Abstract
Interleukin-12 (IL-12) has been shown to play a central role in the innate and acquired immune responses. Its activities include enhancement of natural killer (NK) and cytotoxic T lymphocyte (CTL) activity and promotion of CD4 Th1 cell development. It has also been shown to provide potent activity as a vaccine adjuvant in generating antibody and T cell responses. We have investigated the efficacy of IL-12 protein in promoting CD8 T cell responses when it is used as an adjuvant for immunization. Studies using, as antigen, cDNA from an autologous antigen (P1A) as well as studies of responses to vaccinia virus-delivered self (gp100) and non-self (β-galactosidase) antigens show that the dose and schedule of IL-12 administration can significantly affect adjuvant activity, leading to enhancement or suppression of antigen-specific responses.
INTRODUCTION
Interleukin-12 (IL-12) is a cytokine central to the regulation of innate and acquired immune responses (reviewed in ref. 1). It directly activates natural killer (NK) cells, enhances their cytolytic activity, and importantly, induces interferon-γ (IFN-γ) secretion. In turn, IFN-γ induces expression of IL-12R on T and NK cells, thereby increasing their response to IL-12 and increasing IFN-γ production even more. A key role of IL-12 is its effect on the development of CD4 Th1 responses. IL-12 induces the differentiation from Th0 to Th1 cells, characterized by the secretion of IL-2, tumor necrosis factor-α (TNF-α), and IFN-γ. IL-12 is counterbalanced by IL-4 driving the differentiation of Th2 cells, characterized by the cytokines IL-4, IL-5, and IL-13. Through its effect on CD4 Th cells, IL-12 also drives switching to cytophilic antibody isotypes. Characterization of effects of IL-12 on CD8 T cells has resulted in conflicting evidence. A key role for CD8 responses in IL-12-induced immunotherapy has been demonstrated in many tumor models. Consistent with these findings, many groups have shown enhancement of CD8 activity by IL-12 as adjuvant for peptide antigens, virally delivered antigens, or using IL-12 cDNA in combination with cDNA vaccines. At the same time, suppressive effects of IL-12 in models using IL-12 as adjuvant have also been demonstrated. Kurazawa et al.(2,3) demonstrated that IL-12-mediated increases in nitric oxide (NO) are responsible for the transient inhibition of antitumor and anti-allo-CD8 responses. Orange et al.(4,5) demonstrated an IL-12 dose-dependent toxicity of lymphocytic choriomeningitis virus (LCMV)-induced CD8 cells. Thus, the specific conditions under which IL-12 is applied as an adjuvant for CD8 responses appears to be critical.
To investigate the efficacy of IL-12 protein as an adjuvant for cDNA immunization, we chose to make use of the P815 tumor model. The model is close to the human situation for many tumors in that the P815 tumor expresses a nonmutated self-antigen, P1A. Reagents for assessing T cell responses have been developed, and the model is well characterized. In particular, the P1A antigen has been cloned from the P815 tumor cells, P1A peptides that bind to H2d have been identified, and CD8 cells generated against the P1A peptide have been shown to be sufficient for promoting tumor rejection.(6–10)
In initial studies in this model, we determined the effects of dose and schedule of IL-12 protein on CD8-mediated antitumor responses in tumor-bearing mice treated or not with IL-12. These studies provided a basis for evaluating the effects of IL-12 protein dose and schedule on induction of a P1A-specific response using cDNA immunization. Our observations show that low doses of IL-12 protein enhance CD8 and antitumor activity in tumor-bearing mice and in mice immunized with P1A cDNA. However, higher doses suppress the endogenously generated CD8 responses and promote tumor growth. They also suppress the adjuvant activity seen with the cDNA vaccines. These findings are in contrast with those found for the adjuvant activity of IL-12 for CD4 responses and have important implications for use in the clinic of IL-12 as an adjuvant for promoting CD8 effector pathways.
MATERIALS AND METHODS
Cell lines and mice
Cells (2 × 105) of the mastocytoma tumor cell line(8,11) were injected into the flank of DBA/2 mice, purchased from Charles River Laboratory (Wilmington, MA). Tumor growth was monitored regularly and expressed as the product of two perpendicular measurements of tumor diameter.
cDNA vaccination
The vector pCMV-P1A encoded the gene for the tumor antigen P1A (provided by T. Gajewski, University of Chicago) under the control of the immediate-early cytomegalovirus (CMV) promoter. DNA was purified by EndoFree MegaKits (Qiagen, Chatsworth, CA) according to the manufacturer’s protocol. Endotoxin levels were 1–5 EU/mg as determined by the Quantitative Chromogenic Limulus Amebocyte Lysate Kit (BioWhittaker, Walkersville, MD). Preparation of the DNA-gold particles and cartridges was according to Bio-Rad’s protocol for the Helios Gene Gun (Bio-Rad, Hercules, CA). We used 0.5–1 µg of DNA and 0.5 mg of 1.6-µm gold particles. The final DNA loading rate of the gold particles was determined by gel analysis of DNA eluted from the DNA-gold particles and ranged from 100 to 300 ng DNA per cartridge. The content of the three cartridges of DNA-gold particles was delivered with the Helios Gene Gun to the shaved abdomen of the mice with the helium pressure set to 350 psi.
Cytotoxic T lymphocyte (CTL) assays
Restimulated splenocyte assay
Spleens were harvested and processed into single-cell suspensions by standard protocols. Splenocytes (2 × 107 per mouse) were incubated with 0.1 mg P1A peptide in 10 ml RPMI with 5 U/ml IL-2 for 6–7 days at 37°C in a CO2 incubator set at 5% CO2. A20 cells(12) were pulsed overnight with 10 mg P1A peptide. Restimulated splenocytes (3 × 105) were serially diluted 2-fold with RPMI medium in a duplicate set of 96-well round-bottom plates. Cytolytic activity was tested in [51Cr]release assays against the A20 target cells radiolabeled with Na[51Cr]O4 for 1 h at 37°C, 5% CO2. The cells were then washed and coincubated in U-bottomed microwells at the indicated E/T ratios. After 4 h at 37°C, supernatants were collected and counted in a Wallac Microbeta 1450 plate reader (Gaithersburg, MD). Percent specific lysis was calculated as
Total release was induced by adding Triton × 100 to 1% to control wells.
Peripheral blood lymphocyte (PBL) assay
The assay was carried out as described by Swiniarski et al.(13) Briefly, blood was collected by retroorbital bleed from anesthetized mice. Samples were diluted in phosphate-buffered saline (PBS) containing heparin, washed with PBS to remove heparin, and resuspended in RPMI 1640 medium containing 5 × 10−5M 2-mercaptoethanol , 2 mM l-glutamine, 100 U/ml penicillin, and 100 µ g/ml streptomycin (GIBCO-BRL, Gaithersburg, MD), 10% fetal bovine serum (FBS), 10 U/ml IL-2 (Genzyme, Cambridge, MA), 200 U/ml IL-6 (Genzyme), 4 µ g/ml anti-CD28 (PV1.17) (Genetics Institute, Cambridge, MA), and 10 U/ml murine IL-12 (Genetics Institute), and an optimized concentration of peptide or no peptide was added. Irradiated syngeneic spleen cells (1 × 105) and 8 × 103 white blood cells (WBC) were cultured for 6 days in wells of a U-bottom 96 well-plate (Costar, Cambridge, MA). A20 cells (1 × 10³) pulsed or not with peptide and chromium labeled as described were added to the wells (40 wells with peptide-pulsed target cells, 40 wells with unpulsed targets). P1.204 cells (a P1A antigen-negative derivative of P815) were used at a 20-fold excess over target cells as a cold target inhibitor to reduce nonspecific lysis. Mean specific percent lysis per well was calculated as
IFN-γ Elispot assay
Restimulated splenocytes (3 × 105) and 3 × 105 peptide pulse A20 cells were serially diluted 2-fold on 96-well flat-bottom plates that had been blocked and precoated with R46A2 antibody. The plates were incubated for 24 h at 37°C in 5% CO2 and washed six times with PBS. A biotinylated secondary antibody, XGM1.2 was added to the plates and incubated at ambient temperature for 1.5 h, and the plates were washed four times with PBS. Streptavidin alkaline phosphatase was added to the plates and incubated at ambient temperature for 1 h. The following substrate solution was prepared: 0.6% low melting agarose in 0.1 M 2-amino-2-methyl-1 propanol, pH 10.5, and kept at 60°C. When the plates were ready, 1 mg/ml BCIP (5-bromo-4-chloro-3 -indoly phosphate) was added to the substrate solution, and 100 µ l of the mixture was added to each well. The plates were incubated 30 min in the dark, and the Elispots were counted using a dissecting microscope.
RESULTS
Effect of IL-12 dose and schedule on growth of P815 tumor
IL-12 has been suppressive of tumor growth in essentially all tumor models in which it has been tested.(1) To evaluate IL-12 antitumor vaccine adjuvant activity in this model, studies were carried out to define IL-12 doses and schedules that themselves were not curative. Initial studies demonstrated that DBA/2 mice are more sensitive than some other common mouse strains used for tumor studies. Thus, about 50% of DBA/2 mice treated with 1 µ g IL-12 for 1 week do not tolerate a second week of IL-12 dosing and die between days 10 and 14. However, this level of sensitivity is not unique. Similar sensitivity is found for C3H mice. Dose escalation studies (not shown) established that the maximum dose of IL-12 for DBA/2 mice that causes no obvious side effects is 100 ng given three times per week. In contrast, Balb/c and C57BL/6 mouse strains survive dosing at 0.5–2 µ g/day given 5 days per week (data not shown).
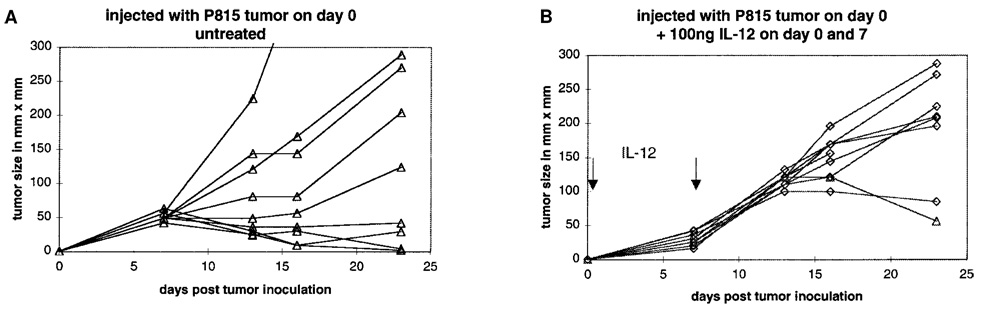
In contrast to most other tumor models, IL-12 was not effective in curing mice of P815. Rather, under some conditions, IL-12 enhanced tumor growth and reduced the frequency of spontaneously curing mice. A typical experiment is shown in Figure 1. Of DBA/2 mice inoculated intradermally with 105 P815 tumor cells, 100% develop palpable tumors by day 7. Twenty to thirty percent of the tumors regress over the next 2–3 weeks. Tumors in the remaining mice grow at highly variable rates but ultimately lead to death or sacrifice of the animals. In contrast, mice inoculated in the same way but administered 100 ng IL-12 (given i.p.) on days 0 and 7 develop palpable but consistently smaller tumors by day 7. However, of these, all the tumors grow at comparable rates, typically faster than most tumors in untreated mice. Moreover, the spontaneous cure rate is dramatically reduced (Fig. 1). This tumor-promoting effect of IL-12 was schedule dependent, as 100 ng or 300 ng IL-12 given only on day 0 or day 7 had no effect on tumor growth (data not shown).
FIG. 1.
Enhancement of P815 tumor growth by administration of IL-12. DBA/2 mice were inoculated on day 0 with tumor cells and treated (B) or not (A) with 100 ng IL-12 given i.p. on days 0 and 7 as described in Materials and Methods. The tumor size of individual mice was monitored. Each line represents a single mouse.
Suppression by IL-12 of endogenous P1A-specific CD8 response and correlation with tumor growth
As P1A-specific CD8 cells have been shown to be key in tumor rejection in this model, we focused on the effect of IL-12 on these cells. For these studies, we made use of an assay that can detect antigen-specific CD8 responses from a 200-µ l blood sample.(13) This assay allowed us to measure CTL responses from the same mouse at different times during development of its tumor.
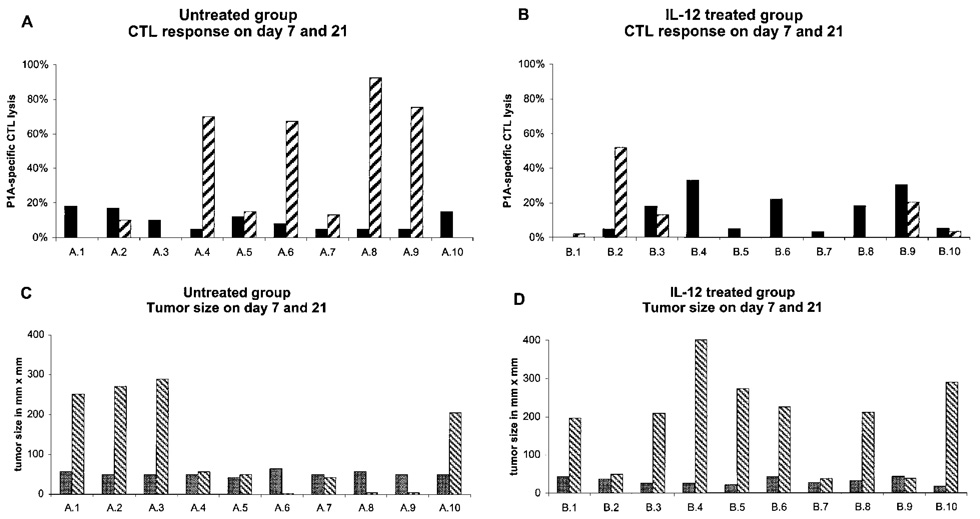
Analysis of P1A-specific CD8 responses in peripheral blood of untreated tumor-bearing mice established that P1A-specific CD8 responses were detectable in all mice (Fig. 2A,C). At day 7 after tumor cell inoculation, when the size of tumors was homogeneous at 20–40 mm², all mice exhibited significant P1A-specific CD8 responses. By day 21, as expected, the tumors in individual mice grew to very different sizes. However, in contrast to the findings on day 7, P1A-specific CD8 responses were detected in only 7 of 10 mice, with an inverse relationship between tumor size and magnitude of the CD8 response. In mice in which the tumors had regressed or were cured, tumor-specific lytic activity was high. In contrast, mice with large and rapidly growing tumors had no or reduced P1A-specific responses relative to that measurable on day 7 (Fig. 2A,C).
FIG. 2.
Correlation between tumor size and CTL response in tumor-bearing and IL-12-treated mice. DBA/2 mice were inoculated on day 0 with P815 tumor cells and treated (B, D) with IL-12 (100 ng on days 0 and 7) or not (A, C). CTL activity was measured from PBMC of the same mice on days 7 (solid bars) and 21 (hatched bars) (A, B). Tumor measurements were taken at the same time from the same mice on day 7 (stippled bars) and 21 (hatched bars) (C, D).
IL-12 treatment of tumor-bearing mice affected significantly the P1A-specific CTL response. On day 7, after only one treatment with IL-12 (on day 0), tumor size and CTL response were comparable to the untreated control mice (Fig. 2B vs. 2A). However, on day 21, after treatment with 100 ng IL-12 on days 0 and 7, appreciable P1A-specific CTL activity was detectable in only 2 mice and absent in the others (Fig. 2B). With untreated mice, the presence of a CTL response correlated with tumor size. In the IL-12-treated group, the 2 mice with detectable CTL responses had the smallest tumors. Seven of 10 mice had tumors larger than 200 mm², compared with only 4 mice in the untreated control group (Fig. 1C,D). Thus, treatment of DBA/2 mice with 100 ng of IL-12 on days 0 and 7 suppressed the development of tumor-specific CD8 T cell responses and resulted in enhanced tumor growth.
Immunization with cDNA encoding a self-antigen promotes antigen-specific CD8 responses
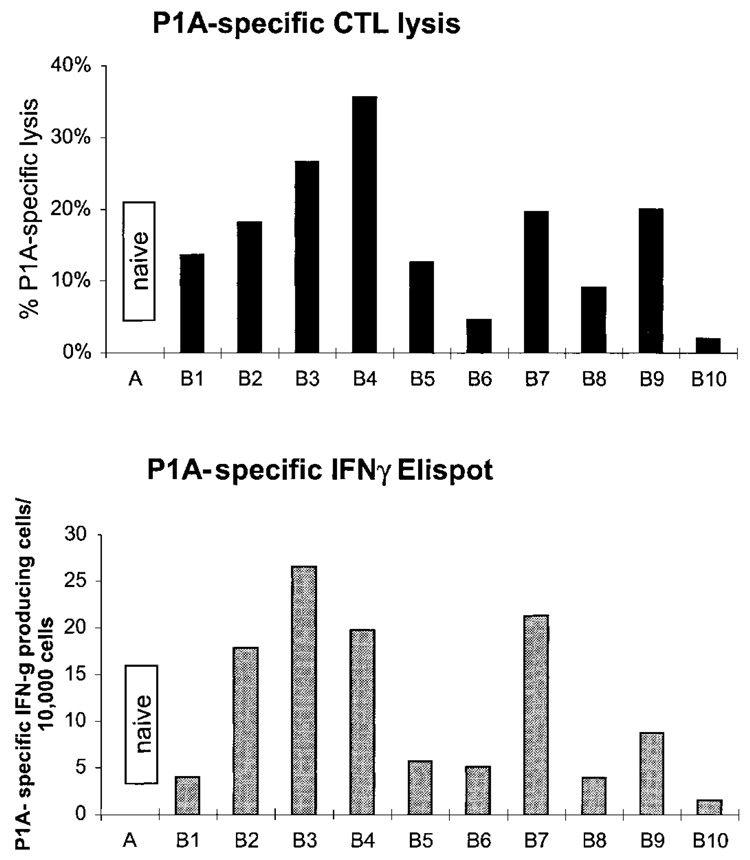
To establish a model to test activity of IL-12 protein as an adjuvant for a cDNA vaccine to induce a CD8 response to self-antigens, we established conditions for immunization of DBA/2 mice with cDNA encoding the nonmutated tumor antigen P1A. Preliminary studies established the efficacy of cDNA vaccination with cDNA coated onto gold beads and delivered to the epidermis by a helium-pressurized gene gun. Two vaccinations of 100 ng DNA each were given separated by 7 or 14 days and caused CTL and IFN-γ responses specific to the MHC class I restricted P1A peptide in 80%–90% of mice (Fig. 3). No P1A-specific responses could be detected 7 days after a primary vaccination or after vaccination with a control plasmid (data not shown). Thus, a strong and reproducible CD8 response could be elicited despite the fact that P1A is a nonmutated self-antigen.
FIG. 3.
Immunization with P1A cDNA induces concordant CTL and MHC class I P1A-specific IFN-γ production. DBA/2 mice were vaccinated with P1A cDNA as described in Materials and Methods. P1A-specific lytic activity was measured from restimulated splenocytes, and IFN-γ production was measured by Elispot as described in Materials and Methods.
IL-12 protein induces a dose-dependent increase in CD8 responses to a P1A cDNA vaccine
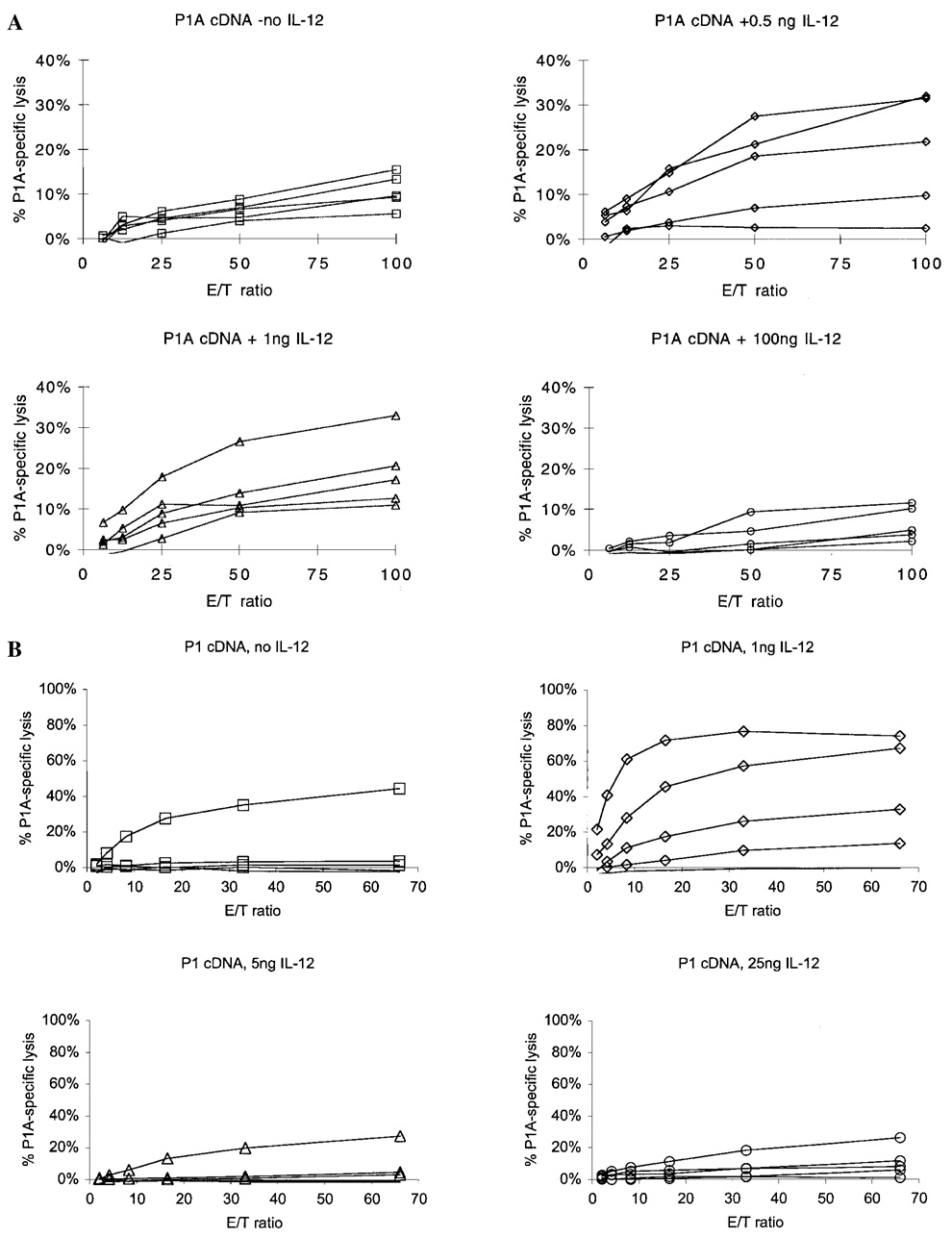
To test the effect of IL-12 protein on the antigen-specific CD8 response, mice were immunized with P1A cDNA as described and given IL-12 protein at various doses and schedules with respect to cDNA immunization. Mice were vaccinated with P1A cDNA on days 0 and 7 or 14. Different doses of IL-12 were given s.c. 1 day after DNA vaccination on days 1 and 8 or 15. As shown in Figure 4, low doses of 0.5 and 1 ng IL-12 increased the P1A peptide-specific CTL response, as determined by two criteria. The maximal lysis at an E/T ratio of 100:1 was increased, and the E/T ratio was reduced at which maximal lysis was reached (Fig. 4A). In the absence of IL-12, P1A cDNA immunization typically induced specific lysis of 5%–15% at an E/T of 100:1 and maximal lysis at the same E/T. The use of IL-12 protein as adjuvant at doses of 0.5 or 1 ng reproducibly increased by a factor of 2 the maximal level of specific lysis and reduced the E/T at which maximal lysis was achieved (Fig. 4B). IL-12 doses of 10–100 ng gave variable results. In some experiments, specific lysis was increased relative to controls, whereas in others, there was no difference or a reduction in activity. IL-12 doses of 300 ng or higher, given on days 1 and 15, consistently suppressed development of P1A-specific CTL responses (Fig. 4B). In all cases, IL-12 enhancement or suppression of P1A peptide-specific CTL responses corresponded closely with increases in P1A-specific IFN-γ responses.
FIG. 4.
Effect of IL-12 dose and schedule on P1A cDNA vaccine-induced P1A-specific CTL response. DBA/2 mice were vaccinated with P1A cDNA (as described in Materials and Methods) on days 0 and 14, and IL-12 given i.p. at the indicated doses on day 1 (A), or cDNA was given on days 0, 1, 2, 7, 8, and 9, and IL-12 protein was given on days 1 and 8 (B). A and B represent independent experiments representative of at least two additional studies of the same schedule and doses.
These data establish that for a given schedule, an optimal dose of IL-12 protein can have adjuvant activity in promoting development of a self-antigen-specific CD8 response. However, high doses of IL-12 suppress the generation of P1A-specific CD8 responses in this mouse model.
Incorrect schedule of IL-12 administration can suppress induction of antigen-specific CD8 responses
As described, 100 ng IL-12 administered on days 1 and 14 or 21 after cDNA vaccination on days 0 and 7 variably enhanced or suppressed induction of P1A-specific responses. We evaluated whether different schedules of IL-12 protein at this dose could change the effect of IL-12 on CTL responses. As shown in Table 1, a single dose of IL-12 given before or on the day of immunization with P1A cDNA was strongly suppressive (4% and 10% specific lysis, respectively, vs. 39% for control). Similarly, dosing with IL-12 protein 1 day before or on the day of each cDNA immunization was suppressive (6% and < 5% specific lysis, respectively, vs. 39% for control). In contrast, dosing with 100 ng IL-12 on day 1 after cDNA immunization or for 3 consecutive days beginning on day 1 after cDNA immunization was neither enhancing nor suppressive. In this and other studies, we could find no dosing schedule for 100 ng IL-12 protein that enhanced the response. However, dosing the day before or on the day of cDNA immunization was clearly suppressive. Thus, the presence of too high levels of IL-12 protein just before or at the time of initiation of a CD8 response appears to suppress development of that response.
Table 1.
Effect of IL-12 Schedule on Suppression of P1A-Specific CTL Responsesa
| P1A cDNA | 100 ng IL-12 | Mean P1A-specific lysis (%)b | Range of lysis (%) | |
|---|---|---|---|---|
| A | Days 0 and 7 | None | 39 | 36–47 |
| B | Days 0 and 7 | Day 0 | 10 | 6–19 |
| C | Days 0 and 7 | Day −1 | 4 | 2–17 |
| D | Days 0 and 7 | Day 1 | 23 | 10–37 |
| E | Days 0 and 7 | Days 1, 2, and 3 | 29 | 22–39 |
| F | Days 0 and 7 | Days −1 and 6 | 6 | 2–12 |
| G | Days 0 and 7 | Days 0 and 7 | <5 | 0–4 |
| H | Days 0 and 7 | Days 1 and 8 | 9 | 3–22 |
P1A cDNA was administered by gene gun as described. 100 ng IL-12 was given s.c. at indicated times. Seven days after the last vaccination, splenocytes were restimulated in vitro as described and measured for P1A-specific CTL response.
Mean P1A-specific lysis for 5 mice (groups A–E) or 3 mice (groups F–H).
High doses of IL-12 suppress generation of antigen-specific CTL responses in C57BL/6 mice
To determine if the suppressive effect of IL-12 on the generation of CD8 responses was unique to cDNA immunization of the IL-12-sensitive DBA/2strain, we tested the effect of IL-12 in C57BL/6 mice vaccinated with a recombinant vaccinia virus encoding human gp100 (rVV-hgp100) or β-galactosidase (rVV-βgal). Unlike DBA/2 mice, C57BL/6 mice tolerate high doses of IL-12 (1–5 µ g/day, 5 days/week).(14) Mice were immunized by i.v. administration of 1 × 107 plaque-forming units (pfu) of either virus on day 0, and CD8 responses were assayed by measuring MHC I peptide-specific IFN-γ secretion from spleen cells collected on day 21. Immunization with rVV-hgp100 induced potent CD8 responses to human gp100 peptide as well as to the corresponding murine gp100 peptide. However, there was no significant response to stimulation of cells from these mice with β-gal peptide 96–103 or to culture and stimulation with β-gal peptide (Table 2). Similarly, immunization with rVV-βgal induced potent CD8 activity specific for βgal but none for human or murine gp100 (Table 2). Administration of a nontoxic dose of 1 µ g IL-12 for 4 consecutive days beginning on the day of immunization ablated both responses (Table 2). Thus, in at least two mouse strains with very different sensitivities to IL-12, higher doses of IL-12 suppress the generation of antigen-specific CD8 responses.
Table 2.
IL-12 Abrogates Generation of Antigen-Specific IFN-γ Responses to Recombinant Vaccinia Vaccines in C57BL/6 Micea
| In vivo vaccination: | rVV-hgp100 |
rVV-hgp100 + IL-12 |
rVV-βgal |
rVV-βgal + IL-12 |
||||
|---|---|---|---|---|---|---|---|---|
| In vitro restimulation: | hgp100 pep | βgal pep | hgp100 pep | βgal pep | hgp100 pep | βgal pep | hgp100 pep | βgal pep |
| Experiment 1 | ||||||||
| Test antigen | ||||||||
| None | 67 | 100 | 565 | 226 | 118 | 62 | 52 | 1,365 |
| hgp100 pep | 19,963 | 42 | 588 | 341 | 49 | 93 | 68 | 471 |
| βgal pep | 71 | 250 | 545 | 386 | 68 | 13,525 | 40 | 821 |
| Experiment 2 | ||||||||
| Test antigen | ||||||||
| None | 288 | 380 | 111 | 130 | 416 | 180 | 150 | 827 |
| hgp100 pep | 38,429 | 476 | 130 | 66 | 3,237 | 693 | 102 | 190 |
| βgal pep | 1,268 | 1,009 | 244 | 150 | 2,084 | 20,275 | 288 | 404 |
C57BL/6 mice were vaccinated with recombinant vaccinia virus expressing either the human gp100 or βgal protein on day 0. Either PBS or 1 µg rMuIL-12 was administered i.p. on days 0–4. On day 21, splenocytes were restimulated in vitro with either hgp100 or βgal peptide and tested 6 days later with the indicated peptide for antigen-specific IFN-γ release.
DISCUSSION
The effect of IL-12 on CD4 responses has been well described.(15–18) However, the adjuvant effect of IL-12 for CD8 responses is not as well defined. Here, we investigated the effect of IL-12 on CD8 responses generated by a cDNA vaccine encoding for the nonmutated tumor antigen P1A. Low doses of IL-12, in the range of 0.5–1 ng/mouse, increased the peptide-specific CTL and IFN-γ responses. Doses of 10–50 ng/mouse of IL-12 had variable effects on CD8 responses. In contrast, higher but nontoxic doses of IL-12 (100–300 ng/mouse) suppressed the generation of CD8 responses to the DNA vaccine. We also investigated the effect of schedule of IL-12 administration on the response. IL-12 (100 ng) given before or on the day of DNA vaccination suppressed the CD8 response. At that dose, only injection of IL-12 1 day after cDNA administration was not suppressive.
The generality of the negative effect of IL-12 protein on CD8 T cell responses is further supported by our observations in the recombinant vaccine model and the P815 tumor model. Immunization with a recombinant vaccinia virus expressing the tumor antigen gp100 or β-galactosidase generated strong antigen-specific CD8 responses that were suppressed when IL-12 was coadministered with the vaccine. In the P815 tumor model, two doses of IL-12 given on the day of tumor inoculation and 7 days later enhanced tumor growth and suppressed tumor-specific CD8 responses. We have recently evaluated the adjuvant effect of IL-12 protein in combination with MHC class I restricted peptide vaccine.(19) IL-12 enhanced the antigen-specific CD8 response but was dramatically improved when used in combination with CD4 T cell helper. We also found that the timing of IL-12 administration is critical.
The mechanisms of the suppressive effect of IL-12 on CD8 T cells is not understood. Kurzawa et al.(20) suggested that the indirect induction of iNOS by IL-12 and the release of NO is responsible for the suppressive effect of IL-12. When iNOS inhibitors were given together with IL-12, normal delayed-type hypersensitivity (DTH) responses were observed. Additionally, the activation of macrophages and their release of radical oxidative metabolites may have an impact on CD8 cells. Clearly, we cannot attribute the suppressive effect of IL-12 on CD8 cells to a general toxicity effect. The DBA/2 mice are sensitive to IL-12. However, at the doses of IL-12 tested for adjuvant activity, we did not observe any toxicity. Second, we observed CD8-suppressive effects of IL-12 in C57BL/6 mice that are resistant to much higher doses of IL-12. Although we have measured antigen-specific responses, our current data do not address the question of whether the suppressive effect of IL-12 is selective for CD8 cells, is antigen-specific, or transiently down-regulates all CD8 responses. These questions are the focus of future studies.
Our findings have important implications for the clinical evaluation of IL-12. Some data from clinical trials suggest that IL-12 may also have negative effects on the generation of tumor-specific CD8 responses in cancer patients.(21) Careful dose-response studies should be considered. At high doses, IL-12 may have direct antitumor activity by inducing IFN-γ, iNOS,(3,22) and IP-10.(23,24) At the same time, it may suppress tumor-specific T cell responses, preventing the development of a beneficial long-term antitumor immunity. Another issue may be variability in the response to the effects of IL-12. Observations in the clinic suggest the level of IFN-γ induced in serum of treated patients can vary significantly between patients.(25) Similarly, the same IL-12 dose has exhibited very different toxicity in different mouse strains (this report; data not shown).
In summary, IL-12 is a potent cytokine central to innate and adaptive immune responses. It is thus not surprising that many regulatory or toxic sequelae of administration of exogenous IL-12 may further modulate the developing immune response by as yet poorly understood mechanisms. Unraveling these pathways remains the effort of future studies.
REFERENCES
- 1.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 2.Kurzawa H, Wysocka M, Aruga E, Chang A, Trinchieri G, Lee WMF. Recombinant interleukin 12 enhances cellular immune responses to vaccination only after a period of suppression. Cancer Res. 1998;58:491–499. [PubMed] [Google Scholar]
- 3.Koblish HK, Hunter CA, Wsocka M, Trinchieri G, Lee WMF. Immune suppression by recombinant interleukin (rIL)-12 involves interferon γ induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of no generation reveal the extent of rIL-12 vaccine adjuvant effect. J. Exp. Med. 1998;188:1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orange JS, Wolf SF, Biron CA. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 5.Orange JS, Salazar-Mather TP, Opal SM, Spencer RL, Miller AH, McEwen BS, Biron CA. Mechanism of IL-12-mediated toxicities during experimental viral infections: role of TNF and glucocorticoids. J. Exp. Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van Den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 7.Chomez P, De Plaen E, Van Pel A, De Smet C, Szikora J, Lurguin C, Libacq-Verheyden A, Boon T. Efficient expression of tum-antigen P1A by transfected subgenic fragments. Immunogenetics. 1992;35:241–252. doi: 10.1007/BF00166829. [DOI] [PubMed] [Google Scholar]
- 8.Boon T, De Plaen D, Lurquin C, Van Den Eynde B, Van Der Bruggen P, Traversari C, Amar-Costesec A, Van Pel A. Identification of tumour rejection antigens recognized by T lymphocytes. Cancer Surv. 1992;13:23–37. [PubMed] [Google Scholar]
- 9.Lethe B, Van Den Eynde B, Van Pel A, Corradin G, Boon T. Mouse tumor rejection antigens P815A and P815B: two epitopes carried by a single peptide. Eur. J. Immunol. 1992;22:2283–2288. doi: 10.1002/eji.1830220916. [DOI] [PubMed] [Google Scholar]
- 10.Boon T, Cerottini J, Van Den Eynde B, Van Der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 11.Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Endogenous IL-12 is necessary for rejection of P815 tumor variants in vivo. J. Immunol. 1996;156:1095–1100. [PubMed] [Google Scholar]
- 12.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 13.Swiniarski H, Sturmhoefel K, Lee K, Wolf SF, Dorner AJ, O’Toole M. A CTL assay requiring 150 µ1 of mouse blood. J. Immunol. Methods. 2000;233:1–11. doi: 10.1016/s0022-1759(99)00186-6. [DOI] [PubMed] [Google Scholar]
- 14.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of IL12 against murine tumors. J. Exp. Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliss J, Vancleave V, Murray K, Wiencis A, Ketchum M, Maylor R, Haire T, Resmini C, Abbas A, Wolf S. Interleukin 12 as an adjuvant promotes a T helper 1 but does not suppress a T helper 2 recall response. J. Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- 16.McKnight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J. Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 17.Gerosa F, Trinchieri G. Mechanisms of T helper cell differentiation induced by interleukin-12. In: Romagnani S, Del Prete G, Abbas AK, editors. Cytokines: Basic Principles and Practical Applications. Rome, Italy: Ares-Serono Symposia Pub.; 1994. [Google Scholar]
- 18.Perez VI, Lederer JA, Lichtman AH, Abbas AK. Stability of Th1 and Th2 populations. Int. Immunol. 1995;7:869–875. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- 19.Swiniarski H, Wolf SF, Stumrhoefel K, Peterson RL, Dorner AJ, O’Toole M. IL-12-Dependent enhancement of CTL response to weak class I-restricted peptide immunogens requires coimmunization with T helper cell immunogens. Clin. Immunol. 2000;94:200–211. doi: 10.1006/clim.2000.4836. [DOI] [PubMed] [Google Scholar]
- 20.Kurzawa H, Wysocka M, Aruga E, Chang A, Trinchieri G, Lee WMF. Recombinant interleukin 12 enhances cellular immune responses to vaccination only after a period of suppression. Cancer Res. 1998;58:491–499. [PubMed] [Google Scholar]
- 21.Gajewski TF, Falarino F, Vogelzang N, Posner M, Ashikari A, Sherman M. Effective melanoma antigen vaccination without dendritic cells (DC): a phase I study of immunization with Mage3 or Melan-A peptide-pulsed autologous PBMC plus rhIL-12; Proc. ASCO; 1999. p. 539a. [Google Scholar]
- 22.Hunter SE, Thibodeaux DK, Schaub RG, Goldman SJ, Leonard JP. Immunoregulation by interleukin-12 in MB49.1 tumor-bearing mice: cellular and cytokine-mediated effector mechanisms. Eur. J. Immunol. 1997;27:3438–3446. doi: 10.1002/eji.1830271244. [DOI] [PubMed] [Google Scholar]
- 23.Tannenbaum CS, Wicker N, Armstrong D, Tubbs R, Finke J, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J. Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 24.Voest ED, Kenyon BM, O’reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiognensis in vivo by interleukin 12. J. Natl. Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, Sandstrom K, Nadeau R, Parjar H, Bukowski R. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin. Cancer Res. 1998;4:1183–1191. [PubMed] [Google Scholar]