Abstract
Background and purpose:
Deletion of TREK-1, a two-pore domain K+ channel (K2P) activated by volatile anaesthetics, reduces volatile anaesthetic potency in mice, consistent with a role for TREK-1 as an anaesthetic target. We used TREK-1 knockout mice to examine the presynaptic function of TREK-1 in transmitter release and its role in the selective inhibition of glutamate vs GABA release by volatile anaesthetics.
Experimental approach:
The effects of halothane on 4-aminopyridine-evoked and basal [3H]glutamate and [14C]GABA release from cerebrocortical nerve terminals isolated from TREK-1 knockout (KO) and littermate wild-type (WT) mice were compared. TREK-1 was quantified by immunoblotting of nerve terminal preparations.
Key results:
Deletion of TREK-1 significantly reduced the potency of halothane inhibition of 4-aminopyridine-evoked release of both glutamate and GABA without affecting control evoked release or the selective inhibition of glutamate vs GABA release. TREK-1 deletion also reduced halothane inhibition of basal glutamate release, but did not affect basal GABA release.
Conclusions and implications:
The reduced sensitivity of glutamate and GABA release to inhibition by halothane in TREK-1 KO nerve terminals correlates with the reduced anaesthetic potency of halothane in TREK-1 KO mice observed in vivo. A presynaptic role for TREK-1 was supported by the enrichment of TREK-1 in isolated nerve terminals determined by immunoblotting. This study represents the first evidence for a link between an anaesthetic-sensitive 2-pore domain K+ channel and presynaptic function, and provides further support for presynaptic mechanisms in determining volatile anaesthetic action.
Keywords: halothane, glutamate release, GABA release, synaptosomes, TREK-1, knockout, MAC, volatile anaesthetics, mechanisms of anaesthesia
Introduction
The synaptic actions of general anaesthetics are agent-specific, but most anaesthetics enhance fast inhibitory synaptic transmission mediated by γ–aminobutyric acid (GABA) and glycine and/or depress fast excitatory synaptic transmission mediated by glutamate (Hemmings et al., 2005; Winegar and MacIver, 2006). The relative contributions of anaesthetic effects on inhibitory vs excitatory synaptic transmission in neuronal networks are unclear (Perouansky and Hemmings, 2002). Recent findings of selective inhibition of glutamate vs GABA release by volatile anaesthetics support a role for presynaptic mechanisms of volatile anaesthetic action, although the specific presynaptic targets involved have not been identified (Westphalen and Hemmings, 2006a, 2006b).
Reduced neuronal excitability through activation of anaesthetic-sensitive K+ currents has been proposed as a mechanism of anaesthesia (Patel et al., 1999; Franks and Honore, 2004). A family of two-pore domain background K+ channels (K2P), many of which are affected by clinical concentrations of volatile anaesthetics, provides baseline regulation of membrane excitability (Patel et al., 1999; Lesage, 2003). Recent attention has focused on TREK-1 (KCNK2; Honore, 2007), which is very sensitive to activation by volatile anaesthetics (Heurteaux et al., 2004) as well as certain gaseous general anaesthetics (Gruss et al., 2004). There are currently no selective antagonists of TREK-1, however deletion through gene knockout technology provides a powerful alternative approach to resolve pharmacological relevance (Docherty, 2007). Reduced volatile anaesthetic potency in vivo in TREK-1 knockout (KO) mice suggests a role for this K2P channel in anaesthesia (Heurteaux et al., 2004). Since TREK-1 is expressed throughout the rodent CNS (Maingret et al., 2000; Lesage, 2003) at both extra-synaptic and synaptic sites (Lauritzen et al., 2000), a role in the presynaptic effects of anaesthetics has been suggested (Patel et al., 1999; Franks and Honore, 2004), but presynaptic localization and function of TREK-1 have not been confirmed. We therefore investigated whether TREK-1 is involved in the presynaptic actions of volatile anaesthetics by comparing the effects of halothane on evoked and basal release of glutamate and GABA from nerve terminals isolated from TREK-1 KO and wild-type littermate mice.
Methods
Animals
All animal procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, as approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. TREK-1 (KCNK2) KO C57BL/6J mice, generated using a Cre/loxp-based strategy, were backcrossed for 10 generations to the C57BL/6J background strain (Heurteaux et al., 2004). Male TREK-1 KO and WT C57BL/6J mice aged 6–24 weeks (median age 14 weeks) were housed together. Control C57BL/6J mice, matched for sex and age, were obtained from Charles River Laboratories (Wilmington, MA, USA).
Nerve terminal preparations
Isolated nerve terminals (synaptosomes) were prepared from cerebral cortex as described (Westphalen and Hemmings, 2006b). The final pellet containing synaptosomes (protein determined by the method of Bradford, 1976) was incubated with 8 nM L-[3H]glutamate and 440 nM [14C]GABA for 15 min at 30 °C in 10 ml Krebs-HEPES buffer (KHB, composition in mM: NaCl 140, KCl 5, HEPES 20, MgCl2 1, Na2HPO4 1.2, NaHCO3 5, EGTA 0.1 and D-glucose 10, pH 7.4 with NaOH). Synaptosomes were collected by centrifugation for 10 min at 20 000 g at 4 °C, resuspended in ice-cold 0.32 M sucrose and loaded into release chambers (see below).
Glutamate and GABA release
Simultaneous release of glutamate and GABA was assayed as described (Westphalen and Hemmings, 2006b). Briefly, samples (0.3–0.8 mg of protein) of dual labelled synaptosomes were confined by Whatman GF/B glass fibre filter disks (Maidstone, UK) and superfused at 0.5 ml min−1 with KHB at 37 °C using a customized Brandel SF12 superfusion apparatus (Gaithersburg, MD, USA) set to collect 1 min fractions. Halothane was prepared by dilution of saturated stock solutions (∼16 mM) in KHB without Ca2+. Tetrodotoxin (1 μM) and/or halothane at aqueous concentrations equivalent to 0.1–8 times minimum alveolar concentration (MAC=0.24 mM for WT; Heurteaux et al., 2004) were delivered through Teflon tubing from glass syringes beginning 12 min prior to 2 min depolarizing pulses of 1 mM 4AP, in the absence or presence of 1.9 mM free extracellular Ca2+. Effects on basal release were determined by perfusion with or without halothane in the absence of Ca2+. Experiments were terminated by synaptosome lysis with 0.2 M perchloric acid. Radioactivity in each fraction was quantified by liquid scintillation spectrometry with dual isotope quench correction (Beckman Coulter LS 6000IC, Fullerton, CA, USA) using BioSafe II scintillation cocktail (RPI, Mt. Prospect, IL, USA). At the end of each experiment, halothane-containing solutions were sampled upon exit from release chambers into a gas-tight glass microsyringe, and were extracted into n-heptane (1:6–1:1 v v−1) for quantification of halothane by gas chromatography (Ratnakumari and Hemmings, 1998).
Data analysis
Release in each fraction was expressed as the fraction of synaptosomal content of labelled transmitter prior to each fraction collected (fractional release (FR); Westphalen and Hemmings, 2006a). The magnitude of evoked release was determined by subtracting baseline release (average of basal fractional release before and after stimulation) from cumulative fractional release values for each evoked release pulse (sum ΔFR). Sum ΔFR data from each experiment were normalized by the ratio of control release to collective mean control release in the presence of Ca2+ prior to curve fitting. Individual halothane concentrations vs 4AP-evoked release were fitted to sigmoidal inhibition curves by least-squares analysis to estimate Imax and IC50 (Prism v.4.0; GraphPad Software, San Diego, CA, USA). Control sum ΔFR values between WT and KO that were not significantly different were shared and constrained in curve-fit comparisons. Independent curve fits were tested for the bottom of the curve (Imax) differing significantly from 0. Values of Imax significantly greater than 0 were compared between WT and KO by curve-fit comparisons. If the null hypotheses (Imax=0 or WT Imax=KO Imax) were not rejected, curve-fit analysis were performed with respective constraints. Significant differences between concentration–effect curve-fit parameters (Imax and IC50) were determined by F-test comparison between best-fit values derived from independent curve fits to values derived from global fits with the parameter shared. Halothane effects on basal release were fitted to sigmoidal maximum effect (Emax) curves with variable slope, and compared between WT and KO curves with normalized control basal release (top of curve, held constant).
TREK-1 immunoreactivity
A synthetic peptide antibody against amino acids 8–28 of human TREK-1 was used to quantify TREK-1 immunoreactivity by immunoblotting. Control C57BL/J6 mouse cortex was homogenized (see nerve terminal preparation, above) in the presence of a protease inhibitor cocktail (4-(2-aminoethyl)benzenesulphonyl fluoride hydrochloride, aprotinin, bestatin, N-trans-epoxysuccinyl-L-leucine 4-guanidinobutylamide, leupeptin, pepstatin A; Sigma-Aldrich). The homogenate was either purified through sucrose gradients (synaptosomes) or washed in an equivalent volume of sucrose (crude homogenate) in the presence of the protease inhibitor cocktail. Protein concentrations of the homogenate and synaptosomes were determined before solubilization in 1% (w v−1) sodium dodecyl sulphate (SDS), and samples (20–60 μg of protein) were solubilized in sample buffer, separated by SDS-polyacrylamide gel electrophoresis (PAGE) (10% acrylamide) and transferred onto polyvinylidine difluoride membrane as described (Hemmings et al., 1992). Membranes were incubated in blocking buffer (5% dried milk powder (w v−1) in PBS (1.9 mM NaH2PO4, 8.4 mM Na2HPO4, 150 mM NaCl, pH 7.3) for 1 h, labelled with rabbit anti-TREK-1 (1:200 dilution), and washed (3 × 20 min) with PBS. Bound antibody was detected with horseradish peroxidase-conjugated goat-anti-rabbit antiserum and enhanced chemiluminescence (ECL, Amersham/GE Life Sciences, Piscataway, NJ, USA). Autoradiograms were scanned with a Typhoon Trio 9410 variable mode imager (Amersham/GE Healthcare, Piscataway, NJ, USA) using the 633 nm He–Ne laser and photomultiplier running at 600 V, with no emission filter selected, giving a digital image of 127 dpi. Signal intensity corresponding to TREK-1 was quantified using ImageQuant v5.2 software (GE Life Sciences, Piscataway, NJ, USA) with local average background correction. Relative density of TREK-1 immunoreactivity was determined for loads of 20–60 μg of protein, averaged from two exposure times and compared by unpaired t-test (Prism 4.0).
Materials
Halothane (thymol-free) was obtained from Halocarbon Laboratories (River Edge, NJ, USA); tetrodotoxin, 4-aminopyridine (4AP) and anti-TREK-1 antibody from Sigma-Aldrich Chemical Co. (St Louis, MO, USA); L-[3H]glutamate from Amersham Radiochemical Centre (42 Ci mmol−1; Buckinghamshire, UK); and [14C]GABA from PerkinElmer Inc. (0.24 Ci mmol−1; Boston, MA, USA).
Results
Evoked transmitter release
Halothane effects on transmitter release in WT mice
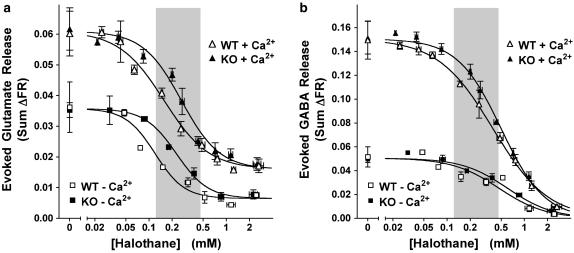
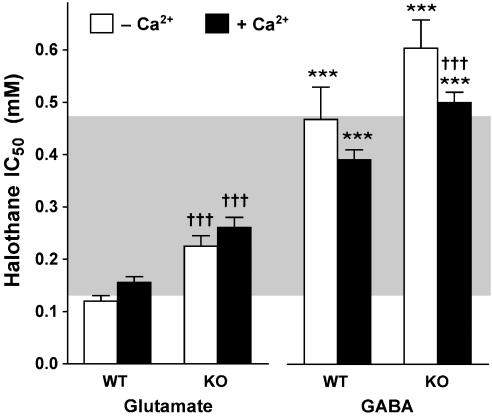
Stimulation of mouse WT cerebrocortical nerve terminals with a 2 min pulse of 1 mM 4AP in the presence of 1.9 mM free external Ca2+ evoked both L-[3H]glutamate and [14C]GABA release (Figure 1). Control release (sum ΔFR) of [14C]GABA was greater than that of L-[3H]glutamate, as reported previously for rat cerebrocortical synaptosomes (Westphalen and Hemmings, 2003). Halothane inhibited 4AP-evoked release of both L-[3H]glutamate and [14C]GABA within the clinical concentration range in the absence or presence of Ca2+ (Figure 1, WT). Halothane was significantly more potent as an inhibitor of glutamate release than of GABA release in the presence of Ca2+ (Figure 2). This figure also shows that, in the absence of Ca2+, halothane inhibition of glutamate release was also significantly more potent than of GABA release.
Figure 1.
Halothane inhibits 4AP-evoked glutamate (a) and GABA (b) release from wild-type littermate (WT) and TREK-1 knockout (KO) mouse cortical nerve terminals. Data are presented binned with curve fits and statistical analyses performed on individually measured halothane concentrations. Sum ΔFR in the presence (+Ca2+) or absence (−Ca2+) of Ca2+, and halothane concentrations are presented as mean±s.e.m. Control data are presented as mean±s.d. Shaded areas highlight the clinical concentration range between 0.5 and 2 times MAC for WT mouse (0.24 mM; Heurteaux et al., 2004). Halothane incompletely inhibited 4AP-evoked glutamate release (Imax≠0) from WT nerve terminals in the presence or absence of extracellular Ca2+ (P<0.0001 and P<0.0001, respectively). Halothane completely (Imax=0) inhibited 4AP-evoked GABA release from WT nerve terminals in the presence (P=0.39) or absence (P=0.40) of Ca2+. In TREK-1 KO nerve terminals, in the presence or absence of Ca2+, halothane also incompletely inhibited 4AP-evoked glutamate release (P=0.005 and P=0.005, respectively), and completely inhibited 4AP-evoked GABA release (P=0.79 and P=0.83, respectively). IC50 values are shown in Figure 2.
Figure 2.
Halothane inhibition of 4AP-evoked glutamate and GABA release from wild-type littermate (WT) and TREK-1 knockout (KO) mouse cortical nerve terminals. IC50 values (mean±s.e.m.) were compared between glutamate and GABA release (***P<0.001), and between WT and KO mice (†††P<0.001) using the F-test (Prism 4.0). Shaded area highlights the clinical concentration range (0.5–2 times MAC) for WT mice (0.24 mM; Heurteaux et al., 2004).
Reduced inhibition of transmitter release by halothane in TREK-1 KO mice
Nerve terminals prepared from TREK-1 KO mice were less sensitive to halothane inhibition of both 4AP-evoked glutamate release and GABA release compared to WT littermates (Figure 1, KO and Figure 2). In the absence of Ca2+, halothane inhibition of 4AP-evoked release was also significantly less potent for glutamate release, but not for GABA release, in KO compared to WT littermates (Figures 1 and 2). The reduced halothane inhibition of 4AP-evoked release produced by TREK-1 deletion was greater for glutamate release (67% increase in IC50) than for GABA release (28% increase) in the presence and in the absence of Ca2+ (88 and 29% increase in IC50, respectively), consistent with a greater role for TREK-1 in anaesthetic action on cortical glutamatergic nerve terminals. TREK-1 deletion did not alter the preferential inhibition by halothane of glutamate vs GABA release in the presence (P<0.0001) or absence of Ca2+ (P<0.0001).
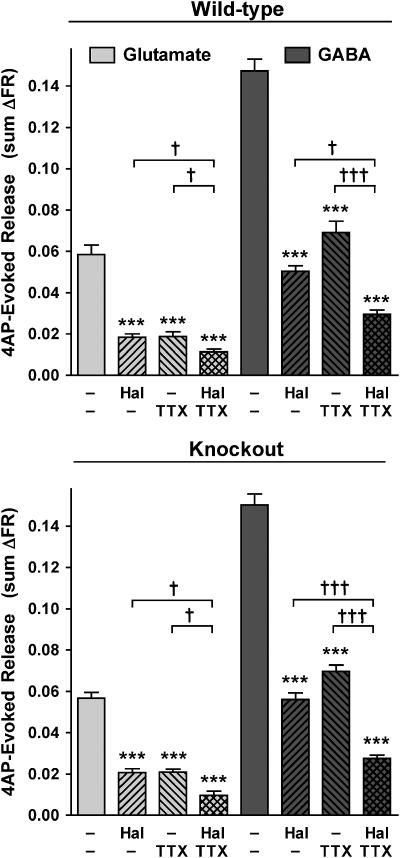
Halothane effects on tetrodotoxin-resistant transmitter release in WT and KO mice
Glutamate and GABA release evoked by 4AP were both inhibited incompletely by a maximally effective concentration of tetrodotoxin (1 μM; data not shown) in WT or KO mice (Figure 3). A nearly maximal inhibitory concentration of halothane (0.7–0.8 mM, see Figure 1) significantly inhibited the tetrodotoxin-resistant fraction of 4AP-evoked glutamate and GABA release from WT cortical nerve terminals (Figure 3). In KO preparations, tetrodotoxin-resistant 4AP-evoked release of both glutamate and GABA was also further inhibited by halothane (Figure 3). This indicates a role for a presynaptic volatile anaesthetic target(s) in addition to tetrodotoxin-sensitive voltage-gated Na+ channels and/or TREK-1 channels in the inhibition of transmitter release. The efficacy of halothane at ∼2 times MAC in inhibiting glutamate and GABA release was similar between WT and KO nerve terminals, as expected from this IC70–90 concentration (see Figure 1). The extent of glutamate and GABA release inhibition by tetrodotoxin was also similar between WT and KO nerve terminals, consistent with no compensatory change in Na+ channel function as a result of TREK-1 deletion.
Figure 3.
Halothane inhibits residual tetrodotoxin-insensitive 4AP-evoked glutamate release. At a nearly maximal inhibitory concentration (∼2 times MAC for WT mouse), halothane (Hal) inhibited 4AP-evoked glutamate and GABA release in the presence of a saturating concentration of tetrodotoxin (TTX, 1 μM) in WT (0.71±0.04 mM) and KO (0.75±0.06 mM) mice, compared to halothane (0.76±0.03 and 0.75±0.04 mM, respectively) or tetrodotoxin alone, in the presence of Ca2+. Statistical analysis of mean±s.e.m. values was performed by one-way ANOVA, with Bonferroni post hoc testing between respective control (***P<0.001) and between indicated groups (†P<0.05, †††P<0.001).
There was no difference in control evoked release of glutamate or GABA between WT and KO, which is consistent with the insensitivity of TREK-1 to 4AP (Fink et al., 1998) and the absence of compensatory changes in transmitter release mechanisms with TREK-1 deletion. Evoked release of glutamate and GABA from control C57BL/6J mouse cerebrocortical synaptosomes was comparable to that from the WT littermate synaptosomes (data not shown). There was no difference in yield between synaptosome preparations from WT littermate (14.6±0.93 mg) or TREK-1 KO mouse (13.4±1.03 mg) cerebral cortex. The ability of either WT or KO synaptosomes to take up L-[3H]glutamate (2.38±0.16 and 2.33±0.27 fmol mg−1, respectively; NS) or [14C]GABA (296±27 and 237±31 fmol mg−1, respectively; NS) was also comparable. A Runs test revealed that 4AP-evoked glutamate and GABA release from WT and KO cortical synaptosomes was significantly linear vs age, and linear regression analysis indicated no correlation between release and age from 6 to 24 weeks (data not shown).
Basal transmitter release
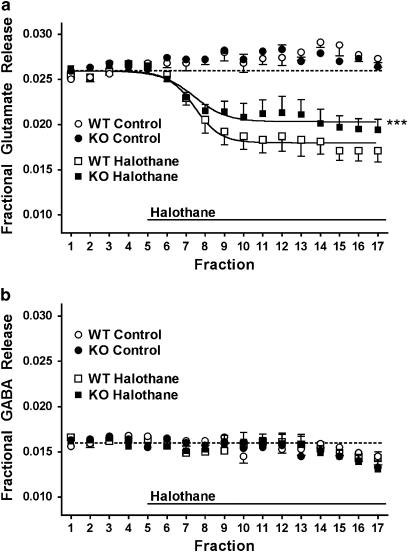
Selective inhibition by halothane of basal glutamate release from WT littermate mouse cerebrocortical synaptosomes (Figure 4a) was similar to that observed previously for rat cerebrocortical synaptosomes (Westphalen and Hemmings, 2006b). At a concentration chosen to maximize the signal-to-noise ratio for the predicted inhibitory effect, halothane (∼3 times MAC) was significantly less effective in inhibiting basal glutamate release from TREK-1 KO compared to WT mice (Figure 4a). Halothane did not significantly affect basal GABA release in either group (Figure 4b). Deletion of TREK-1 did not completely prevent inhibition by halothane of basal glutamate release, which suggests involvement of additional anaesthetic target(s) in the selective inhibition of basal glutamate vs GABA release. Control basal release of glutamate and GABA prior to drug/secretogogue application did not differ between KO and WT, either in the absence or presence of Ca2+ (data not shown).
Figure 4.
Inhibition of basal glutamate release by halothane is reduced in TREK-1 knockout mice. Basal release of glutamate (a) and GABA (b) in the absence of Ca2+ from wild-type littermate (WT) or TREK-1 knockout (KO) mouse cortical nerve terminals was determined in the absence (control, n=5) or presence of halothane (0.99±0.06 mM, n=6, and 1.03±0.03 mM, n=6, respectively). Halothane effects on basal release (Emax) were compared by curve fitting (Prism 4.0). Prestimulus baseline release was standardized and is denoted by a dotted line. ***P<0.001 vs WT.
TREK-1 immunoreactivity
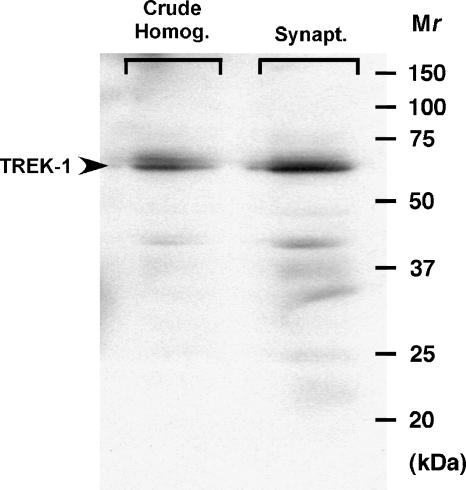
Immunoblots revealed the presence of a 67 kDa band in control C57BL/6J mouse cortical synaptosomes (Figure 5) that corresponds to the reported migration of TREK-1 (Bai et al., 2005). TREK-1 immunoreactivity was greater in synaptosome (n=4) versus crude homogenate (n=4) preparations from control mouse cortex (1.8±0.1-fold enrichment, P<0.01; Figure 5).
Figure 5.
TREK-1 immunoreactivity is enriched in mouse cortical nerve terminals. Mouse cortex was homogenized (crude homogenate) and further purified through sucrose gradients (synaptosomes). Representative immunoblot shows immunoreactive TREK-1 band at 67 kDa (40 μg protein per lane). Quantification by scanning of the immunoblots indicated a 1.8-fold enrichment of TREK-1 in cortical synaptosomes (n=4) vs crude homogenate (n=4; P<0.01).
Discussion and conclusion
Targeted deletion of TREK-1 reduced the inhibition by halothane of depolarization-evoked release of glutamate and GABA and of basal glutamate release from mouse cerebrocortical nerve terminals. TREK-1 KO mice require a significantly higher dose of halothane for anaesthesia (MAC=0.36 mM) vs wild-type control (MAC=0.24 mM; Heurteaux et al., 2004). This 50% increase in halothane EC50 for anaesthesia in vivo corresponds to a 70% increase in IC50 for glutamate release and 30% increase in IC50 for GABA release in TREK-1 KO mice. These results support a role for reduced inhibition of glutamate release, and possibly GABA release, by halothane in its reduced anaesthetic potency in TREK-1 KO mice in vivo. Since clinical concentrations of volatile anaesthetics preferentially inhibit glutamate release vs GABA release in cortical nerve terminals from both rat (Westphalen and Hemmings, 2006a, 2006b) and mouse (this study), reduced glutamate release mediated in part by activation of TREK-1 is a plausible mechanism for the presynaptic effects of volatile anaesthetics. Deletion of TREK-1 could also reduce anaesthetic potency indirectly via increased neuronal excitability due to a net loss of membrane potential stabilising K+ channels. However, this interpretation is less likely given the selective reductions in potency for specific anaesthetics and the absence of CNS hyperexcitability phenotypes in TREK-1 KO mice (Heurteaux et al., 2004). Control release of glutamate or GABA was not altered in TREK-1 KO mice. This suggests that TREK-1 does not play a major role in depolarization-evoked or basal transmitter release under normal conditions and is consistent with the absence of apparent neurological defects in TREK-1 KO mice. The selective inhibition of evoked glutamate vs GABA release by halothane in the absence of TREK-1 expression is consistent with the fact that TREK-1 KO mice are anaesthetized by halothane, albeit at reduced potency, probably due to the involvement of redundant anaesthetic mechanisms.
Halothane selectively inhibited basal glutamate release with no effect on basal GABA release, as reported previously in rat (Westphalen and Hemmings, 2006b). This is consistent with a reduction of net excitatory synaptic tone by volatile anaesthetics. The efficacy of halothane inhibition of basal glutamate release was reduced in TREK-1 KO mice, consistent with a role for TREK-1 in this effect. The Ca2+ independence of the halothane effect on basal glutamate release is consistent with a contributory role for TREK-1, since its gating is independent of Ca2+ (Patel and Honore, 2001). The insensitivity of basal GABA release to halothane suggests a transmitter-specific role for TREK-1 in halothane inhibition of basal glutamate release. In the TREK-1 KO, the specificity and degree of reduction in efficacy of halothane inhibition of basal glutamate and GABA release (14% and no effect, respectively) is distinct from the preferential and substantial reduction in potency of halothane inhibition of evoked glutamate and GABA release (67 and 28%, respectively). This difference is consistent with the distinct presynaptic mechanisms of basal and evoked release (see Westphalen and Hemmings, 2006a). The significantly greater effects of TREK-1 deletion on glutamate vs GABA for both basal and evoked release implies a transmitter-selective nerve terminal function for TREK-1 in glutamatergic and GABAergic synaptic transmission.
Inhibition by halothane of residual 4AP-evoked glutamate and GABA release from WT synaptosomes in the presence of voltage-gated Na+ channel block by tetrodotoxin, as previously shown in rat (Westphalen and Hemmings, 2006a), indicates a role for a presynaptic volatile anaesthetic target in addition to tetrodotoxin-sensitive Na+ channels. Since additive inhibition by halothane and tetrodotoxin was also evident in TREK-1 KO mice, a presynaptic anaesthetic target in addition to both tetrodotoxin-sensitive Na+ channels and TREK-1 K2P channels must be involved. Other volatile anaesthetic-sensitive K2P channels including TREK-2, TASK-1, TASK-2, TASK-3 and TALK-2 (Patel and Honore, 2001; Linden et al., 2006) are reasonable candidates, although their expression and function in nerve terminals are unknown.
A synaptic localization of TREK-1 has been demonstrated by immunocytochemical colocalization of TREK-1 and synapsin 1 (a synaptic vesicle-associated protein) in mouse hippocampus (Lauritzen et al., 2000). Our demonstration of significant enrichment of TREK-1 immunoreactivity in cortical nerve terminal preparations, along with functional evidence for nerve terminal TREK-1 expression in cortical nerve terminals, indicates a previously unrecognized presynaptic role for TREK-1. Together with effects at presynaptic voltage-gated Na+ channels and postsynaptic ligand-gated ion channels (Hemmings et al., 2005), effects at presynaptic TREK-1 channels are likely to contribute to the synaptic mechanisms of volatile anaesthetics including depression of excitatory transmission.
Acknowledgments
This project was supported by the National Institutes of Health (Bethesda, MD, USA) research Grant GM 58055. The generous contribution of the TREK-1 KO and WT littermate mice by Professor Michel Lazdunski (IPMC, Valbonne, France) is gratefully acknowledged. Thanks to Mr Brian Y Pan (WCMC, New York, USA) for his expert technical assistance with the immunoblotting.
Abbreviations
- 4AP
4-aminopyridine
- KO
knockout
- MAC
minimum alveolar concentration
- WT
wild-type
Conflict of interest
The authors state no conflict of interest.
References
- Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, et al. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Use of knockout technology to resolve pharmacological problems. Br J Pharmacol. 2007;150:1–2. doi: 10.1038/sj.bjp.0706941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, et al. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–608. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Girault JA, Nairn AC, Bertuzzi G, Greengard P. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARP-32. J Neurochem. 1992;59:1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK 1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Linden AM, Aller MI, Leppa E, Vekovischeva O, Aitta-Aho T, Veale EL, et al. The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the α2 adrenergic sedative dexmedetomidine, and cannabinoid agonists. J Pharmacol Exp Ther. 2006;317:615–626. doi: 10.1124/jpet.105.098525. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 2001;95:1013–1021. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalation anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Perouansky M, Hemmings HC., JrPresynaptic actions of general anesthetics Neural Mechanisms of Anesthesia 2002Humana Press Inc: Totowa, NJ; 345–369.In: Antognini JF, Carstens EE, Raines DE (eds). [Google Scholar]
- Ratnakumari L, Hemmings HC., Jr Inhibition of presynaptic sodium channels by halothane. Anesthesiology. 1998;88:1043–1054. doi: 10.1097/00000542-199804000-00025. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine evoked release. J Pharmacol Exp Ther. 2006a;316:216–223. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release. J Pharmacol Exp Ther. 2006b;316:208–215. doi: 10.1124/jpet.105.090647. [DOI] [PubMed] [Google Scholar]
- Winegar BD, MacIver MB. Isoflurane depresses hippocampal CA1 glutamate nerve terminals without inhibiting fiber volleys. BMC Neurosci. 2006;7:5. doi: 10.1186/1471-2202-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]