Abstract
Background and purpose:
IKur (Ultra-rapid delayed rectifier current) has μM sensitivity to 4-aminopyridine (4-AP) and is an important modulator of the plateau amplitude and action potential duration in canine atria. Kv1.5 encodes IKur and is present in both atria and ventricles in canines and humans. We hypothesized that a similar plateau outward current with μM sensitivity to 4-AP is present in canine ventricle.
Experimental approach:
We used established voltage clamp protocols and used 4-AP (50 and 100 μM) to measure a plateau outward current in normal canine myocytes isolated from the left ventricular mid-myocardium.
Key results:
Action potential recordings in the presence of 4-AP showed significant prolongation of action potential duration at 50 and 90% repolarization at 0.5 and 1 Hz (P<0.05), while no prolongation occurred at 2 Hz. Voltage clamp experiments revealed a rapidly activating current, similar to current characteristics of canine atrial IKur, in ∼70% of left ventricular myocytes. The IC50 of 4-AP for this current was 24.2 μM. The concentration of 4-AP used in our experiments resulted in selective blockade of an outward current that was not Ito or IKr. β-Adrenergic stimulation with isoprenaline significantly increased the 4-AP sensitive outward current density (P<0.05), suggesting a role for this current during increased sympathetic stimulation. In silico incorporation into a canine ventricular cell model revealed selective AP prolongation after current blockade.
Conclusions and implications:
Our results support the existence of a canine ventricular plateau outward current sensitive to micromolar 4-AP and its constitutive role in ventricular repolarization.
Keywords: ventricular myocyte, potassium current, action potential, canine, ventricle, repolarization, 4-aminopyridine
Introduction
Cardiac action potential repolarization occurs through a multitude of voltage-gated K+ channels with differing activation and inactivation patterns (Nerbonne and Kass, 2005). The transient outward potassium current (Ito) activates and inactivates rapidly; thereby contributing to early phase 1 repolarization (Patel and Campbell, 2005). The rapid (IKr) and slow (IKs) delayed rectifier K+ currents activate towards the end of the action potential plateau (phase 2) and contribute significantly to phase 3 repolarization. In rat, canine and human atria, and in rodent and guinea pig ventricle, an ultra-rapid delayed rectifier current (IKur) has been described (Yue and Marban, 1988; Fedida et al., 2003; Brouillette et al., 2004; Nerbonne and Kass, 2005). IKur is a determinant of the normal canine atrial action potential duration (APD). IKur, like Ito activates very rapidly, but in contrast to Ito inactivates very slowly; IKur along with Ito contributes to phase 1 repolarization in the atria (Nerbonne and Kass, 2005).
Despite the abundant Kv1.5 protein expression in canine and human ventricle, previous studies have not demonstrated functional ventricular IKur in canine or human ventricular myocytes (Yue et al., 1996; Li et al., 1996b). More recently, IKur has received increased attention due to the ‘atrial-specific' expression of the current (in canines and humans), providing a novel therapeutic target for the treatment of atrial arrhythmias without the risk of ventricular proarrhythmia (Page and Roden, 2005).
IKur is sensitive to micromolar concentrations of 4-aminopyridine (4-AP), while Ito is inhibited only at millimolar concentrations (Nattel et al., 2000; Patel and Campbell, 2005). This difference in sensitivity to 4-AP has been used to define IKur in canine and human atria, and in rodent ventricle. We elicited a current that is sensitive to micromolar concentrations of 4-AP, which is activated during the plateau voltages of the action potential in canine left ventricular mid-myocardium. This outward current has a constitutive role in ventricular repolarization.
Materials and methods
Animal procedures and myocyte isolation
All animal procedures were approved by the Institutional Lab Animal Use and Care Committee of The Ohio State University.
Twenty-three adult hound-type dogs (age 9 months to 5 years) weighing between 8 and 20 kg were used for the experiments. Dogs were verified to have normal cardiac function by routine electrocardiograms and echocardiographic examinations during butorphanol tartarate (0.5 mg kg−1 intramuscularly) sedation. On the day of the experiment, dogs were killed by intravenous injection of pentobarbital sodium (120 mg kg−1 for the first 4.5 kg and 60 mg kg−1 for every 4.5 kg thereafter) via the cephalic vein. Following this, the hearts were rapidly excised via thoracotomy and perfused with cold cardioplegic solution (containing 5% glucose, 0.1% mannitol, 22.4 mM NaHCO3 and 30 mM KCl) injected into the coronary ostia. The left circumflex artery was cannulated for myocyte isolation as described previously (Kubalova et al., 2005). Following the washout of blood from the heart, collagenase (Worthington type II, 0.65 mg ml−1) and protease-free bovine serum albumin (BSA) (0.65 mg ml−1) were added to the perfusate (100 ml). After 30–45 min of collagenase perfusion, the digested mid-myocardial section of the lateral wall of the left ventricle was separated from the epicardial and endocardial sections; digested tissue was shaken in a water bath at 37°C for an additional 5–10 min. This typically yielded 70–90% of rod-shaped myocytes with staircase ends and sharp margins. The myocytes were stored at room temperature in a standard incubation buffer solution containing (in mM) NaCl 118, KCl 4.8, MgCl2 1.2, KH2PO4 1.2, glutamine 0.68, glucose 10, pyruvate 5, CaCl2 1, along with 1 μmol l−1 insulin, and 1% BSA until use.
Electrophysiological protocols
Myocytes were placed in a laminin-coated cell chamber (Cell Microcontrols, Norfolk, VA, USA) and superfused with bath solution containing (in mM) 135 NaCl, 5 MgCl2, 5 KCl, 10 glucose, 1 CaCl2, 5 HEPES, pH 7.40 with NaOH, at a temperature of 36±0.5°C. For action potential recordings, the same bath solution was used with CaCl2 increased to 1.8 mM. During voltage clamp experiments to measure potassium currents, L-type calcium current was blocked by 2 μM nifedipine. Solutions were changed with a six-port gravity flow system (∼1 ml min−1). Borosilicate glass micropipettes (tip resistance between 1.5–3 MΩ) were filled with pipette solution containing (in mM) 100 K+-aspartate, 40 KCl, 5 MgCl2, 5 EGTA, 5 HEPES, pH adjusted to 7.2 with KOH. Perforated whole-cell patch clamp (using amphotericin B) was used to minimize alterations in intracellular milieu. For voltage clamp experiments, only recordings with an access resistance <20 MΩ were included in the analyses. For determination of drug-sensitive currents, only cells with less than a 20% change in access resistance were included in the analyses. Series-resistance compensation (∼70%) was used for current recordings. All 4-AP-sensitive currents were recorded after 3–5 min of 4-AP superfusion.
Action potentials were recorded with perforated whole-cell patch techniques, as described above. Action potentials were measured as the average of the last 10 (steady-state) action potentials, recorded during a train of 25 action potentials at each stimulation rate. To analyse beat-to-beat variability in the action potential recordings, standard deviation and coefficient of variation (CV) of the APD at 90% repolarization was calculated. The amplitude of phase 2 was measured as the maximum potential following phase 1 of the action potential.
Ito and the rapid component of the IKr were elicited using voltage protocols as shown in the insets of Figure 2. Currents were recorded both in the presence and absence of 100 μM 4-AP to examine potential inhibition of Ito and IKr by 4-AP.
Sustained outward potassium current was elicited from a holding potential of −40 mV with an 80 ms prepulse to +30 mV to inhibit Ito, followed by 300 ms voltage steps from −20 mV to +50 mV. The interval between each voltage step was 3 s. 4-AP-sensitive plateau outward current was measured as the steady-state difference current after a minimum of 4 min of superfusion with 4-AP. The activation time constant of the 4-AP-sensitive current was determined by fitting the activation to a monoexponential function in Clampfit (v 8.0, Axon Instruments, Union City, CA, USA).
We tested two concentrations of 4-AP: 50 and 100 μM, based on previously published observations (Yue et al., 1996; Brouillette et al., 2004). An envelope-of-tails test was adapted from a previously published method used to evaluate IKur in canine atria (Yue et al., 1996). The envelope-of-tails protocol started from a holding potential of −40 mV with a prepulse to +30 mV, followed 30 ms later by a variable-duration test pulse (60–240 ms) to +20 mV, followed by a step to −30 mV to elicit the tail current. The interval between test pulses was 3 s. The constancy of the ratio of the step current to tail current was evaluated as discussed below.
Data acquisition was performed with Clampex 8.0 software (Axon Instruments) and an Axopatch 200A patch clamp amplifier (Axon Instruments).
Statistical analysis
Acquired data were analysed using Clampfit 8.0 software (Axon Instruments) and Origin 6.1 software (OriginLab, Northampton, MA, USA). Currents were normalized to cell capacitance in picofarads (pF) and are expressed as pA pF−1. All data are presented as mean±s.e.
APDs obtained at baseline and during drug exposure were analysed by one-way analysis of variance (SAS for Windows v9.1, SAS Inc., Cary, NC, USA). 4-AP-sensitive, isoprenaline-modulated and baseline 4-AP-sensitive current densities at each test voltage were statistically compared using Student's t-tests. Dose–response curves for 4-AP were constructed using the Hill equation in Origin 6.1 (OriginLab). To test the envelope-of-tails data, a linear regression was performed (SAS for Windows, v.9.1, SAS Inc.) to calculate the slope and to test whether the slope was statistically different from zero. P<0.05 was the criterion for statistical significance for all tests.
Chemicals and solutions
All chemicals for buffer and stock solution preparation were purchased from Fisher Scientific (Pittsburgh, PA, USA), Sigma-Aldrich (St Louis, MO, USA) and Invitrogen Inc. (Carlsbad, CA, USA). Stock solutions of nifedipine, amphotericin B and 4-aminopyridine were prepared fresh on the day of each experiment. DPO-1, (2-isopropyl-5-methylcyclohexyl diphenylphosphine oxide, Tocris Bioscience, Ellisville, MD, USA), a relatively new, selective IKur blocker (Lagrutta et al., 2006) was used for a separate set of experiments, and was prepared from a stock solution (10 mM) in dimethylsulphoxide prepared on the day of each experiment. Isoprenaline solutions were prepared daily from commercially available injectable solutions (0.2 mg ml−1), which were stored at 4°C until use. All nifedipine, isoprenaline, DPO-1 and amphotericin B solutions were protected from exposure to light.
Computational methods
Because the patch clamp data suggested little inactivation of the current, we used a three-state Markov model:
 |
with two closed states (C1 and C2), and one open state (O) to fit the data. All state transition rates (α1, α2, β, β2) are of the form Px* exp (Py*V), where Px and Py are parameters and V is the membrane potential in mV.
From the recorded difference current, a training data set for the model fitting was constructed. First, data during the pre-pulses and data after the 300 ms pulses were discarded. Second, data demonstrating a capacitance transient were excluded to remove any artifactual changes in the current.
Optimization of the model parameters began with a set of randomly selected parameters. The same voltage clamp protocol that was used in the experiment was applied to the model. The resulting simulated current was compared to the training data. A cost function, CF(M), was defined where M is the vector of the parameters in the model that was to be optimized. This cost function is defined by
 |
Here Train_datai is the training data at the ith time point, Sim_datai(M) is the simulation value at the ith time point using the parameters given by M. The sum is taken over i = 1 to N, the total number of time points in the recording. The goal of the optimization process was to find the parameter values that minimize the cost function CF(M) by iteratively changing the parameters, simulating the model and evaluating the cost function until the simulation nearly matched the data.
The optimization routine used a combination of global and local methods. The global method, differential evolution (Storn, 1996), used the following strategy: a population of parameter sets was generated randomly, and the cost of each parameter set was evaluated. New parameter sets were generated by first adding a weighted difference between two randomly selected parameter sets to a third random parameter set, and then by exchanging a fraction of the resulting parameter set with a member of the population according to a cross-over probability. The cost of the new parameter set was then evaluated and compared to the cost of a randomly chosen set in the population. The lower cost set was kept in the next generation of the population. The optimization strategy consisted of using differential evolution for a total of 4000 generations. After each thousand generations, the Levenberg–Marquardt (Marquardt, 1963; Press et al., 1992) local method was applied to each parameter set in the population to quickly take each parameter vector to a local minimum.
All simulations and optimizations were run on a Dell Inspiron 9100 computer and a 16-node Linux cluster of Intel Xeon dual processors using custom written C++ computer code. Each model is represented by a set of differential equations of the form dx/dt = f(x,t,p), where x is a vector describing the current state of the system, t is time and p is a vector of parameters. The corresponding differential equations are usually quite stiff in the sense that they have widely separated time scales: some variables change rapidly under small perturbations, while others change slowly. To improve the accuracy of our simulations, we used the CVODES package from Lawrence Livermore National Laboratories (Cohen and Hindmarsh, 1994) with the backward differentiation formula, designed for stiff systems. We also used automatic differentiation to calculate the Jacobian derivative of the function for use with the dense Newton-based solver that is included as part of CVODES.
Results
Myocyte capacitance was 163.8±7.7 pF (n=56). Figures 1a and b show representative action potentials recorded at 0.5 and 1 Hz at baseline, in the presence of 100 μM 4-AP and during washout. Superfusion with 100 μM 4-AP decreases the net outward current evident during phase 1. Consistent with 100 μM 4-AP block of an outward current (activated at plateau potentials), phase 2 amplitude was increased from baseline values (Table 1). Figure 1c summarizes the APD data obtained at baseline and after 4-AP superfusion at all tested stimulation frequencies. There was a statistically significant prolongation of APD at 50% (APD50) and 90% (APD90) repolarization seen at 0.5 and 1 Hz (Figures 1c and d). No significant change in the APD was seen at a stimulation rate of 2 Hz during 4-AP superfusion. This effect was reversible after prolonged washout of 7–10 min (Table 1). The resting membrane potential was not affected by 50 or 100 μM 4-AP (−76.5±0.5 mV at baseline, −75.7±0.8 mV and −75.2±0.4mV, with 50 and 100 μM 4-AP, respectively, P=NS). The baseline CVs of the APD90 values were 4.2, 5.9 and 5.8% at 0.5, 1 and 2 Hz respectively. Superfusion with 100 μM 4-AP did not change the CVs of the APD90 values (3.4, 5.8 and 6.7% at 0.5, 1 and 2 Hz respectively).
Figure 1.
Blockade of IKur using 50 or 100 μM 4-AP causes reverse use-dependent action potential prolongation. Representative action potential recordings are shown at 0.5 Hz (a) and 1 Hz (b). (c) Summary data for APD50 plotted as a function of stimulation frequency. (d) Summary data for APD90 plotted as a function of stimulation frequency. The numbers on each bar indicate the number of cells tested. (*P<0.05 compared to baseline recordings). 4-AP, 4-aminopyridine; APD, action potential duration; IKur, ultra-rapid delayed rectifier current.
Table 1.
4-AP at 100 μM prolongs APD and increases phase 2 amplitude at 0.5 and 1 Hz
| Baseline | 100 μM 4-AP | Washout | |
|---|---|---|---|
| 0.5 Hz | |||
| APD50 (ms) | 392.8±34.6 | 543.8±56.6*,† | 367.8±90.5 |
| APD90 (ms) | 576.3±33.9 | 784.6±60.5*,† | 518.9±106.2 |
| Phase 2 amplitude (mV) | 27.5±2.6 | 36.3±3.0*,† | 27.4±2.0 |
| 1 Hz | |||
| APD50 (ms) | 328.9±26.6 | 434.8±23.7*,† | 328.7±36.9 |
| APD90 (ms) | 498.4±21.7 | 633.3±34.6*,† | 483.4±51.9 |
| Phase 2 amplitude (mV) | 26.4±2.7 | 33.1±3.1* | 29.4±1.3 |
| 2 Hz | |||
| APD50 (ms) | 243.7±9 | 253.1±26.8 | 242.5±16.4 |
| APD90 (ms) | 373.8±9.5 | 412.1±14.2 | 386.6±22.2 |
| Phase 2 amplitude (mV) | 21.6±4.4 | 31.4±5.2* | 22.4±4.7 |
Abbreviations: APD, action potential duration; 4-AP, 4-aminopyridine.
*P<0.05 vs baseline, †P<0.05 vs washout (n=11–13 cells in 0.5 and 1 Hz, n=4–9 cells in 2 Hz group).
K+ current-dependent action potential prolongation in the canine ventricle has been attributed to two currents: Ito, where blockade with 2 mM 4-AP prolongs both APD50 and APD90 (Litovsky and Antzelevitch, 1989) or IKr, where blockade selectively prolongs APD90 (Gintant, 2000; Varro et al., 2000). Therefore, we sought to exclude these possibilities and determine whether the 4-AP concentrations used in our experiments affected either Ito or IKr.
Figures 2a and b show Ito and IKr recorded at baseline and during superfusion with 100 μM 4-AP, respectively. There was no significant inhibition of either Ito or IKr amplitude by this concentration of 4-AP, confirming that the observed AP prolongation did not result from blockade of either Ito or IKr.
Figure 2.
4-AP at 100 μM does not block Ito or IKr. (a) Representative Ito traces from a ventricular myocyte (cell capacitance—154 pF) recorded at baseline (left), after superfusion with 100 μM 4-AP (middle, voltage protocol is inset) and the averaged Ito I–V curves (right). (b) The peak ramp IKr from a ventricular myocyte (cell capacitance—148 pF) recorded at baseline and 100 μM 4-AP using the ramp protocol (inset). Current was measured as the peak outward current during the voltage ramp. For purposes of clarity, only the traces obtained at the last test pulse (plateau duration=80 ms) are shown. The current densities plotted as function of the plateau duration are shown on the right. The dashed lines represent the zero current line. The interpulse interval for Ito and IKr was 2 and 7 s respectively. 4-AP, 4-aminopyridine; Ito, transient outward potassium current; IKr, rapid delayed rectifier K+ current.
The 100 μM 4-AP-sensitive difference current, which was obtained by digital subtraction, shows rapid current activation following membrane depolarization (Figure 3a). The 4-AP-sensitive current does not display significant inactivation during the 300 ms test pulse. The current density–voltage relationship of the 100 μM 4-AP-sensitive current is shown in Figure 3b. The outward current begins to activate at −10 mV and increases with increasingly positive test potentials. The threshold for activation (Figure 3b) is consistent with the voltage range occurring during phase 1 of the action potential. The activation time constant was 16.7±11 ms at −10 mV, 4.7±0.81 ms at +10 mV and 3.96±1.44 ms at +50 mV.
Figure 3.
4-AP at 100 μM inhibits canine ventricular-sustained outward K+ current. (a) The baseline (top), 100 μM 4-AP (middle) and difference currents from a ventricular myocyte (cell capacitance—166 pF) using the voltage clamp protocol shown in the inset. The dashed lines represent the zero current line. (b) The averaged I–V curve for the 4-AP-sensitive sustained outward K+ current (from myocytes exhibiting measurable 4-AP-sensitive plateau outward current), with current measured as the average during the last 20 ms of the test pulse. (c) Dose–response relationship for 4-AP inhibition of sustained outward K+ current, fitted to the Hill equation ((r2=0.99), n=5–8 myocytes at each concentration). (d) A representative 4-AP-sensitive current trace from a ventricular myocyte using the voltage clamp protocol shown in the inset. Circles on the step current trace indicate the time at which the tail currents were elicited. (e) The mean envelope-of-tails test data where the ratio of tail to step current amplitude is plotted as a function of step pulse duration. 4-AP, 4-aminopyridine.
Notably, we were unable to measure a 4-AP-sensitive current (100 μM) in 12 of the 41 myocytes tested with this protocol. This finding was evaluated further in secondary experiments. Action potentials were recorded first, followed by voltage clamp experiments (with nifedipine exposure of 3–4 min) in the same cells, to record baseline currents (n=4). Then, 4-AP superfusion (perfusate calcium at 1.8 mM) was performed to washout nifedipine and record action potentials during 4-AP exposure in the same myocytes. Following the second action potential recordings, voltage clamp experiments were repeated to determine the presence of any 4-AP-sensitive current. No action potential prolongation during 4-AP treatment was seen in myocytes lacking a 4-AP-sensitive current. The converse was also true; in myocytes (n=3) exhibiting 4-AP-dependent action potential prolongation, we were able to consistently elicit a 4-AP-sensitive sustained outward current. These results argue against either a nonspecific effect of 100 μM 4-AP on APD or current rundown during the duration of our experiments.
We analysed the concentration–response data to evaluate the inhibition of the sustained potassium current by 4-AP (Figure 3c). This analysis revealed an IC50 value of 24.2 μM. At 50 and 100 μM 4-AP, this fitted curve predicts 78.7 and 96.4% inhibition, respectively. The tested concentrations were therefore close to the maximal blocking concentration, which explains the lack of a significant difference when comparing results from the two concentrations (Figure 1c). We tested only up to a concentration of 500 μM, as a recent publication (Ridley et al., 2003) has shown inhibition of IKr with millimolar concentrations of 4-AP, and Ito is known to be blocked at 1–1.5 mM (Nattel et al., 2000).
Figure 3d shows a representative envelope-of-tails test using the protocol shown. The ratio of the peak tail current to the average steady-state step current as a function of the step duration is shown in Figure 3e. Visual examination of the data revealed a constant ratio as a function of time. This was confirmed statistically by linear regression analysis, which revealed a slope of 0.021±0.015, which did not differ significantly from a slope of zero (P=0.17); this is consistent with a single current component in the 100 μM 4-AP-sensitive current.
In canine atria, IKur is augmented by β-adrenergic stimulation (Yue et al., 1999). To test the β-adrenergic modulation of the 4-AP-sensitive plateau outward current in the ventricle, we used 100 nM and 1 μM isoprenaline (a nonspecific β1- and β2-adrenergic receptor agonist), followed by isoprenaline and 4-AP to obtain the 100 μM 4-AP-sensitive current. Data were obtained after 6–8 min of exposure, which we found in preliminary experiments to result in steady-state activation of the sustained outward K+ current. The baseline 100 μM 4-AP-sensitive current recorded in the absence of isoprenaline (Figure 4a) and isoprenaline stimulated, 4-AP-sensitive current is shown in Figure 4b. Isoprenaline (1 μM) significantly (P<0.05) increased the measured current amplitude at +50 mV from 0.51±0.10 pA pF−1 to 1.53±0.37 pA pF−1 (Figure 4c). Isoprenaline did not significantly alter the activation time constant of the current measured at +50 mV, which was 3.96±1.4 ms at baseline vs 1.3±0.9 ms with 1 μM isoprenaline (P=0.09).
Figure 4.
Canine ventricular plateau outward current is augmented by isoprenaline (Iso). Representative 100 μM 4-AP-sensitive current traces from myocytes recorded at baseline (a; 166 pF) and after exposure to isoprenaline (b; 154 pF). The dashed lines represent the zero current line. Summary I–V curves are shown in (c). (*P<0.05 vs baseline control values). 4-AP, 4-aminopyridine.
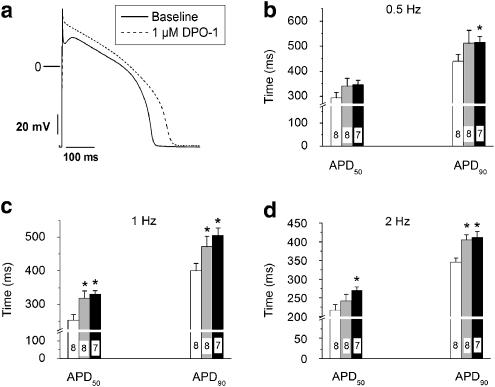
In separate experiments, action potentials were recorded in the presence and absence of DPO-1 (0.3 and 1 μM). We observed significant prolongation of APD50 with DPO-1 (P<0.05) at 1 and 2 Hz (Figure 5b), while significant prolongation of APD90 was evident at all three stimulation frequencies (Figure 5c).
Figure 5.
DPO-1, an IKur-selective blocker, prolongs APD in canine left ventricular myocytes. (a) Representative action potential trace recorded at 1 Hz before and after superfusion of 1 μM DPO-1. Average data, for APD50 and APD90 measured at 0.5 (b), 1 (c) and 2 Hz (d) at baseline (open bar) or after superfusion with 0.3 μM (grey bar), or 1 μM (black bar) of DPO-1. (*P<0.05 vs control). APD, action potential duration; DPO-1 (2-isopropyl-5-methylcyclohexyl diphenylphosphine oxide; IKur, ultra-rapid delayed rectifier current.
Results from computer simulations
A Markov model structure was used to develop a computer simulation of the 4-AP-sensitive ‘IKur-like' current. Parameters in the model were estimated by using the nonlinear optimization routine, described above, to fit the model output to experimental data from patch clamp recordings. Multiple parameter vectors with low cost were obtained after the optimization. The lowest cost parameter vector (Table 2) was used to generate the results below. No significant differences were observed for the other low-cost parameter vectors (data not shown). Figure 6a compares the resulting ion current model and the experimental patch clamp data. The lowest cost ion current model was then incorporated into a canine action potential model (Fox et al., 2002) by replacing the IKp current originally in that model. Action potentials were generated from the model at the same cycle lengths as in the experiment (basic cycle lengths of 2000, 1000 and 500 ms). To study the effect of the ‘IKur-like' current on APD, control action potential simulations were compared with simulations of ‘IKur-like' current block (Figures 6b–d), and to simulations of Ito block (Table 3). Table 3 summarizes the effects of Ito blockade vs ‘IKur-like' current blockade in two different canine action potential models (Fox et al., 2002; Hund and Rudy, 2004). Blockade of ‘IKur-like' current in the action potential models results in significantly more APD prolongation than that of Ito blockade.
Table 2.
Parameter values from the lowest cost fit for the IKur-like current model
| Parameter | Value |
|---|---|
| EK | −94.88 mV |
| GKur | 0.1453 mS/uF |
| α1x | 0.04957 ms−1 |
| α1y | 0.007138 mV−1 |
| α2x | 0.002703 ms−1 |
| α2y | 0.0001 mV−1 |
| β1x | 0.2348 ms−1 |
| β1y | −1.708 mV−1 |
| β2x | 0.02055 ms−1 |
| β2y | −0.0001 mV−1 |
Figure 6.
‘IKur-like' current blockade prolongs canine ventricular action potential in silico. (a) Fitting of a computer model of the 4-AP-sensitive (‘IKur-like') current to current data from patch clamp experiments. For purposes of clarity, current fitting to test steps to −10, 0, 20 and 40 mV are shown. Model parameters after fitting are provided in Table 2. The fitted model was incorporated into the Fox–Gilmour and Hund–Rudy canine action potential models. Steady-state action potential tracings from the Fox–Gilmour model obtained at 2000 (b), 1000 (c) and 500 ms (d) basal cycle lengths. Control action potential traces are shown in black and action potentials in the presence of ‘IKur-like' current blockade are shown in grey. 4-AP, 4-aminopyridine; IKur, ultra-rapid delayed rectifier current.
Table 3.
Effects of Ito blockade versus ‘IKur-like' current blockade on APD90 and plateau amplitude in in silico models
| Model |
Fox–Gilmour |
Hund–Rudy |
||||
|---|---|---|---|---|---|---|
| Basic cycle length | 2000 ms | 1000 ms | 500 ms | 2000 ms | 1000 ms | 500 ms |
| Control APD (ms) | 166 | 163 | 148 | 219 | 211 | 150 |
| Control plateau (mV) | −4.4 | −5.3 | −8.7 | 12.8 | 3.4 | −2.5 |
| Ito blocked APD (ms) | 161 | 163 | 156 | 168 | 163 | 149 |
| Ito blocked plateau (mV) | 7.0 | 8.0 | 8.0 | 10.0 | 8.0 | 7.5 |
| IKur-like current blocked APD (ms) | 227 | 227 | 212 | 243 | 228 | 192 |
| % increase to control | 36 | 39 | 43 | 11 | 8 | 28 |
| IKur-like current blocked plateau (mV) | 5.4 | 5.8 | 1.6 | 21.2 | 16.1 | 11.5 |
Abbreviations: APD, action potential duration; IKur, ultra-rapid delayed rectifier current; Ito, transient outward potassium current.
Discussion
We found that a plateau outward current sensitive to 4-AP exists in the majority of left ventricular mid-myocardial canine myocytes. Based on pharmacological response and activation and inactivation properties (Yue et al., 1996; Nerbonne and Kass, 2005), we suggest that the observed 4-AP-sensitive current has canine ‘IKur-like' properties. Furthermore, this current plays a functional role in AP repolarization in normal canine left ventricle mid-myocardial myocytes, as assessed both in vitro and in silico.
IKur has been found in rodent atria and ventricle, and canine and human atria (Wang et al., 1993; Yue et al., 1996). This current has been shown to initially activate in phase 1 of atrial action potential, and blockade of this current results in prolongation of APD50 and APD90 in canine atria (Yue et al., 1996). In addition, guinea-pig ventricular myocytes exhibit a similar outward K+ current which activates at plateau voltages (Yue and Marban, 1988). This current was named IKp due to its unique open probabilities at plateau potentials, with no detectable inactivation. Similarities between IKp and IKur in canine and human atria have been noted (Nerbonne and Kass, 2005).
Low concentrations of 4-AP (50–100 μM) have been previously used to block IKur in canine and human atria, and in rodent ventricle (Brouillette et al., 2004), and the similar sensitivities to micromolar 4-AP concentrations suggests blockade of the same channel subunit (Nerbonne and Kass, 2005). The IC50 for 4-AP on the plateau outward ‘IKur-like' current that we measured from canine left ventricle was 24.2 μM, which is similar to that previously reported for IKur in canine (5.31±0.74 μM) and human (49 μM) atria (Wang et al., 1993; Yue et al., 1996) and in Kv1.5 expression systems (50 μM) (Bouchard and Fedida, 1995). As reported for canine atrial IKur (Yue et al., 1996), we observed that the plateau outward current is rapidly activating, and did not show significant inactivation during the 300 ms test pulse. While previous reports (Amos et al., 1996; Yue et al., 1996) on IKur demonstrate that the current inactivates more rapidly at physiological temperatures (compared to room temperature), there is still sufficient IKur at physiologically relevant temperatures to contribute to action potential repolarization.
Kv1.5, the protein encoding IKur, is present in canine atria and ventricle (Fedida et al., 2003; Szuts et al., 2004). While Kv1.5-encoded current has not been thought to be normally expressed in the ventricles of large mammals, we note one report of a C9356-sensitive current (a Kv1.5 blocker) in ventricular myocytes isolated from a post-infarction canine model (Dun et al., 2004). In this canine post-infarction model, Kv1.5 has been shown to lateralize from the intercalated disks in the peri-infarct region (Mays et al., 1997). Due to the ‘IKur-like' nature of the 4-AP-sensitive plateau outward current observed, we suggest that the 4-AP-sensitive current may be carried by Kv1.5 channels. Further studies are required to address this issue.
A role for IKur in canine and human ventricle?
We tested two different concentrations of 4-AP on APD at 0.5, 1 and 2 Hz. Both 50 and 100 μM 4-AP produced a reverse use-dependent prolongation of APD50 and APD90 consistent with a previous study (Bouchard and Fedida, 1995), where block of Kv1.5 by 4-AP was relieved at faster stimulation rates, which was attributed to rate-dependent relief of closed channel block. In addition, we evaluated the effects of DPO-1, reportedly a use-dependent, selective IKur blocker (Stump et al., 2005; Lagrutta et al., 2006) to provide additional confirmatory evidence, of the existence of an outward plateau (‘IKur-like') current in canine mid-myocardial left ventricular myocytes. We observed similar effects on the APD with both DPO-1 and 4-AP, and these were quantitatively similar at a stimulation frequency of 1 Hz. In contrast to a previous report, we did not observe use-dependent effects of DPO-1 on APD (Lagrutta et al., 2006). The reported use-dependent properties of DPO-1 are in contrast to the known reverse use-dependent effects of 4-AP. Our simulations revealed a similar degree of frequency-independent prolongation of APD90 during IKur-like current block (Table 3). This suggests that it is the block of the outward plateau current by 4-AP which is reverse-use dependent, as has been reported for IKur (Bouchard and Fedida, 1995), rather than the current itself being fundamentally reverse use-dependent.
Our data are in contrast to data obtained in an in vivo study on the effect of IKur blockade on canine ventricular repolarization, where dogs were anaesthetized with pentobarbital (Stump et al., 2005). Pentobarbital is a known blocker of repolarizing currents, and also prolongs canine ventricular APD50 and APD90 at concentrations required for general anaesthesia (Nattel et al., 1990). Thus, any potential effect of IKur blockade in this study of in vivo canine ventricular repolarization may have been obscured by anaesthesia. The in vivo effects of IKur blockers on in vivo canine ventricular repolarization therefore remain poorly defined.
A study in myocytes from failing explanted human hearts (Li et al., 1996b) used 50 μM 4-AP to detect IKur, which could be detected in atrial, but not in right ventricular myocytes. The authors suggested that the absence of IKur in ventricular myocytes may have resulted from limited sampling (n=5 myocytes) or possible differences in regional distribution. A recent report with a mixed IKur and Ito blocker did not find significant effects on the canine right ventricular APD (Schotten et al., 2007); the difference between this report and our observations could potentially result from differences in the selectivity of the drugs, or differences between right and left ventricular electrophysiology (Volders et al., 1999).
DPO-1, is reported to cause rate-dependent blockade of IKur and action potential prolongation in human atrial myocytes, with no action potential prolongation noted in a human ventricular myocyte, obtained from an end-stage heart at the time of transplantation (Lagrutta et al., 2006). However, the source of the ventricular myocyte (right vs left ventricle) was not stated, and the results are again limited by a small sampling of myocytes. In summary, the contribution of IKur to normal human left ventricular repolarization is undefined at the present time. Notably, recent reports confirm the expression of Kv1.5 mRNA in the ventricle of normal, undiseased, human hearts, suggesting a potential role for Kv1.5 encoded currents in the human ventricle (Szuts et al., 2004; Ordog et al., 2006). In both human atria and ventricle, immunohistochemical studies reveal high expression of Kv1.5 which is localized to the intercalated disks, similar to the distribution in canine atrial myocytes (Mays et al., 1995; Fedida et al., 2003).
4-Aminopyridine and blockade of other K+ currents
Human ether-a-go-go gene current and canine ventricular Ito are blocked by 4-AP, with an IC50 of 4.4 and 1.15 mM respectively (Nattel et al., 2000; Ridley et al., 2003). We found no inhibition of either Ito or IKr at the 4-AP concentrations used in our experiments. Our Ito measurements are consistent with a previous report (Nattel et al., 2000), where a concentration of 50 μM 4-AP only blocked ∼1% of ventricular Ito (Nattel et al., 2000). The following lines of evidence: (1) the previously reported lack of significant inhibition of ventricular Ito with concentrations up to 200 μM 4-AP (Nattel et al., 2000); (2) the characteristics of the 4-AP-sensitive current we recorded; (3) the use of Ito inactivating prepulses and (4) the absence of Ito inhibition with 100 μM 4-AP in our myocytes, all suggest that the 4-AP-sensitive current that we report was not contaminated by Ito. Similarly, the results of the envelope-of-tails test and the absence of 4-AP block of IKr both suggest that the 4-AP-sensitive plateau current was not contaminated by IKr.
β-Adrenergic modulation of ‘IKur-like' current
β-Adrenergic modulation of IKur has been previously reported in human atrial myocytes (Li et al., 1996a). Kv1.5 has been shown to interact with the accessory channel subunits, Kvβ1.2 and Kvβ1.3, which alter the adrenergic responsiveness of the channel (Kwak et al., 1999). Using the β-adrenergic agonist, isoprenaline, we found that the ventricular 4-AP-sensitive plateau current was also modulated by β-adrenergic stimulation. These results suggest a possible role for the current in modulating ventricular repolarization during sympathetic stimulation.
Limitations
We did not examine the presence of this current in either right ventricular or atrial myocytes, and our measurements were confined to one region of the left ventricle myocardium. The purpose of the current study was to establish the presence and functional role of this ‘IKur-like' current in the canine left ventricle, and thus we did not examine myocytes from other regions in these experiments.
The model used in the in silico experiments is based on experimental data derived from canine right and left endocardial tissues (Koller et al., 1998), and therefore the model does not emulate the action potentials from the left ventricular lateral mid-myocardium. The modelling results are therefore more qualitative than quantitative and serve to confirm that block of a small amplitude plateau current increases action potential plateau voltage and prolongs the APD.
The length of our recorded action potentials is longer than that previously reported in canine ventricular muscle (Jost et al., 2005). One possible explanation for this difference is the use of isolated myocytes, rather than multi-cellular preparations, since the APD is modulated by intercellular coupling (Zaniboni et al., 2000). Alternatively, the APD may vary with site of origin of the myocytes (Litovsky and Antzelevitch, 1989). We isolated myocytes from the left lateral mid-myocardium of the left ventricle-free wall, and previous reports have primarily focused on myocytes of anterior left ventricle origin (Kaab et al., 1996; Volders et al., 1999). Notably, while our control APD90 values are longer than those in some previous reports, they are shorter than control values previously published in isolated canine myocytes (Kaab et al., 1996). Lastly, there is the possibility of alterations in APD due to the myocyte isolation procedure, which cannot be excluded in our experiments.
We did not examine the effects of 4-AP on IKs. However, in control myocytes (in the absence of β-adrenergic agonists), there is no contribution of IKs to repolarization of canine or human ventricle (Varro et al., 2000; Stengl et al., 2003; Volders et al., 2003; Jost et al., 2005). Our experimental protocols (250–300 ms test pulses) were designed to minimize the role of IKs during the current (Jost et al., 2005). Furthermore, we did not observe any time-dependent increases in the amplitude of the rapidly activating, 4-AP-sensitive current, as would be expected if IKs were present.
In summary, our study demonstrates the presence of a plateau outward current in canine ventricle, which has several ‘IKur-like' properties. This current activates rapidly at depolarized potentials, and block of the current causes prolongation of both APD50 and APD90. In addition, the β-adrenergic-mediated increase in current density suggests a potential role in rate adaptation of the APD during periods of increased sympathetic stimulation. Our findings suggest that IKur blockers, such as DPO-1, may affect ventricular as well as atrial repolarization. This may have implications for the use of IKur blockers for the treatment of atrial tachyarrhythmias.
Acknowledgments
We thank Drs Sandor Györke and Andriy Belevych for their careful review of the manuscript. This work was supported in part by NIH grant R01HL075515 (JJF).
Abbreviations
- APD
action potential duration
- 4-AP
4-aminopyridine
- DPO-1
(2-isopropyl-5-methylcyclohexyl diphenylphosphine oxide
- IKr
rapid delayed rectifier K+ current
- IKs
slow delayed rectifier current
- IKur
ultra-rapid delayed rectifier current
- Ito
transient outward potassium current
Conflict of interest
The authors state no conflict of interest.
References
- Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491 Part 1:31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard R, Fedida D. Closed- and open-state binding of 4-aminopyridine to the cloned human potassium channel Kv1.5. J Pharmacol Exp Ther. 1995;275:864–876. [PubMed] [Google Scholar]
- Brouillette J, Clark RB, Giles WR, Fiset C. Functional properties of K+ currents in adult mouse ventricular myocytes. J Physiol. 2004;559:777–798. doi: 10.1113/jphysiol.2004.063446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Hindmarsh AC. CVODE User Guide 1994. LLNL Report UCRL-MA-118618
- Dun W, Baba S, Yagi T, Boyden PA. Dynamic remodeling of K+ and Ca2+ currents in cells that survived in the epicardial border zone of canine healed infarcted heart. Am J Physiol Heart Circ Physiol. 2004;287:H1046–H1054. doi: 10.1152/ajpheart.00082.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, et al. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 2003;93:744–751. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- Fox JJ, McHarg JL, Gilmour RF., Jr Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002;282:H516–H530. doi: 10.1152/ajpheart.00612.2001. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Characterization and functional consequences of delayed rectifier current transient in ventricular repolarization. Am J Physiol Heart Circ Physiol. 2000;278:H806–H817. doi: 10.1152/ajpheart.2000.278.3.H806. [DOI] [PubMed] [Google Scholar]
- Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110:3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- Kaab S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, et al. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YG, Hu N, Wei J, George AL, Jr, Grobaski TD, Tamkun MM, et al. Protein kinase A phosphorylation alters Kvbeta1.3 subunit-mediated inactivation of the Kv1.5 potassium channel. J Biol Chem. 1999;274:13928–13932. doi: 10.1074/jbc.274.20.13928. [DOI] [PubMed] [Google Scholar]
- Lagrutta A, Wang J, Fermini B, Salata JJ. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 2006;317:1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res. 1996a;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996b;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Litovsky SH, Antzelevitch C. Rate dependence of action potential duration and refractoriness in canine ventricular endocardium differs from that of epicardium: role of the transient outward current. J Am Coll Cardiol. 1989;14:1053–1066. doi: 10.1016/0735-1097(89)90490-7. [DOI] [PubMed] [Google Scholar]
- Marquardt D. An algorithm for least-squares estimation of nonlinear parameters. Siam J Appl Math. 1963;11:431–441. [Google Scholar]
- Mays DJ, Boyden PA, Tamkun MM. Redistribution of the Kv1.5 K+ channel protein on the surface of myocytes from the epicardial border zone of the infarcted canine heart. CV PathoBiol. 1997;2:79–87. [Google Scholar]
- Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattel S, Matthews C, De Blasio E, Han W, Li D, Yue L. Dose-dependence of 4-aminopyridine plasma concentrations and electrophysiological effects in dogs: potential relevance to ionic mechanisms in vivo. Circulation. 2000;101:1179–1184. doi: 10.1161/01.cir.101.10.1179. [DOI] [PubMed] [Google Scholar]
- Nattel S, Wang ZG, Matthews C. Direct electrophysiological actions of pentobarbital at concentrations achieved during general anesthesia. Am J Physiol. 1990;259:H1743–H1751. doi: 10.1152/ajpheart.1990.259.6.H1743. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- Ordog B, Brutyo E, Puskas LG, Papp JG, Varro A, Szabad J, et al. Gene expression profiling of human cardiac potassium and sodium channels. Int J Cardiol. 2006;28:386–393. doi: 10.1016/j.ijcard.2005.07.063. [DOI] [PubMed] [Google Scholar]
- Page RL, Roden DM. Drug therapy for atrial fibrillation: where do we go from here. Nat Rev Drug Discov. 2005;4:899–910. doi: 10.1038/nrd1876. [DOI] [PubMed] [Google Scholar]
- Patel SP, Campbell DL. Transient outward potassium current, ‘Ito', phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol. 2005;569:7–39. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C 1992Cambridge University Press: Cambridge, UK; 2nd edn, [Google Scholar]
- Ridley JM, Milnes JT, Zhang YH, Witchel HJ, Hancox JC. Inhibition of HERG K+ current and prolongation of the guinea-pig ventricular action potential by 4-aminopyridine. J Physiol. 2003;549:667–672. doi: 10.1113/jphysiol.2003.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotten U, de Haan S, Verheule S, Harks EG, Frechen D, Bodewig E, et al. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc Res. 2007;73:37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Stengl M, Volders PG, Thomsen MB, Spatjens RL, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol. 2003;551:777–786. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storn R. Biennial Conference of the North American Fuzzy Information Processing Society. North American Fuzzy Information Processing Society: Berkeley, CA, USA; 1996. On the usage of differential evolution for function optimization. [Google Scholar]
- Stump GL, Wallace AA, Regan CP, Lynch JJ., Jr In vivo antiarrhythmic and cardiac electrophysiologic effects of a novel diphenylphosphine oxide IKur blocker (2-isopropyl-5-methylcyclohexyl) diphenylphosphine oxide. J Pharmacol Exp Ther. 2005;315:1362–1367. doi: 10.1124/jpet.105.092197. [DOI] [PubMed] [Google Scholar]
- Szuts V, Ordog B, Acsai K, Horvath Z, Virag L, Seprenyi G, et al. Kv1.5 channels in ventricular preparations (abstract) J Mol Cell Cardiol. 2004;36:762. [Google Scholar]
- Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, et al. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol. 2000;523 Part 1:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99:206–210. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Yue DT, Marban E. A novel cardiac potassium channel that is active and conductive at depolarized potentials. Pflugers Arch. 1988;413:127–133. doi: 10.1007/BF00582522. [DOI] [PubMed] [Google Scholar]
- Yue L, Feng J, Li GR, Nattel S. Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization. J Physiol. 1996;496 Part 3:647–662. doi: 10.1113/jphysiol.1996.sp021716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Feng J, Wang Z, Nattel S. Adrenergic control of the ultrarapid delayed rectifier current in canine atrial myocytes. J Physiol. 1999;516 Part 2:385–398. doi: 10.1111/j.1469-7793.1999.0385v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniboni M, Pollard AE, Yang L, Spitzer KW. Beat-to-beat repolarization variability in ventricular myocytes and its suppression by electrical coupling. Am J Physiol Heart Circ Physiol. 2000;278:H677–H687. doi: 10.1152/ajpheart.2000.278.3.H677. [DOI] [PubMed] [Google Scholar]