John Wiley & Sons; New York: 1999. pp. 451–531. [Google Scholar]
- 41.Loew G. Structure, spectra, and function of heme sites. Int J Quant Chem. 2000;77:54–70. [Google Scholar]
- 42.Denisov IG, Hung SC, Weiss KE, McLean MA, Shiro Y, Park SY, Champion PM, Sligar SG. Characterization of the oxygenated intermediate of the thermophilic cytochrome P450 CYP119. J Inorg Biochem. 2001;87:215–226. doi: 10.1016/s0162-0134(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 43.Glascock MC, Ballou DP, Dawson JH. Direct observation of a novel perturbed oxyferrous catalytic intermediate during reduced putidaredoxin-initiated turnover of cytochrome P-450-CAM: probing the effector role of putidaredoxin in catalysis. J Biol Chem. 2005;280:42134–42141. doi: 10.1074/jbc.M505426200. [DOI] [PubMed] [Google Scholar]
- 44.Dawson JH, Andersson LA, Sono M, Hager LP. Systematic trends in the spectroscopic properties of low-spin ferric ligand adducts of cytochrome P-450 and chloroperoxidase: the transition from normal to hyper spectra. New J Chem. 1992;16:577–582. [Google Scholar]
- 45.Roach MP, Pond AE, Thomas MR, Boxer SG, Dawson JH. The role of the distal and proximal protein environments in controlling the ferric spin state and in stabilizing thiolate ligation in heme systems: Thiolate adducts of the myoglobin H93G cavity mutant. J Am Chem Soc. 1999;121:12088–12093. [Google Scholar]
- 46.Hoffman BM. ENDOR of metalloenzymes. Acc Chem Res. 2003;36:522–529. doi: 10.1021/ar0202565. [DOI] [PubMed] [Google Scholar]