Abstract
Background and purpose:
Acemetacin is regarded as a pro-drug of indomethacin and induces significantly less gastric damage but the reasons for this greater gastric safety of acemetacin are unclear. The anti-inflammatory effects of acemetacin have been attributed, at least in part, to its hepatic biotransformation to indomethacin. The aim of this study was to determine the effects of acemetacin and indomethacin in an in vivo model of acute inflammation and to examine the importance of biotransformation of acemetacin (to indomethacin) to its anti-inflammatory actions.
Experimental approach:
The zymosan airpouch model was used in rats. Indomethacin or acemetacin (2.7–83.8 μmol kg−1) were administered orally or directly into the pouch. Leukocyte infiltration, prostaglandin (PG) E2 and leukotriene (LT) B4 levels in exudates, and whole blood thromboxane (TX) B2 synthesis were measured.
Key results:
Acemetacin was rapidly converted to indomethacin after its administration. Both acemetacin and indomethacin elicited comparable, dose-dependent reductions of leukocyte infiltration and of PGE2 and TXB2 synthesis. However, indomethacin induced more gastric damage than acemetacin and elevated LTB4 production in the airpouch.
Conclusions and implications:
The similar effects of acemetacin and indomethacin on leukocyte infiltration and PG synthesis are consistent with rapid biotransformation of acemetacin to indomethacin. Some of this biotransformation may occur extra-hepatically, for instance in inflammatory exudates. Acemetacin probably exerts actions independent of conversion to indomethacin, given the different effects of these two drugs on LTB4 production. Such differences may contribute to the relative gastric safety of acemetacin compared to indomethacin.
Keywords: inflammation, NSAID, acemetacin, indomethacin, cyclooxygenase, gastric ulcer, prostaglandin, thromboxane, leukotriene, leukocyte infiltration
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely employed as analgesic and anti-inflammatory agents, but their use is significantly limited by their propensity to induce ulceration and bleeding in the gastrointestinal tract (Wallace, 1997; Schoenfeld et al., 1999). Selective cyclooxygenase (COX)-2 inhibitors produce less gastrointestinal bleeding and ulceration than conventional NSAIDs, but this benefit may be offset by significant increases in renal and cardiovascular adverse events associated with their use (Zarraga and Schwarz, 2007). Thus, safer alternatives are required. It is well known that non-selective NSAIDs produce their anti-inflammatory and analgesic effects through inhibition of COX-2 and, in some cases, COX-1 (Wallace et al., 1998).
Acemetacin may represent a useful alternative to conventional NSAIDs for the treatment of inflammation and pain. It is a carboxymethyl ester derivative of indomethacin (Boltze et al., 1980; Jacobi and Dell, 1980), an NSAID with modest selectivity for COX-1 (Warner et al., 1999). Acemetacin is often referred to as a pro-drug of indomethacin, possibly explaining why it produces less gastric damage than indomethacin (Bori-Segura et al., 2002; Chou and Tsai, 2002). However, Tavares and Bennett (1993) demonstrated that, in vitro, acemetacin inhibited prostaglandin (PG) synthesis in human leukocytes or gastric mucosa in a concentration-dependent manner that did not differ markedly from the inhibition observed with indomethacin. These observations suggested that either acemetacin itself can inhibit COX activity, or acemetacin was converted into indomethacin in the presence of leukocytes or gastric mucosal tissue. The pharmacological profile of acemetacin remains incomplete, particularly with respect to its gastric-sparing properties and its ability to exert anti-inflammatory activities independent of conversion into indomethacin. Thus, the aim of this study was to characterize the anti-inflammatory effects of both orally and locally administered acemetacin, its effects on COX-1 and COX-2 activity and the relationship of these activities with its bioconversion into indomethacin.
Methods
Animals
All experimental protocols were approved by the Animal Care Committee of the University of Calgary, and the experiments were performed in accordance with the guidelines of the Canadian Council on Animal Care. The minimum sample size per group was five.
Male Wistar rats (175–200 g) were obtained from Charles River Laboratories (Montreal, Quebec, Canada) and were housed in the Animal Care Facility at the University of Calgary. Rats were fed standard laboratory chow and tap water ad libitum.
Zymosan airpouch model
Rats were deprived of food, but not water, for 18–20 h prior to experiments. The airpouch was induced as described previously (Edwards et al., 1981; Wallace et al., 1999). Briefly, 20 ml of air was injected subcutaneously on the back of the rats. Additional injections of 10 ml of air were performed 2, 5 and 6 days after the first injection. Twenty-four hours after the last injection of air, 1 ml of either saline or a 1% (w v−1) solution of zymosan was injected into the pouch. All of the injections were performed under halothane anaesthesia. Six hours after zymosan injection, rats were anaesthetized with sodium pentobarbital (60 mg kg−1 intraperitoneal), and blood was drawn from the inferior vena cava for measurement of whole blood thromboxane B2 (TXB2) synthesis, as an index of COX-1 activity (Wallace et al., 1999). Immediately thereafter, 1 ml of heparinized saline was injected into the pouch. The airpouch was carefully opened by a small incision. The exudate was collected, the volume measured and an aliquot used to quantify leukocyte numbers using a Sysmex KX-21N haematology analyzer. An aliquot was applied to a glass slide and stained with Wright's stain to determine the relative numbers of different leukocyte subtypes. The exudate was centrifuged at 1000 g for 10 min. The supernatant was collected and stored at −80°C for measurement of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) using commercially available enzyme immunoassay kits. An additional aliquot of airpouch exudate was stored for subsequent measurement of indomethacin and acemetacin concentrations by high-performance liquid chromatography.
One hour prior to zymosan injection into the airpouch, rats were treated with vehicle (5% sodium bicarbonate), acemetacin or indomethacin (2.7, 8.3, 27.9 or 83.8 μmol kg−1), either orally or by direct injection into the pouch. Six hours after zymosan injection, the exudate and whole blood were collected, as described above.
In another set of experiments, exudate samples were collected at 0, 1, 2, 3, 4, 6, 12, 24 or 36 h after injection of zymosan into the airpouch.
Gastric damage and prostaglandin synthesis
Groups of at least five rats were given acemetacin or indomethacin orally (8.3, 27.9 and 55.7 μmol kg−1). Control rats received the vehicle (5% sodium bicarbonate). Three hours later, the rats were killed with an overdose of sodium pentobarbital. The stomach was removed and the extent of haemorrhagic damage was scored by an observer unaware of the treatments the rats had received. The length (in mm) of all haemorrhagic lesions was measured and a gastric damage score was calculated for each stomach by summing these values (Wallace et al., 2000). A sample of the corpus region of the stomach was excised, weighed and added to a tube containing 1 ml of sodium phosphate buffer (10 mM; pH 7.4). The tissue was minced with scissors for 30 s, then placed in a shaking water bath (37 °C) for 20 min. The samples were centrifuged (9000 g) for 1 min, the supernatant was snap frozen and then stored at −80 °C. The concentration of PGE2 in the supernatants was determined by enzyme-linked immunosorbent assay.
Whole-blood thromboxane synthesis
Blood was collected from untreated rats and dispensed in 500 μl aliquots into glass tubes containing indomethacin (0.1–10 μM), the same concentrations of acemetacin, or vehicle. The blood was incubated at 37 °C for 45 min, then centrifuged at 9000 g for 3 min. TXB2 concentrations in the supernatants were measured by enzyme-linked immunosorbent assay.
High-performance liquid chromatography analysis samples
Acemetacin and indomethacin concentrations in plasma and exudate were determined by reverse-phase high-performance liquid chromatography with ultra violet detection. Briefly, 100 μl of plasma was spiked with 67.716 μM of carbamazepine (internal standard) and 1100 μl of methanol was added to extract the drugs by vortex agitation during 1 min at maximum speed, then samples were centrifuged. An aliquot (60 μl) of supernatant was injected into the chromatographic system equipped with a Novapak C-18 column (150 × 3.9 mm ID, particle size 4 μm, Waters Assoc., Milford, MA, USA) eluted with a mobile phase consisting of a mixture of 0.025 M phosphate buffer (pH 6.0) with methanol, 45:55 v v−1 at constant flow (1.0 ml min−1) at room temperature. The effluent from the column was monitored spectrophotometrically at 260 nm. Retention times were 2.30, 4.25 and 5.10 min for internal standard, indomethacin and acemetacin respectively.
This method permits simultaneous determination of acemetacin and indomethacin concentrations. The limit of detection of both compounds was 0.64 μg ml−1, and the quantification limit was 1.27 μg ml−1. Sensitivity was the same for both compounds as they exhibit similar spectrophotometric properties. The method was linear in the range of 1.27–102 μg ml−1 (r2 of 0.9998 for indomethacin and 0.9995 for acemetacin). At concentrations of 3.8, 19.1 and 76.5 μg ml−1, the coefficient of variability was less than 13% for acemetacin and less than 11% for indomethacin.
Plasma acemetacin and indomethacin profile after oral or subcutaneous administration of acemetacin
Polyethylene catheters were implanted into the caudal artery to collect blood samples, as described previously (Rivera-Espinosa et al., 2003). Acemetacin (83.8 μmol kg−1) suspended in 0.5% carboxymethyl cellulose (4 ml kg−1) was then administered orally or subcutaneously. Blood samples (200 μl) were drawn prior to and at 0.08, 0.16, 0.25, 0.33, 0.5, 0.75, 1, 2, 4, 8, 10, 24, 27 and 30 h after drug administration. The dose used for this pharmacokinetic study was selected because it was the highest dose administered for the COX-1- and COX-2-inhibition experiments. Plasma was obtained by whole blood centrifugation (1000 g for 10 min), and plasma samples were stored at −80 °C until analysis was performed.
Statistical analysis
All data are expressed as mean±s.e.m. Comparisons among groups were made using a one-way analysis of variance followed by the Newman–Keuls test or using a Student's t-test, when appropriate. Values of P<0.05 were considered to show significant differences between means.
Materials
Indomethacin, acemetacin, zymosan and carbamazepine were obtained from Sigma Aldrich (St Louis, MO, USA). The enzyme-linked immunosorbent assay kits for measuring PGE2, LTB4 and TXB2 were obtained from Cayman Chemical Co. (Ann Arbor, MI, USA).
Results
Time course of leukocyte infiltration, and PGE2 and LTB4 synthesis
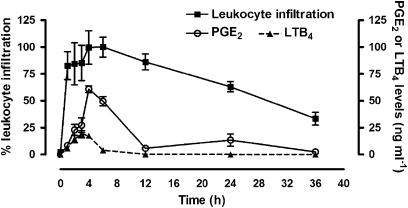
Administration of zymosan into the airpouch resulted in substantial infiltration of leukocytes, peaking at 6 h (Figure 1). Most of the leukocytes were neutrophils (94.4%±0.87) and lymphocytes (5.2%±0.82). PGE2 levels in exudates were maximal between 4 and 6 h after zymosan injection and declined gradually thereafter. The highest levels of LTB4 in the exudates occurred between 3 and 4 h after zymosan administration, decreasing to near-basal levels by 12 h. For the subsequent studies of the effects of acemetacin and indomethacin, we selected the 6 h post-zymosan time point.
Figure 1.
Leukocyte infiltration, PGE2 and LTB4 after zymosan injection into the airpouch. Leukocyte infiltration is expressed as a percentage of the maximal effect, which occurred at 6 h after zymosan administration. Five rats per group. LTB4, leukotriene B4; PGE2, prostaglandin E2.
Acemetacin reduces acute inflammation with the same potency as indomethacin
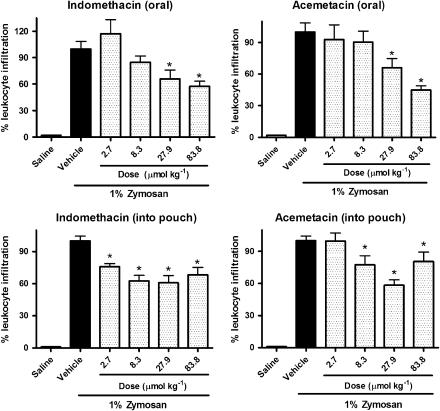
Acemetacin and indomethacin inhibited zymosan-induced leukocyte infiltration into the airpouch to a similar extent. A significant effect was seen with lower doses when given directly into the airpouch than with oral administration, although a clearer dose–response relationship was seen with oral administration (Figure 2).
Figure 2.
Percentage of leukocyte infiltration in rat airpouch exudate following a single oral or local administration of indomethacin or acemetacin. Data are expressed as mean±s.e.m. *P<0.05 vs vehicle; five rats per group.
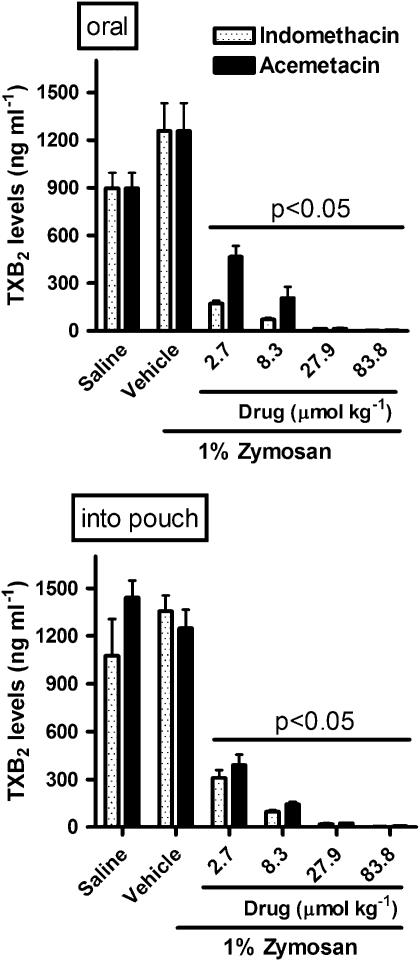
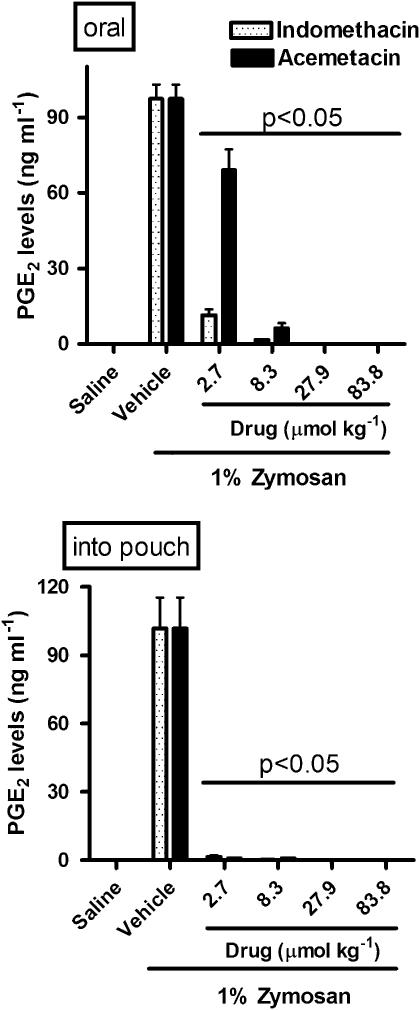
Acemetacin and indomethacin significantly inhibited whole blood TXB2 synthesis (COX-1; Figure 3) and reduced exudate PGE2 levels (COX-2; Figure 4), irrespective of the route of administration of the drugs. With the lowest doses of acemetacin, given orally, there was less inhibition of TXB2 and PGE2 synthesis than was seen with the same doses of indomethacin. Both drugs produced a profound inhibition of PGE2 synthesis when they were administered locally (Figure 4).
Figure 3.
Cyclooxygenase-1 activity, assayed as TXB2 levels, in rat whole blood following a single oral or local (into airpouch) dose of indomethacin or acemetacin. Data are expressed as mean±s.e.m. P<0.05 vs vehicle, as indicated by bar; five rats per group. TXB2, thromboxane B2.
Figure 4.
Cyclooxygenase-2 activity, assayed as PGE2 levels, following a single oral or local (into airpouch) dose of indomethacin or acemetacin. Data are expressed as mean±s.e.m. Bar shows P<0.05 vs corresponding indomethacin group; five rats per group. PGE2, prostaglandin E2.
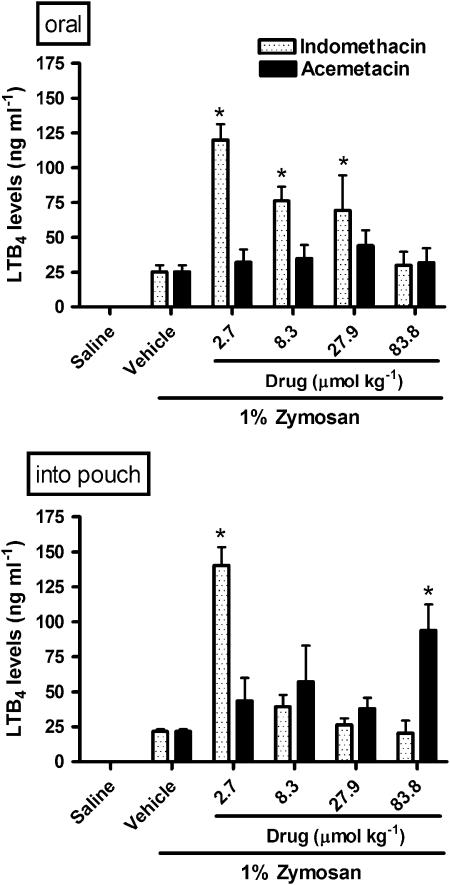
Acemetacin and indomethacin differentially affect LTB4 synthesis
In contrast to their effects on COX products, the effects of acemetacin and indomethacin on LTB4 levels in the exudate were quite different. Orally administered indomethacin significantly increased LTB4 levels in the exudate (by up to fivefold) at all but the highest dose tested. In contrast, LTB4 synthesis was not affected by orally administered acemetacin (Figure 5). When given directly into the airpouch, indomethacin significantly increased LTB4 synthesis only at the lowest dose tested, while acemetacin significantly increased LTB4 synthesis only at the highest dose tested (Figure 5).
Figure 5.
LTB4 levels in rat airpouch exudates following a single oral or local (into airpouch) administration of indomethacin or acemetacin. Data are expressed as mean±s.e.m. *P<0.05 vs vehicle; five rats per group. LTB4, leukotriene B4.
Transformation of acemetacin to indomethacin in rat airpouch exudates
Acemetacin and indomethacin were quantified in exudates collected 7 h after administration of the drugs directly into the pouch or given orally. Acemetacin was not detected in the exudate. The concentrations of indomethacin in the exudate were similar when acemetacin or indomethacin was administered via either route (Table 1). The concentration of indomethacin in the exudate increased in a dose-dependent manner after either oral or local administration of either drug (data not shown). These observations suggest that acemetacin is completely biotransformed to indomethacin, possibly in part within the airpouch. To further examine this possibility, we performed a pilot study as follows: acemetacin (100 μg ml−1) was incubated in airpouch exudate or rat plasma for 45 min at 37 °C, then acemetacin and indomethacin were quantified by high-performance liquid chromatography. Within this short time frame, we found that only a modest amount of indomethacin was detectable in plasma and exudate (1.3±0.1 and 2.2±0.1 μg ml−1, respectively).
Table 1.
Indomethacin levels in inflammatory exudate
| Drug/route of administration | Exudate indomethacin concentration (μg ml−1) |
|---|---|
| Indomethacin (oral) | 8.71±2.03 |
| Acemetacin (oral) | 10.29±2.49 |
| Indomethacin (into pouch) | 16.87±4.44 |
| Acemetacin (into pouch) | 13.21±2.97 |
Indomethacin or acemetacin (83.8 μmol kg−1) was administered orally or by injection into the airpouch. One hour later, zymosan was injected into the airpouch. Exudates were collected 6 h after zymosan administration. Data are expressed as mean±s.e.m., with five rats per group.
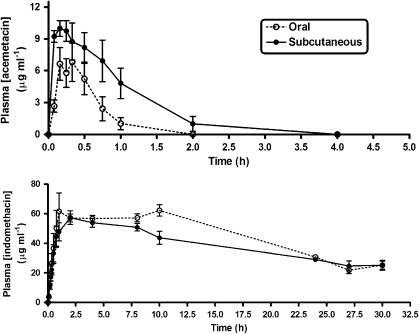
The pharmacokinetic profile of acemetacin was examined following oral or subcutaneous (s.c.) administration at a dose of 83.8 μmol kg−1 (Figure 6). The plasma levels of indomethacin derived from acemetacin were similar for both routes of administration. For example, the area under the curve values were the same: 1193±51 μg h ml−1 for oral administration and 1115±64 μg h ml−1 for s.c. administration. When plasma acemetacin was measured, a clear difference in pharmacokinetic profile was seen. After s.c. administration, the area under the curve was significantly greater than when acemetacin was given orally (Table 2). In addition, Cmax was significantly greater after s.c. acemetacin administration than when it was given orally.
Figure 6.
Plasma acemetacin (top panel) and indomethacin (bottom panel) concentrations following a single oral or subcutaneous dose of acemetacin (83.8 μmol kg−1). Data are expressed as mean±s.e.m. Eight rats per group.
Table 2.
Pharmacokinetics of acemetacin
| Drug measured | Route of administration of acemetacin | Tmax (h) | Cmax (μg ml−1) | AUCt (μg h ml−1) |
|---|---|---|---|---|
| Acemetacin | Oral | 0.28±0.04 | 5.93±1.11 | 4.88±1.08 |
| Acemetacin | Subcutaneous | 0.18±0.04 | 10.44±1.09* | 10.83±2.11* |
| Indomethacin | Oral | 8.50±0.73 | 64.40±3.72 | 1193±51.32 |
| Indomethacin | Subcutaneous | 2.16±0.40* | 61.42±5.47 | 1115±63.66 |
Pharmacokinetic parameters for acemetacin and indomethacin were measured in plasma samples collected 5 min to 30 h after oral or subcutaneous administration of acemetacin (83.8 μmol kg−1). Data are presented as mean±s.e.m. with eight rats per group. Cmax is the maximal plasma concentration, Tmax is the time to reach Cmax, and AUC is the area under the plasma-against-time curve.
*P⩽0.05 (Student's t-test) compared to the group given acemetacin orally.
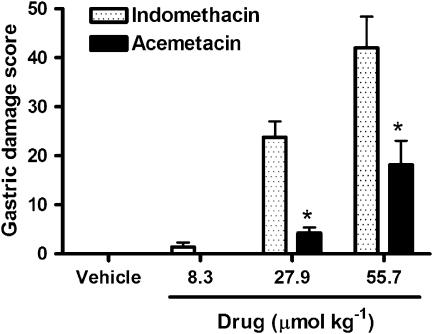
Gastric damage and prostaglandin synthesis
Orally administered indomethacin elicited the formation of extensive haemorrhagic erosions in the stomach, which increased in severity in a dose-dependent manner (Figure 7). Acemetacin caused significantly less gastric damage than indomethacin. Across the range of doses tested, both indomethacin and acemetacin significantly suppressed gastric PGE2 synthesis. For example, indomethacin at a dose of 8.3 μmol kg−1 reduced gastric PGE2 synthesis from 17.4±3.2 pg mg−1 (in vehicle-treated controls) to 5.0±1.1 pg mg−1, while an equimolar dose of acemetacin reduced gastric PGE2 synthesis to 4.4±1.1 pg mg−1. Across the full range of doses shown in Figure 7, there was no significant difference between the extent of inhibition of gastric PGE2 synthesis by acemetacin and indomethacin.
Figure 7.
Gastric damage score 3 h after oral administration of indomethacin or acemetacin. Data are expressed as mean±s.e.m. *P<0.05 vs corresponding dose of indomethacin; five rats per group.
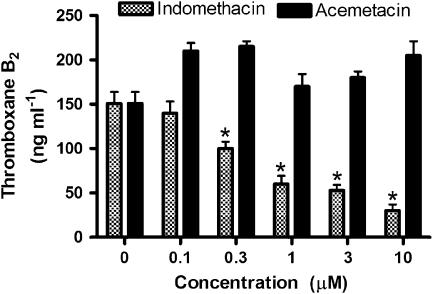
Whole-blood thromboxane synthesis
As shown in Figure 8, indomethacin concentration-dependently reduced TXB2 synthesis in rat whole blood. In this assay, the TXB2 is derived almost entirely from platelets (Wallace et al., 1998). In contrast, acemetacin did not affect TXB2 synthesis at any of the concentrations tested (up to 10 μM).
Figure 8.
Inhibition of whole-blood thromboxane B2 synthesis in vitro by indomethacin and acemetacin. *P<0.05 vs the group that did not receive either drug; five rats per group).
Discussion and conclusions
Indomethacin is a very potent anti-inflammatory drug used for treating painful conditions such as arthritis and gout. However, like other NSAIDs, its use is limited by a relatively high incidence of adverse effects, the most common of which are gastrointestinal ulceration and bleeding (Wallace, 1997). The anti-inflammatory properties of acemetacin, a carboxymethyl ester of indomethacin (Notarianni and Collins, 1987; Jones et al., 1991), have been characterized in many studies, including kaolin-induced oedema in mice (Jacobi et al., 1980). In clinical studies, acemetacin exhibits comparable anti-inflammatory efficacy to indomethacin, but with better gastric tolerance (Bori-Segura et al., 2002; Chou and Tsai, 2002). The reasons for the greater gastric safety of acemetacin relative to that of indomethacin are not clear. One possibility is that acemetacin has less capacity to suppress gastric PG synthesis than indomethacin. Indeed, acemetacin may itself be inactive as a COX inhibitor, with the inhibition occurring only after bioconversion into indomethacin. Based on in vitro studies of acemetacin and indomethacin, Tavares and Bennett (1993) concluded that acemetacin was capable of suppressing COX-1 and COX-2 activity, and was suggested to be ‘anti-inflammatory in its own right'. In the present study, we directly compared the anti-inflammatory properties of acemetacin to equimolar doses of indomethacin in the zymosan airpouch model in rats. When injected directly into the airpouch, acemetacin exhibited similar anti-inflammatory effects as indomethacin (that is, reduction of leukocyte infiltration and suppression of COX-2-dependent PGE2 synthesis). While this might be taken as evidence for acemetacin exerting anti-inflammatory effects independent of bioconversion into indomethacin, we observed that bioconversion of acemetacin into indomethacin occurs rapidly within the inflammatory exudate. On the other hand, the markedly different effects of acemetacin and indomethacin on LTB4 production in the airpouch suggest that the actions of acemetacin cannot be completely attributed to effects secondary to its bioconversion into indomethacin. We also observed that acemetacin produced significantly less gastric damage than indomethacin, and this occurred despite similar inhibition of gastric PG synthesis.
The airpouch model is a well-established tool for studying inflammation. It is a simple model that allows for measurement of several parameters of inflammation (Ferrandiz and Foster, 1991; Payá et al., 1997). When inflammation is induced in an airpouch by injection of zymosan, elevated eicosanoid synthesis can be detected within the first hour (Payá et al., 1997). The PGE2 that is produced in response to zymosan is derived almost entirely from COX-2 (Wallace et al., 2007). As shown in the present study, one can also monitor the resolution of inflammation in the airpouch model; thus, exudate levels of PGE2 and LTB4 had recovered to control levels by 36 h after the injection of zymosan. As reported by others, the resolution of inflammation in this model could be driven by the generation of anti-inflammatory substances, such as PGD2, lipoxins and resolvins (Gilroy et al., 1999; Serhan et al., 2007).
Biotransformation of acemetacin to indomethacin occurs rapidly, whether administered orally or subcutaneously. We observed a very low rate of conversion of acemetacin into indomethacin in vitro in inflammatory exudates and plasma, and such conversion also appeared to be marginal when acemetacin was incubated with blood for a short period of time (that is, no inhibition of TXB2 synthesis during a 45-min exposure to acemetacin). Nevertheless, the similar suppression of COX-1 and COX-2 in vivo by acemetacin and indomethacin, along with the pharmacokinetic data demonstrating rapid conversion of the former into the latter, does support a rapid biotransformation process. Indeed, 7 h after acemetacin administration into the airpouch, there was no detectable acemetacin, but levels of indomethacin were comparable to those observed when an equimolar dose of indomethacin was injected directly into the pouch. There is evidence in the literature to suggest that acemetacin has anti-inflammatory effects independent of its bioconversion into indomethacin. For example, Tavares and Bennett (1993) reported comparable inhibition of gastric PG synthesis and human leukocyte PG synthesis (assumed to be via COX-2) by acemetacin and indomethacin. One cannot, however, rule out the possibility that some conversion of acemetacin into indomethacin occurred in these assay systems.
Pro-drugs of NSAIDs are generally thought to cause less gastric damage by virtue of producing less inhibition of gastric PG synthesis, which occurs primarily via COX-1. The in vitro experiments with whole blood in the present study clearly demonstrate an inability of acemetacin to inhibit COX-1 in platelets. On the other hand, we observed comparable inhibition of gastric PG synthesis 3 h after oral administration of acemetacin vs indomethacin. Of course, it is possible that the onset of the inhibition of gastric PG synthesis may have been delayed somewhat after acemetacin administration, although the pharmacokinetic studies suggest that conversion of acemetacin into indomethacin occurs within an hour. Nevertheless, endothelial injury in the gastric microcirculation can be detected as early as 15 min after administration of indomethacin, a time when significant suppression of gastric PG synthesis has already been achieved (Wallace et al., 1990). Haemorrhagic erosions, such as those scored in the present study, take longer to develop fully. A delay in the onset of inhibition of gastric PG synthesis could, therefore, translate into a reduction of macroscopically visible haemorrhagic erosions.
One of the more surprising observations in the present study was that indomethacin produced radically different effects on LTB4 levels in the airpouch from those of acemetacin, particularly after direct injection of the drugs into the airpouch. It is not clear if this difference is related to differential effects on the synthesis or catabolism of LTB4, although several previous studies have noted elevated leukotriene synthesis in vitro and in vivo when PG synthesis is suppressed by drugs such as indomethacin (Ham et al., 1983; Salmon et al., 1983; Robinson et al., 1986). The underlying mechanism for this effect is not clear, but may be related to a shunting of arachidonate metabolism to lipoxygenase pathways, when COX is inhibited, or to removal of the suppressive effects of PGs on leukotriene synthesis (Ham et al., 1983). Only with the highest dose of acemetacin was an indomethacin-like elevation of LTB4 synthesis seen.
This observation is significant for at least two reasons. First, it is consistent with the notion that acemetacin acts independently of its conversion into indomethacin. Second, it presents another possible mechanism to explain the reduced gastric-damaging effects of acemetacin vs indomethacin. Several previous studies have suggested a role for LTB4 in the pathogenesis of NSAID-induced gastric injury (Vaananen et al., 1992; Asako et al., 1992a; Kirchner et al., 1997). For example, indomethacin was shown to increase LTB4 synthesis in mesenteric venules and this contributed significantly to the increase in leukocyte adherence that was induced by this NSAID (Asako et al., 1992a). This increase in leukocyte adherence has been observed following administration of many different NSAIDs, including selective COX-2 inhibitors (Asako et al., 1992b; Wallace et al., 1993; Muscara et al., 2000), and has been shown to be a critical step in the pathogenesis of NSAID-induced gastric damage. Indeed, prevention of NSAID-induced leukocyte adherence to the vascular endothelium or immunodepletion of circulating neutrophils prevented NSAID-induced gastric damage (Wallace et al., 1990, 1991). Inhibitors of LTB4 synthesis were also shown to significantly diminish NSAID-induced leukocyte adherence and gastric damage (Vaananen et al., 1992; Asako et al., 1992a; Kirchner et al., 1997). Thus, the absence of an increase in LTB4 synthesis following acemetacin administration (except at a very high dose), in contrast to the large increase in LTB4 synthesis following indomethacin administration, could be a significant factor contributing to the gastric safety of acemetacin.
In summary, acemetacin exhibited anti-inflammatory efficacy and potency similar to that of indomethacin in the zymosan airpouch model of inflammation. It has been suggested by others that acemetacin exhibits similar anti-inflammatory effects as celecoxib, with comparable gastric safety (Leeb et al., 2004). However, the results of the present study demonstrate that, unlike celecoxib (at recommended anti-inflammatory doses), acemetacin potently inhibits COX-1 in vivo (to the same extent as, and most likely due to its bioconversion into, indomethacin). Our results confirm that acemetacin is rapidly biotransformed to indomethacin (Jones et al., 1991), and further demonstrate that this occurs extrahepatically, although to a limited extent, in the plasma and in inflammatory exudates. Nevertheless, we observed an important difference in activity between acemetacin and indomethacin; acemetacin (except at a very high dose) did not cause an increase in LTB4 synthesis, as was observed with indomethacin. This may provide an important clue for the mechanism underlying the gastric safety of acemetacin vs indomethacin, given that NSAID-induced gastric damage has been attributed, in part, to LTB4-mediated events in the gastric microcirculation.
Acknowledgments
We thank Lourdes González-Flores for her technical support. Aracely E Chávez-Piña is a CONACyT fellow with Grant number 176515. This work was supported by a grant from the Canadian Institutes of Health Research. Dr Wallace is an Alberta Heritage Foundation for Medical Research Scientist and holds a Canada Research Chair in Inflammation.
Abbreviations
- AUC
area under the curve
- COX
cyclooxygenase
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high-performance liquid chromatography
- NSAID
non-steroidal anti-inflammatory drug
Conflict of interest
The authors state no conflict of interest.
References
- Asako H, Kubes P, Wallace J, Gaginella T, Wolf RE, Granger DN. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol. 1992a;262:G903–G908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- Asako H, Kubes P, Wallace J, Wolf RE, Granger DN. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology. 1992b;103:146–152. doi: 10.1016/0016-5085(92)91107-f. [DOI] [PubMed] [Google Scholar]
- Boltze K, Brendler O, Jacobi H, Optiz W, Raddatz S, Seidel PR, et al. Chemical structure and anti-inflammatory activity in the group of substituted indole-3 acetic acids. Arzneim-Forsch/Drug Res. 1980;30:1314–1325. [PubMed] [Google Scholar]
- Bori-Segura G, Torres A, Herrera LE, Olguín J. Efficacy and tolerability of acemetacin, a non-steroidal anti-inflammatory drug, in Mexican patients: result of the ETAPAM study. Proc West Pharmacol Soc. 2002;45:104–107. [PubMed] [Google Scholar]
- Chou CT, Tsai YY. A double-blind, randomized, controlled parallel group study evaluating the efficacy and safety of acemetacin for the management of osteoarthritis. Int J Clin Pharmacol Res. 2002;22:1–6. [PubMed] [Google Scholar]
- Edwards JW, Sedgwick AD, Willoughby DA. The formulation of a structure with features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Ferrandiz ML, Foster SJ. Tumor necrosis factor production in a rat airpouch model of inflammation: role of eicosanoids. Agents Actions. 1991;32:289–294. doi: 10.1007/BF01980888. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Ham EA, Soderman DD, Zanetti ME, Dougherty HW, McCauley E, Kuehl FA. Inhibition by prostaglandins of leukotriene B4 release from activated neutrophils. Proc Natl Acad Sci USA. 1983;80:4349–4355. doi: 10.1073/pnas.80.14.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi H, Breier P, Dell HD. Anti-inflammatory action of acemetacin. Arzneim-Forsch/Drug Res. 1980;30:1326–1347. [PubMed] [Google Scholar]
- Jacobi H, Dell HD. On the pharmacodynamics of acemetacin. Arzneim-Forsch/Drug Res. 1980;30:1348–1362. [PubMed] [Google Scholar]
- Jones RW, Collins AJ, Notarianni LJ, Sedman E. The comparative pharmacokinetic of acemetacin in young subjects and elderly patients. Br J Clin Pharmacol. 1991;31:543–545. doi: 10.1111/j.1365-2125.1991.tb05577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner T, Aparicio B, Argentieri DC, Lau CY, Ritchie DM. Effects of tepoxalin, a dual inhibitor of cyclooxygenase/5-lipoxygenase, on events associated with NSAID-induced gastrointestinal inflammation. Prostaglandins Leukot Essent Fatty Acids. 1997;56:417–423. doi: 10.1016/s0952-3278(97)90593-7. [DOI] [PubMed] [Google Scholar]
- Leeb BF, Bucsi L, Keszthelyi B, Bohmova J, Valesova M, Hawel R, et al. Treatment of osteoarthritis of the knee joint. Efficacy and tolerance to acemetacin slow release in comparison to celecoxib. Orthopade. 2004;33:1032–1041. doi: 10.1007/s00132-004-0670-z. [DOI] [PubMed] [Google Scholar]
- Muscara MN, Vergnolle N, Lovren F, Triggle CR, Elliott SN, Asfaha S, et al. Selective cyclo-oxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br J Pharmacol. 2000;129:1423–1430. doi: 10.1038/sj.bjp.0703232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarianni L, Collins AJ. Method for the determination of acemetacin, a non-steroidal anti-inflammatory drug, in plasma by high-performance liquid chromatography. J Chromatogr. 1987;413:305–308. doi: 10.1016/0378-4347(87)80244-x. [DOI] [PubMed] [Google Scholar]
- Payá M, García-Pastor P, Coloma J, Alcaraz JM. Nitric oxide synthase and cyclo-oxygenase pathways in the inflammatory response induced by zymosan in the rat airpouch. Br J Pharmacol. 1997;120:1445–1452. doi: 10.1038/sj.bjp.0701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Espinosa L, Muriel P, Ordaz-Gallo M, Perez-Urizar J, Palma-Aguirre A, Castaneda-Hernandez G. Ketorolac pharmacokinetics in experimental cirrhosis by bile duct ligation in the rat. Ann Hepatol. 2003;2:175–181. [PubMed] [Google Scholar]
- Robinson DR, Skoskiewicz M, Bloch KJ, Castorena G, Hayes E, Lowestein E, et al. Cyclooxygenase blockade elevates leukotriene E4 production during acute anaphylaxis in sheep. J Exp Med. 1986;163:1509–1517. doi: 10.1084/jem.163.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon JA, Simmons PM, Moncada S. The effects of BW755C and other anti-inflammatory drugs on eicosanoid concentrations and leukocyte accumulation in experimentally-induced acute inflammation. J Pharm Pharmacol. 1983;35:808–813. doi: 10.1111/j.2042-7158.1983.tb02901.x. [DOI] [PubMed] [Google Scholar]
- Schoenfeld P, Kimmey MB, Scheiman J, Bjorkman D, Laine L. Review article: nonsteroidal anti-inflammatory drug-associated gastrointestinal complications—guidelines for prevention and treatment. Aliment Pharmacol Ther. 1999;13:1273–1285. doi: 10.1046/j.1365-2036.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares IA, Bennett A. Acemetacin and indomethacin: differential inhibition of constitutive and inducible cyclo-oxygenases in human gastric mucosa and leucocytes. Int J Tissue Reac. 1993;15:49–53. [PubMed] [Google Scholar]
- Vaananen PM, Keenan CM, Grisham MB, Wallace JL. Pharmacological investigation of the role of leukotrienes in the pathogenesis of experimental NSAID gastropathy. Inflammation. 1992;16:227–240. doi: 10.1007/BF00918812. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Arfors KE, McKnight GW. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991;100:878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Bak A, McKnight W, Asfaha S, Sharkey KA, MacNaughton W. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol. 1999;126:1200–1204. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J, Anderson DC, et al. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol. 1993;265:G993–G998. doi: 10.1152/ajpgi.1993.265.5.G993. [DOI] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full invitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarraga IG, Schwarz ER. Coxibs and heart disease: what we have learned and what else we need to know. J Am Coll Cardiol. 2007;49:1–14. doi: 10.1016/j.jacc.2006.10.003. [DOI] [PubMed] [Google Scholar]