Abstract
Background and purpose:
In Dahl S rats, high salt increases activity of the tissue renin-angiotensin-aldosterone system (RAAS) in the CNS, heart and kidneys. Here, we assessed the effects of chronic angiotensin converting enzyme (ACE) inhibition on salt-induced hypertension and cardiovascular and renal hypertrophy and fibrosis, relative to the extent of ACE blockade.
Experimental approach:
From 4.5 weeks of age, Dahl S rats received either the lipophilic ACE inhibitor trandolapril (1 or 5 mg kg-1 day-1) or the hydrophilic ACE inhibitor lisinopril (10 or 50 mg kg-1 day-1) and a high salt diet was started 0.5 week later. Treatments ended at 9 weeks of age.
Key results:
High salt diet markedly increased blood pressure (BP), decreased plasma angiotensin II and increased ACE binding densities in brain, heart, aorta and kidneys. Trandolapril and lisinopril prevented 50% of the increase in BP in light and dark period of the day. After the last doses, trandolapril decreased ACE densities by ∼80% in brain nuclei and heart and lisinopril by ∼60% in the brain and by ∼70% in the heart. The two ACE inhibitors prevented right ventricular hypertrophy and attenuated left ventricular hypertrophy but did not affect renal hypertrophy caused by high salt. Both drugs prevented high salt-induced fibrosis in heart, kidney and aorta.
Conclusion and implication:
As the ACE inhibitors could completely prevent tissue fibrosis and partially prevent tissue hypertrophy and hypertension, the tissue RAAS may play a critical role in salt-induced fibrosis, but a lesser role in the hypertrophy.
Keywords: lisinopril, trandolapril, brain, heart, kidneys, aorta, ACE, AT1-receptor, high salt diet, blood pressure
Introduction
In recent years, several studies have demonstrated that high salt diet decreases the activity of the circulating renin–angiotensin–aldosterone system (RAAS), but may activate the tissue RAAS in some organs such as the brain, heart and kidneys. In Dahl S rats, high salt increases the mRNA for angiotensin-converting enzyme (ACE) and its activity as well as AT1 receptor densities in the hypothalamus (Zhao et al., 2001; Wang et al., 2003). Intracerebroventricular infusion of an AT1 receptor blocker prevents both the sympatho-excitation and hypertension (Huang and Leenen, 1998; Leenen and Yuan, 2001). In normotensive WKY rats, high salt increases cardiac AT1 receptor mRNA and aldosterone synthesis (Takeda et al., 2000), and causes widespread fibrosis in the heart, arteries and kidneys (Yu et al., 1998). In Wistar rats, the high salt-induced myocardial fibrosis was prevented by spironolactone (Lal et al., 2003). In Dahl S rats, high salt intake for 3 weeks caused modest increases in angiotensin II levels and clear increases in aldosterone levels in the heart and kidneys (Bayorh et al., 2005) associated with extensive cardiovascular and renal hypertrophy, fibrosis and damage. We hypothesized that in Dahl S rats, this activation of the tissue RAAS by high salt not only contributes to the salt-induced hypertension, but also to the salt-induced tissue hypertrophy and fibrosis. The latter may be—at least in part—independent of the hypertension since central blockade prevented the hypertension but not the high salt-induced increases in weight and ACE mRNA and activity in the left ventricle (LV) (Zhao et al., 2000). If the tissue RAAS indeed mediates these effects of high salt diet, treatment with blockers of the RAAS at high enough doses to cause sufficient and persistent tissue blockade should be able to prevent both the hypertension and the cardiovascular and renal hypertrophy and fibrosis. We previously showed that chronic subcutaneous (s.c.) treatment with the AT1 receptor blocker irbesartan at high doses causing central blockade indeed can prevent the hypertension in Dahl S rats on high salt diet (Leenen and Yuan, 2001). Other studies on the effects of peripheral treatment with blockers of the RAAS in Dahl S rats have shown diverse results. Some reported minimal effects on blood pressure (BP) (De Simone et al., 1996; Sugimoto et al., 1998; Satoh et al., 2003; Yamamoto et al., 2005; Kobayashi et al., 2006), others showed significant attenuation of the increase in BP on high salt (O'Donnell et al., 1992; Ashida et al., 1997). None of these studies assessed BP effects relative to extent of tissue RAAS blockade.
To assess the hypothesis proposed above, the present study had as its main objective the assessment, in Dahl S rats on high salt diet, of the effects of chronic s.c. treatment with an ACE inhibitor on the development of hypertension and on cardiovascular and renal hypertrophy and fibrosis relative to the extent of ACE blockade in relevant tissues. The extent of inhibition of tissue ACE may vary between ACE inhibitors with high lipophilicity and high affinity for ACE and those inhibitors, which are hydrophilic and exhibit low ACE affinity. This difference has been postulated to affect intermediate and clinical outcomes (Dzau et al., 2001). As a secondary objective, we therefore evaluated two ACE inhibitors, trandolapril being very lipophilic and having high ACE affinity (Conen and Brunner, 1993) and lisinopril being very hydrophilic and having low ACE affinity (Dzau et al., 2001), each at medium- and high-dose levels (Tan et al., 2005).
Methods
Animals
All experimental procedures were carried out in accordance with the guidelines of the Canadian Institutes of Health Research outlined in the ‘Guide for the Care and Use of Experimental Animals' and were approved by the University of Ottawa Animal Care Committee. Male Dahl S rats (Harlan Sprague Dawley, Madison, WI, USA; 3.5–4 weeks of age), were housed in a temperature-controlled environment at 24 °C on a 12:12-h light–dark cycle, fed regular rat chow, and allowed tap water ad libitum for 3–5 days before entering the study.
Experimental protocols
Protocol I
Two sets of male Dahl S rats were randomly divided into six treatment groups (6 rats per group): Trandolapril (1 and 5 mg kg−1 day−1), lisinopril (10 and 50 mg kg−1 day−1) or vehicle (0.9% saline, for 2 groups). Treatments started at 4.5 weeks of age with daily s.c. injections in the morning. At 5 weeks of age, five groups (the four drug groups and one vehicle group) were started on high (1370 μmol Na+ g−1) salt diet (Harlan Teklad, Madison, WI, USA), and one vehicle group remained on regular (101 μmol Na+ g−1) salt diet (Harlan Teklad, Madison, WI, USA). Treatments continued until 9 weeks of age and final assessments were made in one set of rats in the afternoon, 4 h after the last dose and in the second set of rats in the morning, 24 h after the last dose. The doses of trandolapril and lisinopril were based on our study in Wistar rats (Tan et al., 2005) which showed that at medium doses trandolapril caused better inhibition of tissue ACE binding densities than lisinopril, while at the higher doses the two drugs caused similar inhibition.
Blood pressure, heart rate and plasma angiotensin II measurements
At the end of the four-week dietary period, rats were anaesthetized by isoflurane–oxygen inhalation, and a polyethylene catheter was inserted into the left carotid artery, tunnelled s.c., and exteriorized at the nape. After a recovery period of 3 h (for the 4 h post dosing groups) or overnight (for 24 h post dosing groups), resting mean arterial pressure and heart rate were recorded for 30 min in conscious, freely moving animals as described previously (Leenen and Yuan, 2001). Blood samples were then obtained from conscious unstressed rats via the arterial catheter. The plasma was stored at −80 °C until measurement of angiotensin II by RIA after separation by HPLC as described previously (Ruzicka et al., 1995).
Protocol II
Male Dahl S rats were randomly divided into four groups (5–7 rats per group): Trandolapril (5 mg kg−1 day−1), lisinopril (50 mg kg−1 day−1) and vehicle (0.9% saline) in two groups. The study design was otherwise the same as in protocol I. Baseline 24 h food and water intake was measured at the last day of regular salt intake and then once weekly.
Telemetry
After two weeks of high salt diet, a BP probe (DSI model TA11PA-C40, Data Science International, St Paul, MN, USA)) was positioned intra-abdominally, under isoflurane anaesthesia and secured to the ventral abdominal muscle with the catheter inserted into the lower abdominal aorta (Huang et al., 2007). The telemetry signal was processed using an analogue adaptor and computerized data acquisition system to calculate and store the mean values of BP and heart rate over a 1 min interval every 1 h. Recordings were started 1 week following the probe implantation.
Echocardiography
A VisualSonics Vevo-770 high-resolution imaging echocardiography system (Visualsonics, Toronto, ON, Canada) with a 33-MHz transducer at a depth setting of 3–4 cm was used. At 2 and 4 weeks on high salt diet, under light isoflurane anaesthesia, an LV M-mode tracing just below the tips of the mitral leaflets was obtained and recorded on videotape. Simultaneous M-mode recording of intraventricular septum (LVIVS) and posterior wall (LVPW) thickness and LV internal dimension in systole and diastole (LVIDd, LVIDs) was performed for at least six cardiac cycles. LV volume in systole and diastole (Vs, Vd) were used to measure ejection fraction (EF) according to the following formula: EF=[(Vd−Vs)Vd−1] × 100%. LVID, LVIVS and LVPW in diastole were used to measure LV mass according to the following formula: LV mass=1.053*((LVIDd+LVIVSd+LVPWd)3−LVIDd3).
Tissue sampling
After blood sampling, rats in protocol I were killed by decapitation and the kidneys, brain, heart and abdominal aorta were excised quickly. The LV+RV and both kidneys were immediately rinsed in ice-cold saline and weighed. The LV and RV together were cut into two parts. The upper sections of the LV and RV were placed in 10% formalin for fibrosis studies. The lower parts were frozen in methylbutane/dry ice and stored at −80 °C for autoradiography. The brain, one kidney, lower parts of the left and right atrium and half of the abdominal aorta were also stored for autoradiography. The other kidney, upper part of left and right atrium and remaining part of abdominal aorta were kept in 10% formalin for fibrosis studies.
Rats in protocol II were decapitated at 4 h after the last dose, and LV, RV and kidney weights measured. Lungs were frozen in methylbutane/dry ice and stored at −80 °C for autoradiography.
Quantitative in vitro AT1 receptor and ACE autoradiography
Binding densities for ACE and AT1 receptors were determined using the ACE inhibitor derivative 125I-351A (a derivative of lisinopril, iodinated by the chloramine T method) and 125I-Sar1I1e8-AngII, respectively, as described recently (Dean et al., 2005; Tan et al., 2005). The preincubation step was omitted to maintain the ACE blockade.
Tissue fibrosis
Transverse sections of the heart, kidney and aorta (4 μm thick) were stained with Sirius red F3BA (0.5%. in saturated aqueous picric acid). The slides of the LV and RV, LA and RA were pictured in their entirety, with a digital camera connected to the microscope, using Adobe Photoshop 6.0 imaging software (Adobe System Canada, Ottawa, ON, Canada). Interstitial fibrosis and perivascular fibrosis were determined separately (Lal et al., 2003), using Image Pro Plus 4.1 imaging software (Media Cybernetics, Silver Spring, MD, USA) and the results were expressed as percentages. Approximately 12–16 images for interstitial fibrosis and 10–12 images for perivascular fibrosis were analysed. In the kidney, fibrosis was measured in 50–60 tubules from each animal selected randomly. To evaluate glomerular size, 20–25 glomeruli per kidney were measured. Fibrosis in the aorta was measured in the tunica media and tunica adventitia. For each animal, one average value for each area was calculated.
Statistical analysis
Values are expressed as mean±s.e.mean. Differences between groups were evaluated by two-way ANOVA. The Bonferroni test was used to locate significant differences for post hoc analysis when applicable. P<0.05 was considered statistically significant.
Materials
Trandolapril and isoflurane were from Abbott Laboratories, Montreal, QC, Canada; lisinopril from Apotex Inc, Toronto, ON, Canada. Angiotensin II and PD 123319 were supplied by Sigma Aldrich Canada, Oakville, ON, Canada; 125I-AngII and 125I-NaI by GE Healthcare, Mississauga, ON, Canada; 125I-Sar1I1e8-AngII was supplied by the University of Mississippi, MS, USA. The derivative of lisinopril, 351A was provided by Dr Y Sun, Division of Cardiovascular Diseases, Department of Medicine, University of Tennessee Health Sciences Centre, Memphis, TN, USA and the antibody against AngII was kindly provided by Dr M Schalekamp's group, Rotterdam, the Netherlands. All other reagents were from Sigma Aldrich Canada, Oakville, ON, Canada.
Results
Body weight and 24 h food and water intake
On high salt diet, body weight increased less over the experimental period, whereas 24 h food intake was similar on regular and high salt diet. The baseline water intake was similarly increased by both ACE inhibitors. High salt diet significantly increased water intake, similarly in vehicle and ACE inhibitor-treated groups (Table 1).
Table 1.
Effects of high salt intake and ACE inhibitors on body weight and 24 h food and water intake in Dahl S rats
| Regular salt diet |
High salt diet |
|||
|---|---|---|---|---|
| Vehicle | Vehicle | Trandolapril | Lisinopril | |
| BW (g) | ||||
| 2 weeks | 213±2 | 179±3* | 200±3*† | 197±2*† |
| 4 weeks | 356±2 | 316±4* | 312±3* | 310±7* |
| Food intake (g 100 g−1 BW) | ||||
| Baseline | 11±1 | 12±1 | 12±1 | 12±1 |
| 1 week | 10±1 | 12±1 | 10±1 | 11±1 |
| 2 weeks | 9±1 | 11±1 | 9±1 | 9±1 |
| 3 weeks | 7±1 | 6±1 | 7±1 | 7±1 |
| 4 weeks | 8±1 | 8±1 | 8±1 | 7±1 |
| 5 weeks | 8±1 | 7±1 | 8±1 | 7±1 |
| Water intake (ml 100 g−1 BW) | ||||
| Baseline | 16±1 | 16±1 | 22±3*† | 22±2*† |
| 1 week | 15±1 | 57±2* | 57±1* | 57±2* |
| 2 weeks | 13±1 | 51±3* | 50±2* | 46±2* |
| 3 weeks | 10±1 | 34±1* | 42±2* | 43±3* |
| 4 weeks | 10±1 | 34±2* | 39±5* | 37±2* |
| 5 weeks | 9±1 | 34±2* | 36±2* | 37±2* |
Abbreviations: ACE, angiotensin-converting enzyme; BW, body weight.
Values are expressed as mean±s.e.mean, n=6 rats per group.
*P<0.05 vs regular salt. †P<0.05 vs high salt +vehicle.
Blood pressure
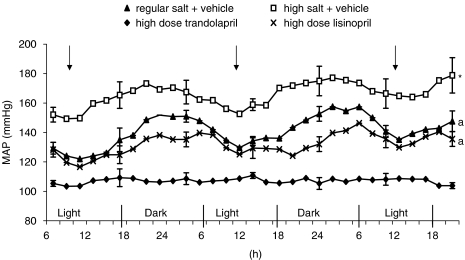
By telemetry, the BP showed little changes during the 24 h light–dark cycle on regular salt diet, but showed a clear diurnal cycle on high salt diet with higher BP in the dark time (P<0.001 for BP variation on high vs regular salt; Figure 1). High salt caused the expected marked increase in BP (P<0.001). Both ACE inhibitors partially (P<0.001 vs regular and vs high salt) prevented the increase in BP, similarly for the light and dark period and did not affect the salt effect on the diurnal cycle. Lisinopril tended (P=0.10) to be more effective during the dark period (Figure 1).
Figure 1.
MAP (mean arterial blood pressure) in Dahl S rats on regular salt intake or on high salt intake treated with vehicle, lisinopril or trandolapril from days 31 to 33 of high salt diet. The arrow indicates the time of vehicle or ACE inhibitor injection. Values are expressed as mean±s.e.mean; n=6 rats per group. *P<0.001 vs other groups, a P<0.001 vs regular and high salt+vehicle.
In protocol I, BP was measured by intra-arterial catheter at the end of the study and results obtained showed a pattern similar to that measured by telemetry (data not shown). Both doses of the two ACE inhibitors prevented most of the increase in BP at 4 h after dosing (∼140 vs 185 mm Hg in vehicle group) and to a less extent at 24 h after dosing (∼160 vs 190 mm Hg).
Plasma angiotensin II
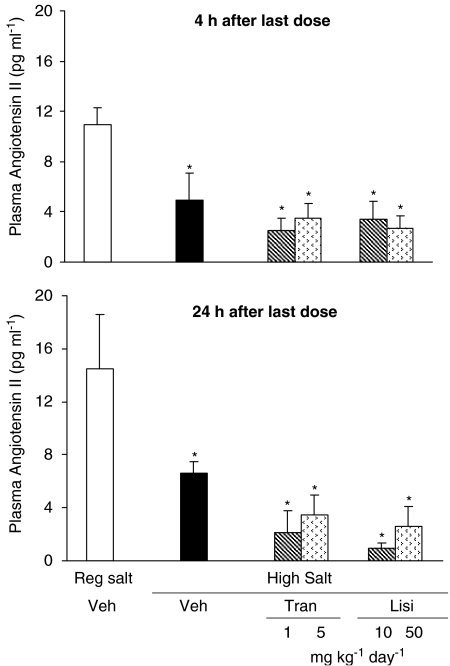
High salt diet for 4 weeks significantly decreased plasma Angiotensin II levels. At either 4 h (NS) or 24 h (P=0.06) after the last dose, the two ACE inhibitors caused a modest further decrease in angiotensin II levels (Figure 2).
Figure 2.
Plasma angiotensin II levels at 4 and 24 h after the last dose in Dahl S rats on regular salt intake or on high salt intake treated with vehicle, lisinopril or trandolapril at two dose levels for 5 weeks. Values are expressed as mean±s.e.m.; n=6 rats per group. *P<0.05 vs regular salt diet.
Tissue ACE densities
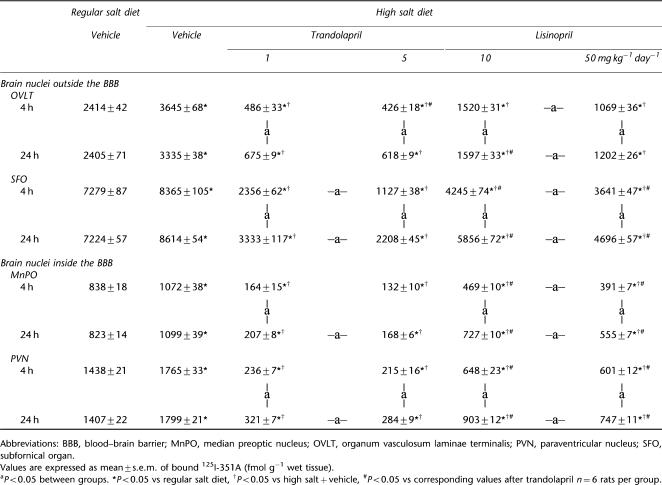
High salt diet caused significant increases in ACE densities by around 20% in brain nuclei both inside and outside the blood–brain barrier (BBB) (Table 2) and (s.c.) administration of the two ACE inhibitors markedly reduced ACE binding densities (Table 2). For trandolapril, the two doses caused nearly similar inhibition of ACE binding, at 4 h only significantly different for SFO and at 24 h for SFO, MnPO and PVN. At 24 h after the last dose, significant inhibition of ACE in these nuclei remained with only moderate increases in the binding densities compared to 4 h. For lisinopril, there was a dose-dependent inhibition apparent at 4 h after dosing in three of four nuclei and at 24 h in all four nuclei (Table 2). The extent of inhibition had diminished significantly at 24 h after the last dose. In comparison with lisinopril, trandolapril caused greater inhibition of ACE densities at both doses and time points. At 24 h after the last dose, the differences in blockade by trandolapril and lisinopril were larger in the nuclei inside the BBB than the ones outside the BBB (Table 2).
Table 2.
ACE densities in brain nuclei in Dahl S rats treated with either vehicle, lisinopril or trandolapril at two dose levels for 5 weeks
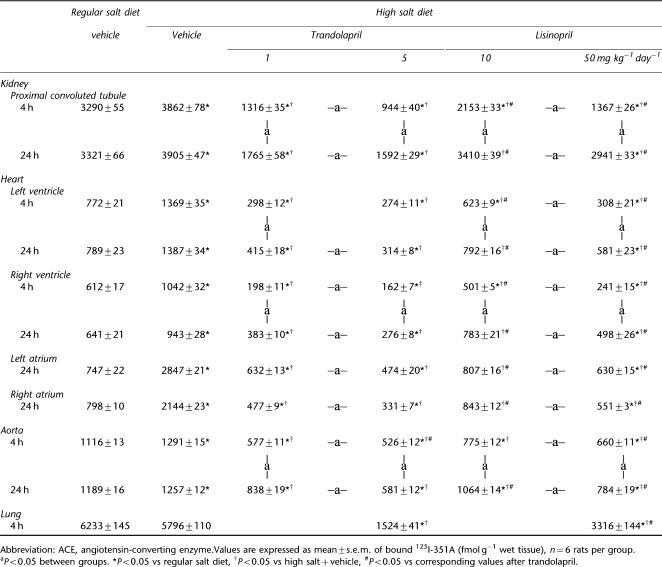
In peripheral tissues, high salt diet caused a twofold increase in ACE binding densities in both ventricles, three- to fourfold increase in atria and 10–20% increases in the aorta and kidney (Table 3). Both ACE inhibitors markedly lowered binding densities at 4 h after the last dose (Table 3). Both trandolapril and lisinopril caused a dose-dependent inhibition in heart, aorta and kidney at 4 and 24 h after the last dose. At 24 h after the last dose of trandolapril, the extent of inhibition had diminished somewhat mainly for the lower dose in the aorta and for both doses in the kidney. By 24 h after the last dose of lisinopril, the extent of inhibition had clearly diminished, particularly in the kidney. At 4 and 24 h after the last dose, trandolapril showed better inhibition of ACE binding densities than lisinopril at both doses in heart, aorta and kidney (Table 3). In the lung, high salt diet did not change ACE-binding densities, and trandolapril showed better inhibition of ACE densities than lisinopril at 4 h after the last dose.
Table 3.
ACE densities in kidney, heart and aorta in Dahl S rats treated with either vehicle, lisinopril or trandolapril at two dose levels for 5 weeks
Tissue AT1 receptor densities
High salt diet increased AT1 receptor-binding densities by 10–20% in nuclei both inside and outside the BBB. The two ACE inhibitors decreased AT1 receptor densities in all nuclei compared to the high-salt vehicle group, almost back to levels on regular salt diet (Table 4).
Table 4.
AT1 receptor densities in Dahl S rats treated with vehicle, lisinopril or trandolapril at two dose levels for 5 weeks
| Regular salt diet |
High salt diet |
||||||
|---|---|---|---|---|---|---|---|
| vehicle | Vehicle |
Trandolapril |
Lisinopril |
||||
| 1 | 5 | 10 | 50 mg kg−1 per day | ||||
| Brain nuclei outside the BBB | |||||||
| OVLT | 830±18 | 932±7* | 856±29 | 798±36† | 839±12 | 814±23 | |
| SFO | 1335±29 | 1617±39* | 1251±24† | 1265±23 | 1370±24†# | –a– | 1293±32† |
| Brain nuclei inside BBB | |||||||
| MnPO | 654±13 | 814±9* | 747±18*† | 738±27*† | 768±11* | 758±10* | |
| PVN | 812±5 | 1021±33* | 911±17*† | 887±24† | 967±19*† | –a– | 866±18† |
| Kidney | |||||||
| Medulla | 2499±23 | 1772±40* | 1902±14† | 2014±51† | 2004±27† | 1932±24† | |
| Cortex | 1454±30 | 949±43* | 1214±23† | 1335±37† | 1218±49† | 1262±48† | |
| Heart | |||||||
| LV | 70±3 | 67±2 | 63±2 | 65±2 | 64±3 | 67±2 | |
| RV | 18±1 | 17±1 | 16±1 | 16±1 | 16±1 | 17±1 | |
| LA | 69±4 | 66±5 | 67±3 | 74±6 | 73±6 | 76±1 | |
| RA | 56±2 | 72±4 | 63±4 | 65±2 | 57±4 | 63±4 | |
| Aorta | 174±6 | 218±10* | 166±6† | 146±5†* | 165±5† | 160±5† | |
Abbreviations: BBB, blood–brain barrier; MnPO, median preoptic nucleus; OVLT, organum vasculosum laminae terminalis; PVN, paraventricular nucleus; SFO, subfornical organ.
Values are expressed as mean±s.e.mean of bound 125I Angiotensin II (fmol mg−1 wet tissue). n=6 rats per group.
aP<0.05 between groups. *P<0.05 vs regular salt diet, †P<0.05 vs high salt+ vehicle, #P<0.05 vs corresponding values after trandolapril.
In the peripheral tissues, high-salt intake caused an increase in AT1 receptor densities in the aorta, but a decrease in the renal medulla and cortex. Both ACE inhibitors decreased the AT1 receptor densities in the aorta to levels somewhat below those in rats on regular salt intake. In contrast, both drugs increased AT1 receptor binding in the kidney, but densities remained below those in rats on regular salt intake. The heart demonstrated low AT1 receptor densities, not changed by either high salt diet or ACE inhibitor treatment (Table 4). Results at 24 h after dosing was fairly similar to those at 4 h (not shown).
LV function and geometry
On high salt diet, LV wall thickness and mass showed progressive increases. EF was still normal at 2 weeks but was decreased after 4 weeks (Table 5). Treatment with either ACE inhibitor completely prevented the initial increase in cardiac geometry and most of the increase at 4 weeks on high salt diet. The decrease in EF was completely prevented by either ACE inhibitor (Table 5).
Table 5.
Left ventricular parameters by echocardiography in Dahl S rats after 2 and 4 weeks of high salt diet and ACE inhibitor treatment
| Regular salt diet |
High salt diet |
||||
|---|---|---|---|---|---|
| vehicle | Vehicle | Trandolapril | Lisinopril | ||
| LVIDd | |||||
| Absolute (mm) | 2 weeks | 6.3±0.2 | 6.3±0.1 | 6.1±0.2 | 5.8±0.1 |
| 4 weeks | 6.7±0.2 | 6.9±0.1 | 6.4±0.2 | 6.6±0.2 | |
| Relative (mm 100 g−1 BW) | 2 weeks | 3.0±0.1 | 3.5±0.1* | 3.1±0.1 | 2.9±0.1† |
| 4 weeks | 1.9±0.1 | 2.1±0.1* | 1.9±0.1 | 2.0±0.1 | |
| LVIDs | |||||
| Absolute (mm) | 2 weeks | 2.9±0.1 | 3.1±0.1 | 2.8±0.1 | 2.5±0.1† |
| 4 weeks | 3.2±0.2 | 3.6±0.1 | 2.6±0.1*† | 2.6±0.2*† | |
| Relative (mm 100 g−1 BW) | 2 weeks | 1.4±0.1 | 1.9±0.1* | 1.4±0.1† | 1.3±0.1† |
| 4 weeks | 0.9±0.1 | 1.1±0.1* | 0.8±0.1† | 0.8±0.1† | |
| IVS+PW (d, mm) | 2 weeks | 2.3±0.1 | 2.7±0.1* | 2.4±0.1† | 2.4±0.1† |
| 4 weeks | 2.6±0.1 | 3.5±0.1* | 2.9±0.1*† | 2.8±0.1† | |
| Heart rate (b.p.m.) | 2 weeks | 350±12 | 407±3* | 349±7† | 356±8† |
| 4 weeks | 373±6 | 431±10* | 369±8† | 379±9† | |
| Ejection fraction (%) | 2 weeks | 83±2 | 79±2 | 84±1 | 86±1† |
| 4 weeks | 82±2 | 74±3* | 87±1† | 88±2† | |
| LV mass | |||||
| Absolute (mg) | 2 weeks | 407±25 | 483±12* | 405±13† | 373±14† |
| 4 weeks | 527±26 | 843±21* | 563±28† | 575±35† | |
| Relative (mm 100 g−1 BW) | 2 weeks | 191±11 | 270±4* | 203±8† | 189±8† |
| 4 weeks | 149±7 | 259±5* | 170±10† | 173±10† | |
Abbreviations: ACE, angiotensin-converting enzyme; BW, body weight; IVS, interventricular septum; LVIDd, left ventricular internal dimension in diastole; LVIDs, left ventricular internal dimension in systole; PW, posterior wall.
Values are expressed as mean±s.e.mean, n=6 rats per group. *P<0.05 vs regular salt. †P<0.05 vs high salt +vehicle.
Tissue weight
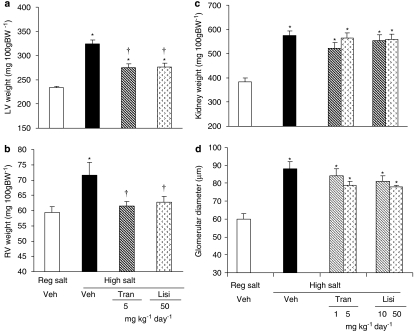
High salt diet caused a marked increase in LV+RV weight from 283±5 to 435±11 mg 100 g−1 (P<0.01). Treatment with either ACE inhibitor partially prevented the increase in LV+RV weight (to ∼350 mg 100 g−1, P<0.01), similarly for both doses and both ACE inhibitors. In protocol II, high salt diet significantly increased both LV and RV weights. Treatment with either ACE inhibitor partially prevented the increase in LV weight (Figure 3a), but completely prevented the increase in RV weight (Figure 3b). High salt diet significantly increased kidney weight and glomerular size. These increases were not prevented by either ACE inhibitor (Figures 3c and d).
Figure 3.
Left (LV) and right (RV) ventricular and kidney changes in Dahl S rats on regular salt intake or on high salt intake treated with vehicle, lisinopril or trandolapril. In (a) LV weight and in (b) RV weight in the protocol II study are shown. In (c), the left kidney weight and in (d) the glomerular size (d) in protocol I studies are shown. Values are expressed as mean±s.e.mean, n=6 rats per group. *P<0.05 vs regular salt diet, †P<0.05 vs high salt diet+vehicle.
Tissue fibrosis
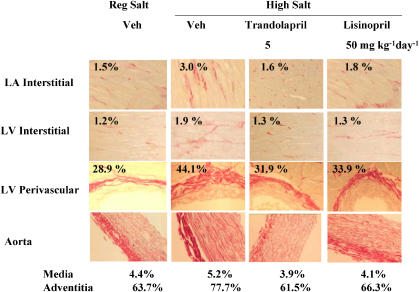
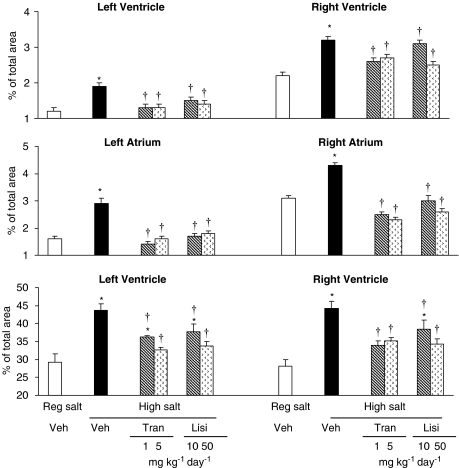
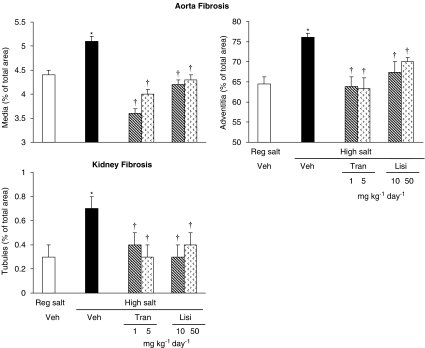
See Figure 4 for representative images. High salt diet increased interstitial fibrosis in all four chambers of the heart. Both trandolapril and lisinopril prevented this increase. High salt diet increased perivascular fibrosis in both ventricles. Both drugs partially prevented this increase at the lower dose and fully at the higher dose (Figure 5). In the aorta, fibrosis increased in both the media and adventia. Kidney tubular fibrosis increased on high salt diet. Both ACE inhibitors at both doses prevented these increases (Figure 6).
Figure 4.
Representative images of cardiac and aorta fibrosis in Dahl S rats on regular salt intake or on high salt intake treated with vehicle, lisinopril or trandolapril. % refers to % of total area. LA: left atrium; LV: left ventricle.
Figure 5.
Cardiac interstitial fibrosis (upper two panel) and perivascular fibrosis (bottom panel) in Dahl S rats on regular salt intake or on high salt intake treated with vehicle, lisinopril or trandolapril at two dose levels. Values are expressed as mean±s.e.mean, n=6 rats per group. *P<0.05 vs regular salt diet, †P<0.05 vs high salt diet+vehicle.
Figure 6.
Aorta tunica media and tunica adventitia fibrosis and kidney tubular fibrosis in Dahl S rats on regular salt intake or on high salt intake treated with vehicle, lisinopril or trandolapril at two dose levels. Values are expressed as mean±s.e.mean, n=6 rats per group. *P<0.05 vs regular salt diet, †P<0.05 vs high salt diet+vehicle.
Discussion
In Dahl S rats, high salt diet for 4 weeks caused marked increases in BP, cardiac and renal weight and cardiovascular fibrosis. Chronic treatment with the two ACE inhibitors prevented ∼50% of the increase in BP and cardiac weight, whereas increases in tissue fibrosis were fully prevented.
Salt and tissue RAAS
Consistent with other studies, a high salt diet markedly reduced plasma angiotensin II levels (Strehlow et al., 1999; Zhao et al., 2000; Kobori et al., 2003; Nishiyama et al., 2004; Bayorh et al., 2005), and, in parallel, increased ACE and AT1 receptor-binding densities in brain nuclei (Wang et al., 2003) of Dahl S rats. Central infusion of angiotensin II increases AT1 receptor mRNA and protein (Porter, 1999; Gao et al., 2005). On the other hand, both ACE inhibitors prevented the increase in AT1 receptor densities in brain nuclei of Dahl S rats on high salt. Similarly, AT1 receptor-binding densities in hypothalamic nuclei of SHR and adrenal of Wistar rats decreased significantly after treatment with ACE inhibitors (Wilson et al., 1988; Nazarali et al., 1989; Regitz-Zagrosek et al., 1994). High salt diet may therefore enhance local release of angiotensin II in these brain nuclei and thereby upregulate AT1 receptor densities.
High salt diet also increased ACE and AT1 receptor-binding densities in the abdominal aorta. Most (Nickenig et al., 1998; Tamura et al., 1999; Takai et al., 2001) but not all (Strehlow et al., 1999) studies reported similar effects of high salt diet. Both ACE inhibitors prevented the salt-induced increases in AT1 receptor densities in the aorta. Consistent with previous studies (Tamura et al., 1999; Yamamoto et al., 2000; Zhao et al., 2000; Wang et al., 2003) high salt diet caused a two-fold increase of ACE densities, but little change in AT1 receptors in both ventricles and atria. The present study is the first reporting increases in ACE densities by high salt intake in the atria and this increase is more marked than the increase in ventricles. In Sprague–Dawley rats, s.c. administration of angiotensin II increased ACE-binding densities in the atria without changes in AT1 receptor levels (Sun et al., 1997).
In the kidney, high salt diet increased renal ACE-binding densities but in contrast decreased AT1 receptor densities. This pattern of change is consistent with previous studies in Dahl rats (Strehlow et al., 1999; Tamura et al., 1999; Nishikimi et al., 2002; Wang et al., 2003). Considering that chronic infusion of angiotensin II increases renal ACE densities, but decreases AT1 receptor densities (Harrison-Bernard et al., 2002), the opposite changes in renal ACE and AT1 receptor densities may reflect an increase in tissue angiotensin II levels in the kidney by high salt (Nishikimi et al., 2002; Bayorh et al., 2005). Consistent with this concept, both ACE inhibitors attenuated the salt-induced decreases in renal AT1 receptor densities.
Effect of ACE inhibitors on tissue ACE
Trandolapril caused marked and persistent inhibition (∼80%) of ACE densities, which was the same in brain regions inside and outside the BBB and in the heart. Even at the higher dose, lisinopril was less effective, causing ∼60% inhibition. In contrast, both drugs caused less and less persistent inhibition of ACE densities in the kidney and the aorta, possibly reflecting faster clearance from the peripheral than from the central compartment.
At 24 h, but not 4 h, after the last dose, the difference in the extent of central ACE inhibition between trandolapril and lisinopril inside the BBB is larger than their difference in nuclei outside the BBB. These findings suggest that both drugs can easily get into the BBB, but differences in ACE affinity and tissue retention contribute to the extent and persistence of ACE inhibition. In the peripheral tissues (heart, kidney and aorta), trandolapril caused more effective and persistent inhibition than lisinopril at both time points and both doses. A 5-fold higher dose of lisinopril was only marginally more effective than the lower dose. Both higher tissue retention and higher binding affinity of trandolapril are likely to contribute to these differences.
ACE inhibitors and BP
Dahl S rats on high salt diet for 4 weeks showed the expected marked increases in mean arterial pressure up to 170 mm Hg at night and 150 mm Hg during the day as compared to 100–110 mm Hg in Dahl S rats on regular salt diet. This enhanced diurnal variation on high salt is consistent with previous studies (Mohri et al., 2003) and may reflect enhanced sympathetic reactivity in Dahl S rats on high salt (Huang et al., 2004) and enhanced pressor responses related to the three- to fourfold higher water intake. ACE inhibition did not affect the high water intake and the enhanced diurnal variation in BP persisted as well. However, the overall increase in BP on high salt was markedly inhibited by both ACE inhibitors. Variable effects of ACE inhibitors on development of hypertension in Dahl S rats have been reported in previous studies. Some studies reported minimal effects (Sugimoto et al., 1998; Satoh et al., 2003; Yamamoto et al., 2005; Kobayashi et al., 2006), some reported partial inhibition of increase in BP (O'Donnell et al., 1992; Ashida et al., 1997). None of these studies assessed extent of ACE inhibition. Neither did any report time of day or time after the last dose when BP was measured.
Interestingly, both ACE inhibitors were similarly effective in attenuating the increases in BP despite that lisinopril caused lower and less persistent ACE inhibition. These findings may indicate that the lowest level of ACE inhibition (about 50–60%) achieved by the treatment regimens is already sufficient to inhibit angiotensin II production and release in relevant tissues and further inhibition may not cause further inhibition of angiotensin II and thus not lower BP further. Chronic central infusion of an AT1 receptor antagonist (Huang and Leenen, 1998; Leenen and Yuan, 2001) at low doses prevents the increase in BP in Dahl S rats on high salt diet. Acute intracerebroventricular administration of captopril lowers the BP in Dahl S rats on high salt diet, but not in Dahl R or Dahl S rats on regular salt diet (Lark and Weyhenmeyer, 1992; Zhao et al., 2001). Inhibition of brain ACE therefore is likely to contribute to the antihypertensive effect of the two ACE inhibitors. However, inhibition of arterial and renal ACE, decreasing local angiotensin II levels and/or increasing local kinin levels probably contributes as well.
In contrast to AT1 receptor blockade (Huang and Leenen, 1998; Leenen and Yuan, 2001), both ACE inhibitors only partially prevented the increase in BP on high salt, despite a high degree of ACE inhibition. It is possible that some persistent angiotensin II production and release may occur through ACE or alternative enzymes such as chymase (Baltatu et al., 1997). Alternatively, persistent ACE inhibition may cause bradykinin accumulation in the CNS and thereby an increase in sympathetic activity and BP (Zhao et al., 2001).
ACE inhibitors and cardiovascular and renal hypertrophy and fibrosis
Both an increase in BP and activation of the cardiac RAAS may contribute to the high salt-induced cardiac hypertrophy and fibrosis. The two ACE inhibitors markedly inhibited ACE in both ventricles and atria, partially inhibited the LV hypertrophy, but fully prevented the development of RV hypertrophy and of interstitial and perivascular fibrosis in both ventricles and atria. Previous studies showed that ACE inhibitor treatment at subdepressor doses attenuates the development of both interstitial (Yamamoto et al., 2005) and perivascular (Kobayashi et al., 2006) fibrosis in the ventricles of Dahl S rats on high salt diet. Altogether, these findings suggest that activation of the cardiac RAAS is largely responsible for the fibrosis in the four chambers of the heart as well as the RV hypertrophy. The LV hypertrophy is likely also to be load dependent and was therefore only partially prevented. On the other hand, the decrease in LV systolic function was fully prevented, possibly as a result of the lower BP and perhaps the attenuation of LV hypertrophy.
Both ACE inhibitors also prevented the development of fibrosis in the media and adventitia of the aorta and the tubular fibrosis in the kidney, indicative of a primary role of the local RAAS in these tissues as well. In contrast, neither ACE inhibitor affected the increase in kidney weight or in glomerular size. Thus, other factors besides BP and local RAAS appear to play a key role in the development of renal and glomerular hypertrophy on high salt diet. Both increased expression of kallikrein (Bledsoe et al., 2006) and immune suppression (Tian et al., 2007) have been shown to prevent salt-induced renal inflammation, fibrosis and hypertrophy.
Both ACE inhibitors showed fairly similar effects on salt-induced cardiovascular and renal hypertrophy and fibrosis. The lower doses showed most of the effects, suggesting that the 50–60% ACE inhibition caused by the lower dose of lisinopril is sufficient to prevent salt-induced fibrosis. One may also conclude that at the doses used at least in this model, lipophilicity or ACE affinity do not influence outcomes (Dzau et al., 2001). However, these findings do not exclude that differences would be apparent at lower doses or in other diseases states such as congestive heart failure.
In conclusion, peripheral administration of either a hydrophilic or a lipophilic ACE inhibitor partially prevented the increase in BP in Dahl S rats on a high salt diet. Both ACE inhibitors prevented tissue fibrosis but only partially protected against LV hypertrophy and had no effect on renal hypertrophy, indicating that salt-induced tissue fibrosis is largely determined by activity of tissue RAAS while tissue hypertrophy may be determined by other factors. In the heart, salt-induced fibrosis may represent an anatomical substrate for atrial fibrillation and diastolic dysfunction. In the aorta and arteries, salt-induced fibrosis may decrease distensibility and thereby increase systolic BP over time, whereas renal fibrosis may lead to renal dysfunction. These phenomena also occur with aging in western, high salt societies. It is possible that high salt intake by activating tissue RAAS also contributes to these cardiovascular changes over time in humans.
Acknowledgments
We thank Dr Ed O' Brien, Vascular Biology Laboratory, University of Ottawa Heart Institute, Ottawa for use of his laboratory for quantification of fibrosis, Dr Y Sun, Division of Cardiovascular Diseases, Department of Medicine, University of Tennessee Health Sciences Centre (Memphis, TN, USA) for providing the derivative of lisinopril, 351A and Dr M Schalekamp's group in Rotterdam for antibody against AngII. We also thank Junhui Tan, Monir Ahmad, Bing S Huang and Roselyn White for technical support. Trandolapril was a generous gift from Abbott Laboratories. Lisinopril was generously supplied by Apotex Inc. This work was supported by operating grant #T-5341 from the Heart and Stroke Foundation of Ontario, Canada. Frans Leenen holds the Pfizer Chair in Hypertension Research, an endowed chair supported by Pfizer Canada, the University of Ottawa Heart Institute Foundation and the Canadian Institutes of Health Research.
Abbreviations
- ACE
angiotensin-converting enzyme
- AT1-receptor
angiotensin II type 1 receptor
- BBB
blood-brain barrier
- EF
ejection fraction
- HPLC
high-performance liquid chromatography
- icv
intracerebroventricular
- LV
left ventricle
- LVIDd
left ventricular internal dimension in diastole
- LVIDs
left ventricular internal dimension in systole
- LVIVS
left ventricle interventricular septum
- LVPW
left ventricle posterior wall
- MAP
mean arterial pressure
- MnPO
median preoptic nucleus
- OVLT
organum vasculosum laminae terminalis
- PVN
paraventricular nucleus
- RAAS
renin–angiotensin aldosterone system
- RIA
radioimmunoassay
- RV
right ventricular
- SFO
subfornical organ
- WKY
Wistar–Kyoto
Conflict of interest
The authors state no conflict of interest.
References
- Ashida T, Yoshimi H, Kawano Y, Matsuoka H, Omae T. Effect of cilazapril and salt on Ca2+ extrusion in arterial smooth muscle of Dahl rats. Am J Hypertens. 1997;10:107S–111S. [PubMed] [Google Scholar]
- Baltatu O, Nishimura H, Hoffmann S, Stoltenburg G, Haulica ID, Lippoldt A, et al. High levels of human chymase expression in the pineal and pituitary glands. Brain Res. 1997;752:269–278. doi: 10.1016/s0006-8993(96)01474-6. [DOI] [PubMed] [Google Scholar]
- Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie II. Alterations in aldosterone and angiotensin II levels in salt-induced hypertension. Clin Exp Hypertens. 2005;27:355–367. [PubMed] [Google Scholar]
- Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, Chao J. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum Gene Ther. 2006;17:545–555. doi: 10.1089/hum.2006.17.545. [DOI] [PubMed] [Google Scholar]
- Conen H, Brunner HR. Pharmacologic profile of trandolapril, a new angiotensin-converting enzyme inhibitor. Am Heart J. 1993;125:1525–1531. doi: 10.1016/0002-8703(93)90450-n. [DOI] [PubMed] [Google Scholar]
- De Simone G, Devereux RB, Camargo MJ, Wallerson DG, Sealey JE, Laragh JH. Reduction of development of left ventricular hypertrophy in salt-loaded Dahl salt-sensitive rats by angiotensin II receptor inhibition. Am J Hypertens. 1996;9:216–222. doi: 10.1016/0895-7061(95)00338-x. [DOI] [PubMed] [Google Scholar]
- Dean SA, Tan J, O'Brien ER, Leenen FH. 17beta-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R759–R766. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlof B, Deanfield J, et al. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 2001;88:1L–20L. doi: 10.1016/s0002-9149(01)01878-1. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BS, Ahmad M, Deng AY, Leenen FH. Neuronal responsiveness to central Na+ in 2 congenic strains of Dahl salt-sensitive rats. Hypertension. 2007;49:1315–1320. doi: 10.1161/HYPERTENSIONAHA.106.086363. [DOI] [PubMed] [Google Scholar]
- Huang BS, Leenen FH. Both brain angiotensin II and ‘ouabain' contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension. 1998;35:1028–1033. doi: 10.1161/01.hyp.32.6.1028. [DOI] [PubMed] [Google Scholar]
- Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Honda T, Yoshida K, Nakano S, Ohno T, Tsubokou Y, et al. Critical role of bradykinin-eNOS and oxidative stress-LOX-1 pathway in cardiovascular remodeling under chronic angiotensin-converting enzyme inhibition. Atherosclerosis. 2006;187:92–100. doi: 10.1016/j.atherosclerosis.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Veinot JP, Leenen FH. Prevention of high salt diet-induced cardiac hypertrophy and fibrosis by spironolactone. Am J Hypertens. 2003;16:319–323. doi: 10.1016/s0895-7061(02)03268-5. [DOI] [PubMed] [Google Scholar]
- Lark LA, Weyhenmeyer JA. The antihypertensive effect of acute intracerebroventricular administration of captopril in Dahl salt-sensitive rats. Eur J Pharmacol. 1992;222:33–37. doi: 10.1016/0014-2999(92)90459-h. [DOI] [PubMed] [Google Scholar]
- Leenen FH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension. 2001;37:981–984. doi: 10.1161/01.hyp.37.3.981. [DOI] [PubMed] [Google Scholar]
- Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, et al. Alterations of circadian expressions of clock genes in Dahl salt-sensitive rats fed a high-salt diet. Hypertension. 2003;42:189–194. doi: 10.1161/01.HYP.0000082766.63952.49. [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Gutkind JS, Correa FM, Saavedra JM. Enalapril decreases angiotensin II receptors in subfornical organ of SHR. Am J Physiol Heart Circ Physiol. 1989;256:H1609–H1614. doi: 10.1152/ajpheart.1989.256.6.H1609. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Strehlow K, Roeling J, Zolk O, Knorr A, Bohm M. Salt induces vascular AT1 receptor overexpression in vitro and in vivo. Hypertension. 1998;31:1272–1277. doi: 10.1161/01.hyp.31.6.1272. [DOI] [PubMed] [Google Scholar]
- Nishikimi T, Mori Y, Kobayashi N, Tadokoro K, Wang X, Akimoto K, et al. Renoprotective effect of chronic adrenomedullin infusion in Dahl salt-sensitive rats. Hypertension. 2002;39:1077–1082. doi: 10.1161/01.hyp.0000018910.74377.93. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, et al. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell MP, Kasiske BL, Katz SA, Schmitz PG, Keane WF. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension. 1992;20:651–658. doi: 10.1161/01.hyp.20.5.651. [DOI] [PubMed] [Google Scholar]
- Porter JP. Chronic intracerebroventricular infusion of angiotensin II increases brain AT1 receptor expression in young rats. Brain Res Dev Brain Res. 1999;112:293–295. doi: 10.1016/s0165-3806(98)00182-5. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Auch-Schwelk W, Neuss M, Fleck E. Regulation of the angiotensin receptor subtypes in cell cultures, animal models and human diseases. Eur Heart J. 1994;15 Suppl D:92–97. doi: 10.1093/eurheartj/15.suppl_d.92. [DOI] [PubMed] [Google Scholar]
- Ruzicka M, Skarda V, Leenen FH. Effects of ACE inhibitors on circulating versus cardiac angiotensin II in volume overload-induced cardiac hypertrophy in rats. Circulation. 1995;92:3568–3573. doi: 10.1161/01.cir.92.12.3568. [DOI] [PubMed] [Google Scholar]
- Satoh S, Ueda Y, Suematsu N, Oyama J, Kadokami T, Sugano M, et al. Beneficial effects of angiotensin-converting enzyme inhibition on sarcoplasmic reticulum function in the failing heart of the Dahl rat. Circ J. 2003;67:705–711. doi: 10.1253/circj.67.705. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Nickenig G, Roeling J, Wassmann S, Zolk O, Knorr A, et al. AT(1) receptor regulation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 1999;277:H1701–H1707. doi: 10.1152/ajpheart.1999.277.5.H1701. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Fujimura A, Takasaki I, Tokita Y, Iwamoto T, Takizawa T, et al. Effects of renin-angiotensin system blockade and dietary salt intake on left ventricular hypertrophy in Dahl salt-sensitive rats. Hypertens Res. 1998;21:163–168. doi: 10.1291/hypres.21.163. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- Takai S, Sakonjo H, Miyazaki M. Beneficial effect of trandolapril on the lifespan of a severe hypertensive model. Hypertens Res. 2001;24:559–564. doi: 10.1291/hypres.24.559. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Yoneda T, Demura M, Miyamori I, Mabuchi H. Sodium-induced cardiac aldosterone synthesis causes cardiac hypertrophy. Endocrinology. 2000;141:1901–1904. doi: 10.1210/endo.141.5.7529. [DOI] [PubMed] [Google Scholar]
- Tamura K, Chiba E, Yokoyama N, Sumida Y, Yabana M, Tamura N, et al. Renin-angiotensin system and fibronectin gene expression in Dahl Iwai salt-sensitive and salt-resistant rats. J Hypertens. 1999;17:81–89. doi: 10.1097/00004872-199917010-00013. [DOI] [PubMed] [Google Scholar]
- Tan J, Wang JM, Leenen FH. Inhibition of brain angiotensin-converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens. 2005;18:158–164. doi: 10.1016/j.amjhyper.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiology. 2007;292:H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- Wang JM, Veerasingham SJ, Tan J, Leenen FH. Effects of high salt intake on brain AT1 receptor densities in Dahl rats. Am J Physiol Heart Circ Physiol. 2003;285:H1949–H1955. doi: 10.1152/ajpheart.00744.2002. [DOI] [PubMed] [Google Scholar]
- Wilson KM, Magargal W, Berecek KH. Long-term captopril treatment. Angiotensin II receptors and responses. Hypertension. 1988;11:I148–I152. doi: 10.1161/01.hyp.11.2_pt_2.i148. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Mano T, Yoshida J, Sakata Y, Nishikawa N, Nishio M, et al. ACE inhibitor and angiotensin II type 1 receptor blocker differently regulate ventricular fibrosis in hypertensive diastolic heart failure. J Hypertens. 2005;23:393–400. doi: 10.1097/00004872-200502000-00022. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Masuyama T, Sakata Y, Doi R, Ono K, Mano T, et al. Local neurohumoral regulation in the transition to isolated diastolic heart failure in hypertensive heart disease: absence of AT1 receptor downregulation and ‘overdrive' of the endothelin system. Cardiovasc Res. 2000;46:421–432. doi: 10.1016/s0008-6363(00)00024-9. [DOI] [PubMed] [Google Scholar]
- Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98:2621–2628. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- Zhao X, White R, Huang BS, Van Huysse J, Leenen FH. High salt intake and the brain renin—angiotensin system in Dahl salt-sensitive rats. J Hypertens. 2001;19:89–98. doi: 10.1097/00004872-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Zhao X, White R, Van Huysse J, Leenen FH. Cardiac hypertrophy and cardiac renin-angiotensin system in Dahl rats on high salt intake. J Hypertens. 2000;18:1319–1326. doi: 10.1097/00004872-200018090-00018. [DOI] [PubMed] [Google Scholar]