Abstract
Classical ischaemic preconditioning, delayed or second window preconditioning and postconditioning are forms of cardioprotection that are dependent on cell surface receptors, intracellular signalling molecules and kinases that ultimately block formation of the mitochondrial permeability transition. The latter is presumed to cause myocardial necrosis as well as apoptosis, so prevention of its formation upon resumption of perfusion after a prolonged coronary occlusion should be cardioprotective. In all of these forms of cardioprotection, formation of cGMP and activation of protein kinase G (PKG) are recognized to be key steps in the signal transduction pathway. Burley et al. highlight the roles of cGMP and PKG in their comprehensive review. They describe the basic biology of PKG and emphasize its compartmentalization, which may be responsible for the frustration induced by assays for PKG in whole cell lysates and for the spurious conclusions about the role of PKG in cardioprotection. This review will be useful to both the novice and the seasoned investigator.
Keywords: cardioprotection, cGMP, mitochondrial permeability transition, preconditioning, protein kinase G, signal transduction
Prior to 1986, the concept of cardioprotection in the setting of acute myocardial infarction was purely theoretical. Many had tried to describe interventions that could salvage ischaemic myocardium but, despite reports of success in individual laboratories, no particular intervention's efficacy could be consistently confirmed when examined in other laboratories. Then in 1986, the team of Murry et al. (1986) reported a most unusual observation. They noted that several cycles of brief ischaemia/reperfusion before a long coronary occlusion in dogs resulted in infarcts 75% smaller than those in dogs subjected to only the long occlusion. In other words, more cumulative ischaemia was better! Although this report by accomplished investigators from a well-respected experimental laboratory appeared in a rigorously reviewed scientific journal, there was still the anticipated scepticism. It actually took several more years before others even tried to duplicate the study. But slowly, other laboratories began to examine this ischaemic preconditioning phenomenon, perhaps to bury it rather than to support it. Eventually, the reports from all investigators in all species examined confirmed preconditioning's cardioprotective quality.
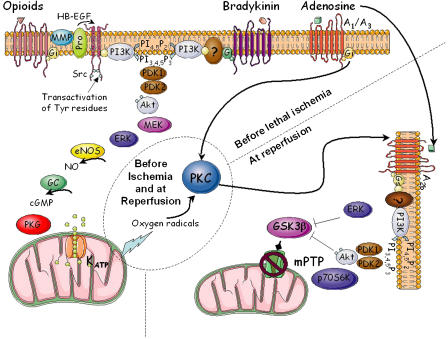
But how could preconditioning protect the heart? Our lab made the first conceptual breakthrough when it was observed that a brief infusion of an adenosine analogue in lieu of brief ischaemia protected the heart, whereas an adenosine receptor antagonist abolished the protection of ischaemic preconditioning (Liu et al., 1991). After this seminal report many laboratories worked to establish the intracellular signalling involved, with the intent and hope that an understanding of the signalling would provide the opportunity to harness the phenomenon for clinical use. Signalling in preconditioning occurs in two phases: the trigger phase, occurring before the long ischaemia, initiated either by endogenous agonists released during the brief cycles of ischaemia or by a number of exogenous compounds activating the pathway at a more distal locus and the mediator phase, occurring during and, more importantly, just after the lethal ischaemia. As shown in Figure 1, much is known about the trigger phase. It involves transactivation of a growth factor receptor, activation of membrane enzymes and metabolism of lipids, activation of Akt, stimulation of nitric oxide production and subsequent activation of protein kinase G (PKG), opening of mitochondrial ATP-dependent K+ channels and release of reactive oxygen species. The latter are not detrimental but rather these free radicals act as second messengers to initiate the mediator phase by activating protein kinase C. At reperfusion, further signalling occurs and many of the steps appear to recapitulate events that occurred during the trigger phase. It is now believed that all of this signalling leads to prevention of formation of mitochondrial permeability transition pores, which normally kill mitochondria in the first minutes of reperfusion in the non-preconditioned heart.
Figure 1.
Simplified signalling pathways of myocardial preconditioning. ERK, extracellular-signal regulated kinase; GC, guanylyl cyclase; GSK-3β, glycogen synthase kinase-3β; HB-EGF, heparin-binding epidermal growth factor-like growth factor; MEK, mitogen-activated protein kinase kinase; MMP, matrix metalloproteinases; NO, nitric oxide; NOS, NOS synthase; eNOS, endothelial NOS; PI3K, phosphatidylinositol 3-kinase; PI4,5P2, phosphatidylinositol bisphosphate; PI3,4,5P3, phosphatidylinositol trisphosphate; PKG, protein kinase G; PKC, protein kinase C; Pro, pro-HB-EGF; p70S6K, p70S6 kinase; mPTP, mitochondrial permeability transition pore. Modified from Tissier et al. (2007).
This complex signalling depends on multiple components interacting in a particular fashion and likely in specific subcellular compartments. Many of these components have been highlighted in prior reviews, but the roles of cGMP and PKG have been under-appreciated. Burley et al. (2007) manage to correct this oversight in their comprehensive review of PKG and its importance to cardioprotection. In a methodical fashion, they remind us of the basic biology of PKG and the central role it plays in cardioprotection. Although PKG is clearly needed in preconditioning (Figure 1), Burley also points out that it is critical in both the trigger and mediator phases of delayed preconditioning in which protection is realized 1–3 days after the triggering stimulus. And very recently, it has also been shown to be part of the signalling cascade in postconditioning in which brief cycles of reperfusion/ischaemia follow rather than precede the prolonged period of ischaemia (Penna et al., 2007). PKG is obviously critical to many forms of cardioprotection. The authors of this review emphasize another important point about PKG, which is only recently being uncovered. PKG activation is not occurring throughout the cell but rather in specific subcellular compartments (Piggott et al., 2006). Thus assays of cGMP or PKG, or for that matter almost any protein or kinase, in whole cell lysates may be unreliable indicators of the significant role played by the object of the assay. To mediate the signalling steps, biochemical assays of phosphorylated proteins or enzymes may be far less specific or accurate than studies with pharmacological antagonists of those signalling steps, heretofore, a radical and often challenged position. But this bold review by Burley et al. (2007) helps to set the record straight on this subject.
Hence, the review by Burley et al. should be useful to both the novice and the seasoned investigator. Both will learn and each will appreciate the multiple insights of the authors.
References
- Burley DS, Ferdinandy P, Baxter GF.Cyclic GMP and protein kinase-G in myocardial ischaemia–reperfusion: opportunities and obstacles for survival signalling Br J Pharmacol 2007152855–869.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GS, Thornton J, Van Winkle DM, Stanley AWH, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75:168–177. doi: 10.1016/j.cardiores.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol. 2006;128:3–14. doi: 10.1085/jgp.200509403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier R, Ghaleh B, Couvreur N, Cohen MV, Downey JM.Protecting the acutely ischemic myocardium beyond reperfusion therapies: are we any closer to realizing the dream of infarct size elimination Arch Mal Coeur Vaiss 2007(in press) [PubMed]