Abstract
It was investigated whether an observer would simulate another person's inhibitory and error processes. Two participants sitting next to each other performed a stop signal task in which they occasionally had to try and inhibit their response when indicated to do so by a stop signal. They could either successfully stop the response or fail to stop and, thereby, make an error. An aftereffect of the other person's successful action inhibition and error was obtained: The participants became slower and more accurate when they observed the other person make an error on the previous trial and when they observed a successful stop. The results suggest that observing another person successfully inhibit an action or make an error evokes processes similar to those that occur when these behaviors are produced.
For successful social interactions, people need accurate information about the internal states that drive other people's behavior. It has been suggested that such knowledge can be gained by simulating the processes that occur in another person's brain (Blakemore & Decety, 2001; Frith & Frith, 1999; Gallese & Goldman, 1998; Jeannerod, 2001). The general idea is that the observation of another person's behavior induces internal states in the observer that are similar to those that would occur if the observer undertook the action him- or herself.
So far, evidence for such a simulation mechanism has been found in the domain of visuomotor processes. In monkeys, the visuomotor circuits involved in producing actions have been shown to also be activated when another individual is observed performing the same action (e.g., Rizzolatti, Fogassi, & Gallese, 2001). Single-unit studies in monkeys have shown that cells in inferior frontal region F5 that encode specific grasping actions are activated when the monkey merely observes another individual making the same grasping action (di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992). As for humans, there is evidence from electrophysiological studies (e.g., Kilner, Vargas, Duval, Blakemore, & Sirigu, 2004), transcranial magnetic stimulation studies (e.g., Fadiga, Fogassi, Pavesi, & Rizzolatti, 1995), and functional magnetic resonance imaging (f MRI) studies (e.g., Grèzes, Armony, Rowe, & Passingham, 2003) that the same visuomotor circuits that are involved in producing actions are also activated when these same actions are observed in another human being. On the behavioral level, it has been reported that people execute actions more easily and fluently when simultaneously looking at another person who is performing the same action (e.g., Bach & Tipper, 2007; Brass, Bekkering, Wohlschläger, & Prinz, 2000; Castiello, 2003; Kilner, Paulignan, & Blakemore, 2003; for an overview, see Wilson & Knoblich, 2005).
Simulation of Higher Level Cognitive Processes
The finding of simulation of visuomotor processes raises the question as to whether other cognitive processes are also simulated. Simulation of another person's brain processes might be a general mechanism in which higher level systems, which control visuomotor processes, are being simulated as well. Indeed, there is recent evidence from electrophysiological studies that other people's errors are processed in a similar way as our own errors. In particular, van Schie, Mars, Coles, and Bekkering (2004) and Bates, Patel, and Liddle (2005) reported that the error-related negativity (ERN) signal observed in frontal regions when people make an error was also found when people observed someone else's error. It remains to be shown whether the observation of another person's error also influences the observer's overt behavior. In the present study, we had people observe simple visual–motor behavior (i.e., another person reaching toward and depressing a key) and measured the observer's overt subsequent behavior. We investigated two situations in which the identical action was observed. In one situation, performance was correct; in the other, an error had been made. Thus, the two situations differed only with respect to the meaning of the observed action. If any differences were found in the observer's subsequent behavior, this would mean that the simulation of error processing went beyond simulations of just the action observed and that it represented the cognitive processes associated with the action.
Given the possibility that error processing is simulated, the question arises as to whether other higher level control processes might be simulated as well. One important group of control mechanisms are inhibitory processes (see Houghton & Tipper, 1996; Tipper, 1985). Inhibitory control in general provides the basis for goal-directed behavior, such as responding to the appropriate object at the appropriate moment in time or disengaging from a current task and switching to another task. If simulation is a ubiquitous basic mechanism for social interaction, one would expect that such inhibitory processes are simulated as well. One kind of inhibition that is easy to observe in another person is the stopping of an ongoing action. The inhibitory processes involved in the stopping of actions have been studied extensively (e.g., De Jong, Coles, Logan, & Gratton, 1990; Logan & Cowan, 1984; for a review, see Logan, 1994). In the present study, we investigated whether similar inhibitory processes are evoked in an observer when he or she is watching another person stopping an action.
The Present Paradigm
We investigated the processes involved in observing another person when this person is stopping an action and when this person is making an error. To this end, we applied the stop signal procedure (Logan, 1994; Logan & Cowan, 1984), which allows investigation of both inhibition processes and error processes (see van Boxtel, van der Molen, & Jennings, 2005). In the stop signal paradigm, participants are instructed to respond to a target as quickly as possible but to stop the ongoing response when a stop signal is presented shortly after the target. The stop signal occurs at a variable delay, which is adjusted in such a way that the participants manage to stop the response on some trials (stop trials) but fail to stop on other trials (error trials). As a measure of inhibition, we analyzed the performance on the trials following a stop trial. It has been found in previous studies that participants are slower after a stop trial than after a go trial, reflecting an aftereffect of the inhibition that was applied on the previous trial (Rieger & Gauggel, 1999; see also Ivanoff & Klein, 2001; Taylor & Ivanoff, 2003). Likewise, participants are slower and more accurate after an error trial (when they failed to stop) than after a go trial, reflecting an aftereffect of the error (Rabbitt, 1966; Rabbitt & Rogers, 1977; Schachar et al., 2004; see Botvinick, Braver, Barch, Carter, & Cohen, 2001, for a theoretical model of error-related effects).
In the present study we assessed the aftereffects in a stop signal paradigm in which two persons alternated their responding. The question was whether one person would be slowed after having observed the other person stopping or after having observed the other person making an error. Such findings would provide, first, evidence for the simulation of inhibition and, further, behavioral evidence for the simulation of error processing.
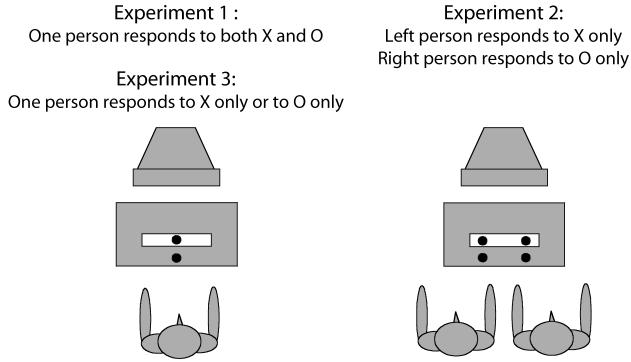
We adapted the standard stop signal paradigm by using two alternating go signals, each of which would turn into a stop signal on a random 50% of the trials (see Figure 1). Experiment 1 served as a pilot experiment to establish the basic effects. One person responded to both go signals. In Experiment 2, two persons performed the experiment together, and the go signals indicated whose turn it was to respond. Thus, one person responded to the X, and the other person responded to the O. Experiment 3 served as a control experiment in which one person responded to the X only or to the O only, without another person responding to the other stimulus (see Figure 2).
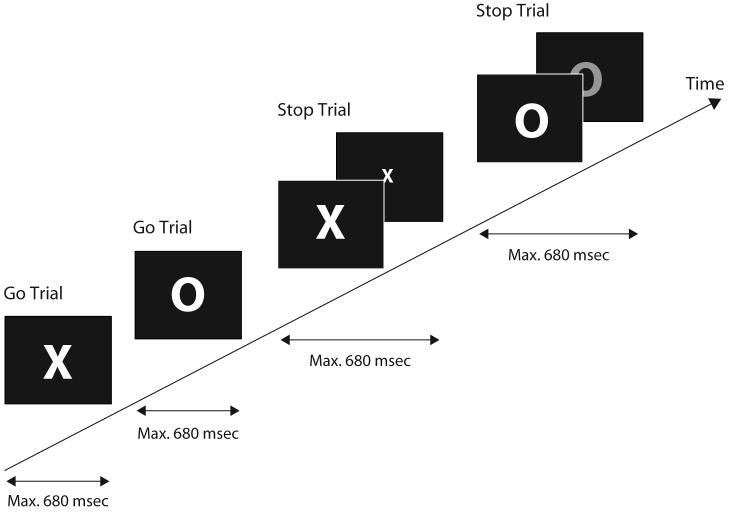
Figure 1.
Experimental procedure. Shown is an example sequence of four trials: go–go–stop–stop. The stimuli X and O were alternating. The stop signal (indicated by the X's becoming smaller or the O's changing color) occurred in a random 50% of the trials, equally often for X and O. The stop signal delays were adjusted adaptively on a trial-by-trial basis. The mean interval between one stimulus and the next was 3,600 msec, varying randomly between 3,400 and 3,600 msec (not shown in the figure).
Figure 2.
Schematic overview of the experiments.
EXPERIMENT 1
In Experiment 1, we expected to find the same aftereffects as those reported in previous stop signal studies (Rieger & Gauggel, 1999; Schachar et al., 2004). That is, we expected that reaction time (RT) would be longer and that the probability of stopping a response would be higher after an action had been stopped on the previous trial. These effects are thought to reflect residual inhibition. Moreover, we expected a slowing of RT and increased ability to prevent a response after an error (i.e., failure to stop a response in the previous trial), in line with previous findings of more accurate performance after errors (Botvinick et al., 2001; Rabbitt, 1966; Rabbitt & Rodgers, 1977). These aftereffects of one's own stopping and of one's own errors in Experiment 1 would serve to demonstrate the effects that take place within an individual brain. The critical question, of course, is to examine whether similar effects are produced when merely observing another person successfully inhibit an action or make an error in Experiment 2.
Method
Participants
Thirty-two undergraduate students from the School of Psychology at the University of Wales, Bangor, participated in the experiment (23 of them female, 9 male; mean age, 19.1 years) and received course credit in exchange. All were naive with respect to the purpose of the experiment. The data of 2 participants were excluded from the analysis because these participants did not follow the instructions to try and stop their response on stop signal trials, resulting in an insufficient number of trials for statistical analysis.
Stimuli and Responses
A white X and a blue O served as go stimuli. They were approximately 4 cm in height and 2 cm in width and appeared centrally on the screen. All the stimuli were presented on a black background. On 50% of the trials, the stimuli changed after a variable delay. The X shrank to approximately 75% of its original size; the O changed its color from blue to green, indicating that the response should be stopped.
Responses were made by pressing the space bar of the keyboard with the index finger of the preferred hand. There was a target pad fixed to the middle of the space bar indicating the location at which the space bar had to be pressed. A starting pad was fixed to the lower part of the keyboard, 5 cm in front of the target pad, indicating where the finger had to be rested before and after responding. For responding, the participants had to move their finger from the starting pad to the target pad, press the space bar, and move it back to the starting pad.
Design
Performance was assessed as a function of the previous trial. The previous trial was a go trial, a successful stop trial, or an error trial (i.e., a failed stop trial). The inhibitory aftereffect was computed as the difference between trials preceded by a go trial and trials preceded by a successful stop trial. The error aftereffect was computed as the difference between trials preceded by a go trial and trials preceded by a failed stop trial.
Three dependent variables were assessed. RT was measured as the time between the onset of the go stimulus and the response on go trials. The participants were required to respond within the 680 msec following the onset of the go stimulus; after that, an error message would appear on the screen. The percentage of go trials on which the participants did not respond within the given time window was assessed as another dependent variable (too long trials; TLs). Finally, stopping performance (SP) was measured as the percentage of successfully stopped trials among all stop trials.
Procedure
The X and the O alternated. A random 50% of the trials were stop trials. The requirement to stop was indicated by the X's becoming smaller or by the O's becoming green after a certain delay. The stop signal delay (SSD) was adjusted continuously so that there were roughly equal numbers of successful and failed stop trials for every participant. Two separate SSDs were used for the X and the O. Both SSDs were initially set to 340 msec and always stayed in the range of 0–500 msec. The SSDs were altered in steps of 17 msec. After a successful stop trial, the respective SSD was increased by one step. After a failed stop trial, the SSD was decreased by two steps.
The stimuli were presented for a maximum of 680 msec on both go trials and stop trials. On go trials, the go stimulus appeared on the screen for 680 msec at maximum or until a response was made. On stop trials, the go stimulus appeared for the duration of the SSD; then the respective stop stimulus appeared for (680 – SSD) msec at maximum or until a response was made. If a response occurred, stimulus presentation was aborted immediately on both go trials and stop trials. If no response had occurred within 680 msec on go trials, a message appeared on the screen for 500 msec saying “too late.” This was done to keep the participants from adopting a waiting strategy in which they would defer their responses until sure that no stop signal would occur. The interstimulus interval was random, so that the onset of the next stimulus was not predictable. This served to prevent premature responding. Since the task was a simple RT task, the participants could have started to prepare their response in advance if they had been able to predict the onset of the next stimulus. The mean interval between one stimulus and the next was 3,600 msec (ranging between 3,400 and 3,800 msec).
The experiment consisted of six blocks of 80 trials each. After each block, the participants were encouraged to take a short rest. Before the first block, the participants performed a practice block of 20 trials.
Instructions
The participants were instructed to rest the index finger of the preferred hand on the starting pad and, if an X or an O occurred, to respond as quickly as possible by pressing the target key. They were asked to try to stop their response if the X became smaller or the O changed its color. They were also told that the task was difficult and that they would not always be able to stop their response when required but that they should try to stop as often as possible.
Results
Data Analysis
In all the analyses, significance was tested at an alpha level of .05. An ANOVA was conducted. If the assumption of sphericity was violated, degrees of freedom (dfs) were computed according to the Huynh–Feldt epsilon. For the sake of simplicity, the uncorrected dfs are reported, together with the epsilon values.
The data were filtered in the following way. In all the analyses, the first and second trials of each block were excluded. For analysis of RTs, only the go trials were included. The go trials on which the participants did not respond within the given time window were excluded, as well as the following trial. Furthermore, all trials with an RT of less than 200 msec and the following trial were excluded. It was assumed that when the participants responded within 200 msec, they had started to respond before the go stimulus occurred. On the remaining trials, RT was computed as the time between the onset of the go stimulus and the response.
The proportion of TL trials was computed as the percentage of go trials not responded to within the given time window among all go trials. For analysis of SP, only the stop trials were included in the analysis. SP was computed as the proportion of stop signal trials on which the response was successfully stopped. The analyzed data are shown in Table 1.
Table 1.
Experiment 1: One Person Responding to Both X and O
| Previous Trial (Performed by Same Person) |
Difference Score |
|||||||
|---|---|---|---|---|---|---|---|---|
| Go |
Stop |
Error |
Inhibition Aftereffect (Stop – Go) |
Error Aftereffect (Error – Go) |
||||
| M | SD | M | SD | M | SD | |||
| RT | 510 | 69 | 545 | 45 | 537 | 59 | 35** | 27** |
| TL | 19.3 | 8.9 | 24.1 | 9.8 | 30.6 | 12.9 | 4.8** | 11.3** |
| SP | 51.5 | 21.1 | 64.0 | 13.8 | 66.2 | 17.0 | 12.5** | 14.7** |
Note—Means (with standard deviations) and difference scores are shown for reaction times (RTs, in milliseconds), percentages of too long trials (TLs), and percentages of successful stops (stopping performance, SP). Preplanned contrasts in ANOVA:
p < .05.
In all the analyses, the trials were sorted according to the nature of the previous trial. The previous trial could be a go trial, a stop trial (i.e., a stop signal trial on which the response was successfully stopped), or an error trial (i.e., a stop signal trial on which a response occurred). For each dependent variable, a 1 × 3 ANOVA was computed with the within-subjects independent variable of previous trial (go, inhibition, or error). If the main effect was significant, preplanned contrasts were computed. The inhibitory aftereffect was computed as the difference between trials preceded by a stop trial and trials preceded by a go trial. The error aftereffect was computed as the difference between trials preceded by an error trial and trials preceded by a go trial.
Reaction Time
The ANOVA yielded a significant main effect of the previous trial [F(2,58) = 19.2, p < .01, ε = .85]. The preplanned contrasts revealed both a significant inhibitory aftereffect [responses were 35 msec slower after stop than after go; F(1,29) = 23.4, p < .01] and a significant error aftereffect [responses were 27 msec slower after error than after go; F(1,29) = 33.0, p < .01].
Too Long Trials
There was a significant main effect of the previous trial in the ANOVA [F(2,58) = 19.3, p < .01, ε = .98]. The preplanned contrasts revealed both a significant inhibitory aftereffect [4.7% higher proportion of TL trials after stop than after go; F(1,29) = 6.3, p < .02] and a significant error aftereffect [11.2% higher proportion of TL trials after error than after go; F(1,29) = 52.7, p < .01].
Stopping Performance
Again, the ANOVA revealed a significant main effect of the previous trial [F(2,58) = 16.7, p < .01, ε = .96]. The preplanned contrasts showed both a significant inhibitory aftereffect [12.5% higher proportion of successful stopping after stop than after go; F(1,29) = 15.7, p < .01] and a significant error aftereffect [14.8% higher proportion of successful stopping after error than after go; F(1,29) = 37.5, p < .01].
Thus, the aftereffects of stopping an action and of making an error that have been reported in previous studies were replicated with the present paradigm. Extending previous studies, the effects were found in three dependent variables: RTs, proportions of TL trials, and SP.
EXPERIMENT 2
Experiment 2 served to explore whether aftereffects of inhibition and of error similar to those in Experiment 1 would be obtained when the participants were watching another person stopping an action or performing an error. The same experimental procedure as that in Experiment 1 was applied, but two persons alternated in responding. One responded to the X, the other to the O. The two persons were sitting next to each other in front of the same computer screen and used the same key (space bar) for responding.
Method
Participants
Thirty-two participants were tested (24 of them female, 8 male; mean age, 20.1 years). They were randomly coupled in pairs.
Stimuli and Responses
The stimuli and responses were the same as those in Experiment 1. The X indicated that it was the left person's turn; the O indicated that it was the right person's turn to respond. Two target pads were attached to the space bar, one at the left end and one at the right end. Two starting pads were attached to the keyboard 5 cm in front of each target pad.
Design and Procedure
The design and procedure were the same as those Experiment 1. As before, performance was assessed as a function of the previous trial (go, stop, or error). However, in Experiment 2, the previous trial was performed by the other person.
Results
The data were filtered in the same way as in Experiment 1; they are reported in Table 2A. The same ANOVAs and preplanned contrasts were conducted as before.
Table 2A.
Experiment 2: Two Participants, One Responding to X, the Other Responding to O
| Previous Trial (Performed by Other Person) |
Difference Score |
|||||||
|---|---|---|---|---|---|---|---|---|
| Go |
Stop |
Error |
Inhibition Aftereffect (Stop – Go) |
Error Aftereffect (Error – Go) |
||||
| M | SD | M | SD | M | SD | |||
| RT | 534 | 52 | 533 | 57 | 541 | 49 | −1 | 7* |
| TL | 22.6 | 8.8 | 28.4 | 12.7 | 26.3 | 13.0 | 5.8** | 3.7** |
| SP | 53.4 | 19.5 | 55.0 | 22.2 | 58.3 | 20.7 | 1.6 | 4.9** |
Note—Means (with standard deviations) and difference scores are shown for reaction times (RTs), percentages of too long trials (TL), and percentages of successful stops (stopping performance, SP). Preplanned contrasts in ANOVA:
p < .10.
p < .05.
Reaction Time
No significant results were obtained in the RT data. There was only a tendency for a main effect of previous trial in the ANOVA [F(2,62) = 1.9, p < .16, ε = .99]. The preplanned contrasts revealed no inhibitory aftereffect [F(1,31) < 1] but a tendency for an error aftereffect [F(1,31) = 3.2, p < .09], indicating that participants responded more slowly after the other person had made an error than after the other person had performed a go trial.
Too Long Trials
The data of 2 participants had to be excluded due to an insufficient number of trials (i.e., fewer than 10 trials) in one of the conditions. There was a significant main effect of previous trial in the ANOVA [F(2,58) = 5.5, p < .01, ε = .93]. The preplanned contrasts revealed a significant inhibition aftereffect: There were more TL trials when the other person had successfully stopped in the previous trial than when he/she had performed a go trial [F(1,29) = 13.3, p < .01]. Moreover, a significant error aftereffect was obtained: There were more TL trials after the other person had performed an error as opposed to a go trial [F(1,29) = 5.6, p < .03].
Stopping Performance
The ANOVA on the SP data yielded a marginally significant main effect of previous trial [F(2,62) = 2.8, p < .08, ε = .92]. The preplanned contrast showed no significant inhibition aftereffect [F(1,31) < 1]. However, there was a significant error aftereffect [F(1,31) = 8.4, p < .01], indicating that the participants were better at stopping after the other person had made an error than after the other person had performed a go trial.
To summarize, we found significant aftereffects of the other person's performance. In particular, the participants were more likely not to respond within the given response window on go trials when the other person had stopped or performed an error on the previous trial, as compared with when the other person had performed a go trial before. There were no such differences in RTs, presumably because the time window for responding was very strict, with the participants having to respond within 680 msec after stimulus onset. Regarding the SP, again an error aftereffect was found (but no inhibitory aftereffect): The participants were more likely to successfully stop their response after the other person had made an error than after the other person had performed a go trial.
Controlling for Aftereffects of the Participant's Own Previous Performance
An additional analysis was conducted to ensure that the observed aftereffects were indeed due to the other person's previous performance (on trial n−1), and not to the participants' own previous performance (on trial n−2). To this end, only those trials were included in the analysis on which the participants had performed a go trial on trial n−2 (and had responded within the time window). In other words, the participants' own prior errors and prior successful stops were removed from the data. Table 2B shows the results of this additional analysis.
Table 2B.
Additional Analysis of Experiment 2 Considering Only Those Trials That Were Preceded by a Valid Go Trial on n−2
| Previous Trial (Performed by Other Person) |
Difference Score |
|||||||
|---|---|---|---|---|---|---|---|---|
| Go |
Stop |
Error |
Inhibition Aftereffect (Stop – Go) |
Error Aftereffect (Error – Go) |
||||
| M | SD | M | SD | M | SD | |||
| RT | 493 | 79 | 522 | 96 | 508 | 76 | 29* | 15** |
| TL | 15.9 | 12.4 | 23.9 | 15.6 | 14.6 | 9.9 | 8.0* | −1.3 |
| SP | 36.5 | 19.8 | 42.9 | 25.4 | 48.6 | 17.9 | 6.4 | 12.1** |
Note—RT, reaction time in milliseconds; TL, too long trials in percentages; SP, stopping performance in percentages. Preplanned contrasts in ANOVA:
p < .10
p < .05
The same analyses were conducted as before; that is, 1 × 3 ANOVAs with the independent variable of previous trial (go, stop, or error) and preplanned contrasts were computed for RT, TL, and SP. Because this was a post hoc analysis, there were not many trials to use in this analysis. Only the data of those participants were included that had at least 10 trials in each of the three conditions of the design (i.e., previous trial being go, stop, or error). For RT analysis, only 5 of the 32 participants could be included. For TL analysis, 11 participants, and for SP analysis, 8 participants were included.
RT
The ANOVA yielded a marginally significant main effect of previous trial [F(2,8) = 4.6, p < .09, ε = .60]. The preplanned contrasts revealed a marginally significant main effect of inhibition [F(1,4) = 7.1, p < .06] and a significant main effect of error [F(1,4) = 22.0, p < .01].
TL
Again, the main effect in the ANOVA was marginally significant [F(2,20) = 2.9, p < .08, ε = .98]. The preplanned contrasts showed a tendency for an aftereffect of inhibition [F(1,10) = 3.3, p < .10] and no aftereffect of error [F(1,10) < 1].
SP
The ANOVA yielded a significant main effect of previous trial [F(2,14) = 3.8, p < .05, ε = 1.0]. In the preplanned contrasts, the aftereffect of inhibition did not reach significance [F(1,7) = 2.0, p < .21]. The aftereffect of error was significant [F(1,7) = 12.8, p < .01].
Note that there was very little power in this post hoc analysis (e.g., there were only 5 participants in the RT analysis); hence, some contrasts did not attain standard significance levels. However, note that in all but one contrast, the numerical size of the effects became larger when controlling for the effects of the participants' own previous errors and inhibition of action.
In summary, even though there were few participants available for this post hoc analysis and, hence, very limited power, nevertheless the data generally support the previous pattern of results. That is, the participants tended to be slower in responding (i.e., longer RT and higher percentage of TL trials) after observing the other person's stop trials than after the other person's go trials. Moreover, the participants were slower and more accurate after observing the other person's errors. These effects could not be due to the participants' own previous performance, because all trials preceded by a stop or error on trial n−2 had been excluded from the analysis. Thus, the obtained effects are indeed solely due to the observation of the other person's previous performance and are not simply due to the participants' own previous performance.
Comparing the Aftereffects of the Participant's Own and the Other Person's Previous Performance
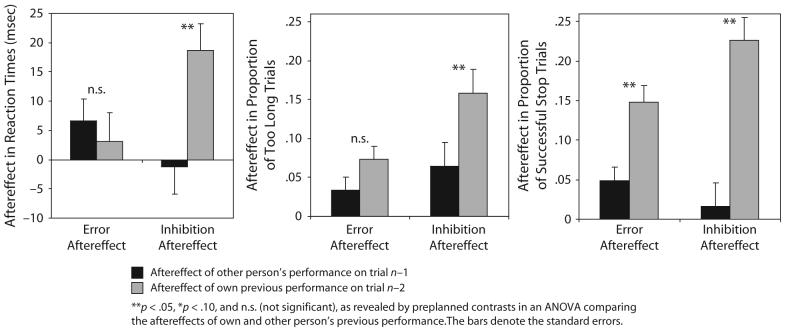
Finally, we directly compared the aftereffects of the other person's performance (on trial n−1) with the aftereffects of the participant's own last performance (on trial n−2). The latter were computed by considering only the nature of trial n−2 (error, stop, or go trial), regardless of the other person's performance on trial n−1. Figure 3 shows the data pattern.
Figure 3.
Experiment 2: Comparison of the aftereffects of the other person's performance and the aftereffects of the participant's own previous performance.
Statistical analysis revealed a significant interaction between the stopping and the error aftereffects of the participant's own versus the other person's previous performance: The difference scores (i.e., the difference between the error and the go conditions for the error aftereffect and the difference between the stopping and the go conditions for the stopping aftereffect, respectively) were subjected to a 2 × 2 ANOVA with the independent variables of aftereffect (stopping vs. error aftereffect) and person (own vs. other person's previous performance) and preplanned contrasts. The ANOVA on the RT data showed a significant interaction [F(1,31) = 8.5, p < .01], indicating that the participant's own previous stopping produced a larger effect than did the other person's previous stopping [F(1,31) = 10.2, p < .01], whereas the aftereffects of the participant's own and the other's previous errors did not differ statistically [F(1,31) < 1]. Moreover, there was a marginally significant main effect of person in the ANOVA [F(1,31) = 3.5, p < .08], indicating that the participant's own previous performance tended to have a larger effect than did the other person's previous performance.
For the analysis of TL data, 5 of the 32 participants had to be excluded because there were fewer than 10 trials in one of the conditions. The interaction did not reach significance [F(1,26) = 2.4, p < .14]. For illustrational purposes, the preplanned contrasts were still conducted. The stopping aftereffect differed between the participant's own and the other person's [F(1,26) = 8.1, p < .01]; the difference in the error aftereffect failed to reach significance [F(1,26) = 2.5, p < .13], essentially replicating the RT data. The ANOVA also revealed a significant main effect of person [F(1,26) = 8.1, p < .01], again indicating that the participant's own previous performance tended to have a larger effect than did the other person's previous performance. Moreover, there was a significant main effect of aftereffect [F(1,26) = 6.8, p < .02], showing that the stopping aftereffect was larger than the error aftereffect in the TL data. The latter result again reflects clear differences between inhibition and error effects.
The SP data showed a significant interaction [F(1,31) = 9.1, p < .01]. The preplanned contrasts showed that both the stopping aftereffect and the error aftereffect of the participant's own previous performance were larger than the effects of the other person's previous performance [F(1,31) = 27.0, p < .01, for the stopping aftereffect; F(1,31) = 13.6, p < .01, for the error aftereffect].
To summarize, the participants showed strong aftereffects of their own previous performance, even though they observed another person undertaking the task before they again responded. In particular, the aftereffects of self-produced inhibition lasted for several seconds (the mean interval was 7.2 sec in the present study) and survived the intervening event of observing another person's action. Most remarkably, there was a dissociation between the effects produced by the participant's own previous behavior (on trial n−2) and that produced by observing another person's behavior (on trial n−1). We will get back to this interesting result in the General Discussion section.
EXPERIMENT 3
Another experiment was undertaken to confirm that the effects of observing another person inhibit an action are produced by the action observation and not by the visual stimuli. That is, it might be the case that the sudden change in the stimulus that signals inhibition of action (the blue O turning green or the X getting smaller) causes slower and more accurate responses on the next trial independently of what another person might be doing. Therefore, we reran Experiment 2. However, in this case, the participants performed the task alone. Thus, they were required to respond to only one of the stimuli and to ignore the other, irrelevant stimulus. Half of the participants responded to the O throughout the experiment and ignored the X; the other half responded to the X and ignored the O.
Method
Another 32 participants were tested. The design and procedure were the same as those in the previous experiments.
Results
The data were analyzed according to whether a go signal or a stop signal had been presented on the previous trial. Table 3A shows the results.
Table 3A.
Experiment 3: One Person Responding to X Only (or O Only)
| Previous Trial (Not Responded To) |
Difference Score: Aftereffect of Stop Signal (Stop – Go) |
||||
|---|---|---|---|---|---|
| Go Signal |
Stop Signal |
||||
| M | SD | M | SD | ||
| RT | 513 | 70 | 511 | 73 | −2 |
| TL | 24.4 | 9.0 | 25.7 | 9.5 | 1.3 |
| SP | 55.4 | 17.1 | 57.2 | 17.8 | 1.8 |
Note—Means (with standard deviations) and difference scores are shown for reaction times (RTs, in milliseconds), percentages of too long trials (TLs), and percentages of successful stops (stopping performance, SP).
Reaction Time
A 1 × 2 ANOVA was conducted with the independent variable of previous trial (go signal vs. stop signal). There was no significant difference [F(1,31) < 1]. Thus, whether the previous stimulus had been a go signal or a stop signal had no influence on the participants' RT performance.
Too Long Trials
Again, there was no significant effect in the ANOVA [F(1,31) < 1].
Stopping Performance
There was no significant effect in the ANOVA [F(1,31) = 2.0, p < .17].
Moreover, an additional analysis was conducted to control for the aftereffects of the participants' previous stopping and errors performed on trial n−2. Only those trials were included on which the participants had performed a valid go trial on n−2. For RT analysis, 27 of the 32 participants were included in the analysis; the remaining 5 had fewer than 10 trials in one of the conditions. For the TL analysis and SP analysis, 30 participants were included. The ANOVA on the RT data did not reveal a significant effect [F(1,26) = 1.1]. Likewise, the ANOVA on the TL data did not show a significant effect [F(1,30) = 2.0], nor did the ANOVA on the SP data [F(1,30) < 1]. Table 3B shows the results of this additional analysis.
Table 3B.
Additional Analysis of Experiment 3 Considering Only Those Trials That Were Preceded by a Valid Go Trial on n−2
| Previous Trial (Not Responded To) |
Difference Score: Aftereffect of Stop Signal (Stop – Go) |
||||
|---|---|---|---|---|---|
| Go Signal |
Stop Signal |
||||
| M | SD | M | SD | ||
| RT | 498 | 76 | 503 | 84 | 5 |
| TL | 20.7 | 11.9 | 24.5 | 10.9 | 3.8 |
| SP | 52.6 | 18.9 | 54.3 | 19.7 | 1.7 |
Note—RT, reaction times (in milliseconds); TL, too long trials in percentages; SP, stopping performance in percentages.
To summarize, the results show that whether the irrelevant stimulus contained the sudden onset signal to stop a response (color or size change) had no effect on the participant's subsequent response. Thus, the inhibitory and error aftereffects obtained in Experiment 2 must have been due to another person's responding to the irrelevant stimuli.
When the inhibitory aftereffect in Experiment 2 (in which another person stopped the participant's response to the irrelevant stimulus) was compared directly to the aftereffect in Experiment 3 (in which no other person was present), the effect was found to be significantly larger in the former than in the latter experiment. A 2 × 2 ANOVA with the independent variables of previous trial (stop vs. go) and experiment (other person vs. no other person) was computed. On the TL trials (for which the inhibitory aftereffect was significant in Experiment 2), a significant interaction was found [F(1,60) = 4.6, p < .04]. Thus, the inhibitory aftereffect was significantly larger when another person was present and successfully stopped his or her response (5.8% more TL trials after the other person's stop than after the other person's go) than when there was only the stop signal on the screen (1.3% more TL trials after an irrelevant stop signal than after an irrelevant go signal). Therefore, the inhibition effect in Experiment 2 must have been produced by the presence of another person's stopping an action and not by the physical change in the target stimulus.
GENERAL DISCUSSION
To investigate whether people simulate another person's higher level control processes, we measured trial-to-trial priming effects in a stop signal paradigm. Experiment 1 served to establish the priming effects within one person. When participants stop their response on one trial, they respond more slowly and more accurately on the next trial, indicating an aftereffect of the inhibition applied on the previous trial. Likewise, when participants fail to stop and, therefore, make an error, their performance on the following trial is slower and more accurate, indicating an aftereffect of the error.
The key issue was whether similar aftereffects would be detected when participants observed another person stop his or her response or fail to stop and, therefore, make an error. In Experiment 2, involving two persons alternating with responding, similar aftereffects of stopping and of error were indeed obtained, although, as might be expected, they were smaller in magnitude than the effects produced within a person. These aftereffects provide evidence for a simulation mechanism—that is, for the idea that observing another person's behavior activates similar neural circuits as performing that behavior oneself. Experiment 3 served to check that the aftereffects were not produced simply by the stop signal stimulus itself. No aftereffects of perceiving the visual stimulus itself were obtained, confirming that the aftereffects obtained in Experiment 2 were, indeed, produced by observing another person's responses to the stimulus.
The key Experiment 2 showed that the participants responded more slowly when they had observed the other person successfully stop his or her response: In particular, they were more likely not to respond within the given time window when the other person had stopped his or her response before than when the other person had fully executed his or her response before. Thus, the present study provides evidence for the first time that observing another person inhibiting an action causes similar aftereffects as inhibiting an action oneself. We concluded that the principle that observing an action in another individual activates similar action tendencies in oneself, which has been shown many times in the domain of visuomotor behavior, seems to apply to higher level cognitive processes as well.
In addition to the aftereffect of another person's successful inhibition of an action, we obtained clear evidence that the participants were slowed when they had observed the other person making an error—that is, failing to stop an action. This finding is in line with those of van Schie et al. (2004) and Bates et al. (2005), who found that observing another person making an error elicits an ERN in the observer, the ERN being an electrophysiological signature usually found in people who have just made an error. Extending van Schie et al.'s and Bates et al.'s electrophysiological findings, the present data provide evidence that observing another person's error also affects subsequent overt behavior in the observer. This evidence is in line with the notion that humans have evolved systems that enable learning from other individuals' errors via mere observation. This way, humans can adjust their behavior in an attempt to avoid errors in the future, without actually having carried out the error themselves. As was noted by van Schie et al., neural structures such as the anterior cingulate have a specialized role in detecting such errors and conflict (in self and in others) and provide inputs to other neural systems for adaptive responses (e.g., Holroyd, Nieuwenhuis, Mars, & Coles, 2004).
It should be noted that attending to the other person's behavior was not required in the present paradigm. Rather, the other person's error and inhibitory processes were simulated even though they were not relevant to the participant's own task. This automatic simulation would make sense with respect to the learning-via-observation mechanism discussed above. For instance, the person walking toward us who slips on ice provides a powerful error signal, enabling us to avoid the same hazard, even if we had not been explicitly monitoring this person's behavior.
The finding that simulating another person's mental processes occurs incidentally has been corroborated by other studies applying paradigms with two people performing a task together (e.g., Knoblich & Jordan, 2003; Sebanz, Knoblich, & Prinz, 2003, 2005). For instance, Sebanz et al. (2005) found that knowing that the other person had to perform a response to the same stimulus at the same time slowed down the participants' responses. The slowing occurred even when they could not see or hear their own and the other person's responses, suggesting that it was not caused by the processing of additional visual or auditory information. Importantly, the other person's response was not relevant to the participants' own task, suggesting that people “unintentionally engage in … simulating others' mental states, even when their current action goal does not require to take others' actions or intentions into account” (Sebanz et al., 2005, p. 1234).
Are the Inhibitory Aftereffect and the Error Aftereffect Different Effects?
An interesting question is whether the inhibitory aftereffects and the error aftereffects observed in the present study are driven by different mechanisms. Since they are similar in nature (both being reflected by slower and less accurate responses), one might argue that they could be driven by one and the same mechanism. However, evidence from imaging literature suggests that response inhibition and error processes involve different cortical systems (e.g., Garavan, Ross, Murphy, Roche, & Stein, 2002, investigating manual responses; Curtis, Cole, Rao, & D'Esposito, 2005, investigating saccadic eye movements). Clearly, further studies using fMRI and EEG are necessary to test whether the neural signals of inhibition and error caused by observing another person are also dissociable.
Behavioral evidence that our effects indeed reflect different error and inhibition processes comes from the additional analysis of Experiment 2. Here, we directly compared the aftereffects of the other person's performance (on trial n−1) with the aftereffects of the participants' own previous performance (on trial n−2). The logic was that a dissociation between the error and the inhibition aftereffects would indicate different underlying processes. A constant relationship of the effects within a person and between persons, however, would indicate that the error and inhibition aftereffects reflect the same mechanism. As can be seen from Figure 3, there was clearly a dissociation between the two effects. First, the aftereffects of the participants' own previous inhibition were much larger than the aftereffects of their own previous errors. Thus, the inhibition aftereffects remain more stable over a longer period than do the aftereffects of making an error, suggesting that the two aftereffects are not the same. Second, whereas the inhibition aftereffect of the participant's own previous stopping was more than twice the size of the aftereffect of the other person's stopping, the difference between the participant's own and the other person's error aftereffects was much smaller. Thus, inhibition carryover effects were significantly larger when produced by the participant's own previous behavior than when produced by another person's behavior. In contrast, error carryover effects were less influenced by who had made the error. This dissociation between inhibition carryover effects and error carryover effects provides evidence that the two effects observed in the present study indeed reflect different mechanisms.
Conclusion
In search of the mechanisms underlying social interactions, it has been reported that processing another individual's action involves similar neural circuits as producing this same action. This simulation mechanism, which was originally postulated for the domain of visually perceived motor actions, has recently been suggested to provide the basis for making inferences about another person's mental states in general (Gallese, Keysers, & Rizzolatti, 2004; Iacoboni et al., 2005; Ramnani & Miall, 2004; but see Jacob & Jeannerod, 2005, for a critical evaluation). If this were true, simulation of another person's behavior would have to go beyond pure visuomotor processes and would have to encompass higher level processes as well. Two such higher level processes were investigated in the present study: the inhibition of an ongoing action and the processing of an error. Evidence for the simulation of another person's inhibition and error processes was obtained.
Acknowledgments
This work was supported by a Wellcome Trust Programme Grant awarded to S.P.T. We thank three anonymous reviewers for their helpful comments.
REFERENCES
- Bach P, Tipper SP. Implicit action encoding influences personal-trait judgments. Cognition. 2007;102:151–178. doi: 10.1016/j.cognition.2005.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates AT, Patel TP, Liddle PF. External behavior monitoring mirrors internal behavior monitoring: Error-related negativity for observed errors. Journal of Psychophysiology. 2005;19:281–288. [Google Scholar]
- Blakemore S-J, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001;2:561–567. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschläger A, Prinz W. Compatibility between observed and executed finger movements: Comparing symbolic, spatial, and imitative cues. Brain & Cognition. 2000;44:124–143. doi: 10.1006/brcg.2000.1225. [DOI] [PubMed] [Google Scholar]
- Castiello U. Understanding other people's actions: Intention and attention. Journal of Experimental Psychology: Human Perception & Performance. 2003;29:416–430. doi: 10.1037/0096-1523.29.2.416. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M. Canceling planned action: An fMRI study of countermanding saccades. Cerebral Cortex. 2005;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MGH, Logan GD, Gratton G. In search of the point of no return: The control of response processes. Journal of Experimental Psychology: Human Perception & Performance. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: A magnetic stimulation study. Journal of Neurophysiology. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. NeuroImage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Mars RB, Coles MGH. Anterior cingulate cortex, selection for action, and error processing. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford; 2004. pp. 219–231. [Google Scholar]
- Houghton G, Tipper SP. Inhibitory mechanisms of neural and cognitive control: Applications to selective attention and sequential action. Brain & Cognition. 1996;30:20–43. doi: 10.1006/brcg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. Public Library of Science. 2005;3:529–535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff J, Klein RM. The presence of a nonresponding effector increases inhibition of return. Psychonomic Bulletin & Review. 2001;8:307–314. doi: 10.3758/bf03196166. [DOI] [PubMed] [Google Scholar]
- Jacob P, Jeannerod M. The motor theory of social cognition: A critique. Trends in Cognitive Sciences. 2005;9:21–25. doi: 10.1016/j.tics.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: A unifying mechanism for motor cognition. NeuroImage. 2001;14:103–109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Paulignan Y, Blakemore SJ. An interference effect of observed biological movement on action. Current Biology. 2003;13:522–525. doi: 10.1016/s0960-9822(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore SJ, Sirigu A. Motor activation prior to observation of a predicted movement. Nature Neuroscience. 2004;7:1299–1301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Knoblich G, Jordan JS. Action coordination in groups and individuals: Learning anticipatory control. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2003;29:1006–1016. doi: 10.1037/0278-7393.29.5.1006. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users' guide to the stop-signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego: Academic Press; 1994. pp. 214–249. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA, Rodgers B. What does a man do after he makes an error? An analysis of response programming. Quarterly Journal of Experimental Psychology. 1977;29:727–743. [Google Scholar]
- Ramnani N, Miall RC. A system in the human brain for predicting the actions of others. Nature Neuroscience. 2004;7:85–90. doi: 10.1038/nn1168. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S. Inhibitory after-effects in the stop-signal paradigm. British Journal of Psychology. 1999;90:509–518. [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding of imitation of action. Nature Reviews Neuroscience. 2001;2:661–669. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Knoblich G, Prinz W. Representing others' actions: Just like one's own? Cognition. 2003;88:B11–B21. doi: 10.1016/s0010-0277(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Knoblich G, Prinz W. How two share a task: Corepresenting stimulus–response mappings. Journal of Experimental Psychology: Human Perception & Performance. 2005;31:1234–1246. doi: 10.1037/0096-1523.31.6.1234. [DOI] [PubMed] [Google Scholar]
- Taylor TL, Ivanoff J. The interplay of stop signal inhibition and inhibition of return. Quarterly Journal of Experimental Psychology. 2003;56A:1349–1371. doi: 10.1080/02724980343000099. [DOI] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: Inhibitory priming by ignored objects. Quarterly Journal of Experimental Psychology. 1985;37A:571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJM, van der Molen MW, Jennings JR. Differential involvement of the anterior cingulate cortex in performance monitoring during a stop-signal task. Journal of Psychophysiology. 2005;19:1–10. [Google Scholar]
- van Schie HT, Mars RB, Coles MGH, Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nature Neuroscience. 2004;7:549–554. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- Wilson M, Knoblich G. The case for motor involvement in perceiving conspecifics. Psychological Bulletin. 2005;131:460–473. doi: 10.1037/0033-2909.131.3.460. [DOI] [PubMed] [Google Scholar]