Abstract
Recent neuroimaging research shows that older adults exhibit recruitment, or increased activation on various cognitive tasks. The current study evaluated whether a similar pattern also occurs in semantic memory by evaluating age-related differences during recognition of Recent (since the 1990s) and Enduring (1950s to present) famous names. Fifteen healthy older and 15 healthy younger adults performed the name recognition task with a high and comparable degree of accuracy, although older adults had slower reaction time in response to Recent famous names. Event-related functional MRI showed extensive networks of activation in the two groups including posterior cingulate, right hippocampus, temporal lobe and left prefrontal regions. The Recent condition produced more extensive activation than the Enduring condition. Older adults had more extensive and greater magnitude of activation in 15 of 20 regions, particularly for the Recent condition (15 of 15; 7 of 15 also differed for Enduring); young adults did not show greater activation magnitude in any region. There were no group differences for non-famous names, indicating that age differences are task-specific. The results support and extend the existing literature to semantic memory tasks, indicating that older adult brains use functional recruitment to support task performance, even when task performance accuracy is high.
Keywords: Semantic memory, Event-related fMRI, Functional recruitment, Aging, Posterior cingulate, Frontal lobes, Neuroimaging, Cognition
1. Introduction
Cognitive neuroscience research, which focuses on revealing brain–behavior relationships, is now being applied toward understanding age-related declines in cognitive abilities such as memory and attention. The extant neuroimaging literature on cognitive aging thus far is small but rapidly growing. Most of the available studies have employed perceptual or short-term episodic memory paradigms, with little attention devoted to the study of age-related changes in semantic memory performance in the neuroimaging literature.
Some studies report that older adults exhibit activation in comparable areas as younger adults, but the extent of activation in these areas is reduced in older adults [17,50]. Other studies report comparable levels of activation between young and healthy older adults, but older adults produce additional regions of activation, which are frequently in contralateral homologues and particularly in prefrontal areas [5,6,11,15–17,29,30,34,38,41,42]. However, at least when healthy participants are examined and higher-order cognitive tasks are used (rather than perceptual-motor), differences in elders have been task-dependent rather than generalized. Such activations have typically been associated with compensation, sometimes referred to as “recruitment”, positing that additional task-specific circuits can be recruited transiently as task demands increase [5,6,41], although alternative interpretations cannot yet be ruled out [41]. This finding has also recently been replicated and retested after approximately 1 year [29].
Memory decline in aging is thought to result from multiple factors, including executive functioning changes associated with frontal–striatal systems and alterations in the medial temporal lobe memory system [3]. Age-related losses in the medial temporal lobe have been noted in some studies [48], but other studies suggest there may be functional changes without structural changes in aging [54,55]. Fronto-striatal systems have more consistently been reported to show structural losses in both white matter and gray matter [18,48]. Notably, many of the cognitive deficits that older adults exhibit are associated with the frontal lobes [33,39,44,47]. Indeed, some theories focus exclusively on frontal lobe changes to explain age-related cognitive decline [1,21,28,40,57], although a number of imaging studies also report increased activation in older adults in the inferior parietal lobule, medial temporal lobe, dorsomedial nucleus of the thalamus, basal ganglia, cerebellum, and occipital lobe [4,11,15,17,23,29,30,32,33,38,41,42]. One recent study showed that increased inferior frontal activations during remembering were associated with decreased parahippocampal activity in elders, suggesting that the frontal activity is compensatory for medial temporal lobe impairment [19].
We recently developed a task to examine recognition of names famous in distinct time epochs using event-related fMRI [12]. We found increased signal activity bilaterally for both hippocampal and parahippocampal regions for famous names from both time epochs compared to unfamiliar names. In addition, the right medial temporal lobe also showed a temporal gradient for famous names, with greater activity for Recent famous names (famous since the 1990s; e.g., Britney Spears) as compared to Remote famous names (famous in the 1950s; e.g., Tab Hunter). The results suggested that the bilateral medial temporal lobes are important in the mediation of retrieval of person-specific information, which combines both semantic and autobiographical components of memory, as compared with the retrieval processes associated with general semantic memory [2,14,22,31,36,58].
In the current study, we compare the findings for older and younger adults with a similar version of the famous names task using whole-brain event-related fMRI. The current versions used Recent names and Enduring famous names (continuous fame since the 1950s, e.g., Frank Sinatra), relative to unfamiliar names (Foils). We hypothesized that a bilateral network for person-specific memory retrieval, including anterior, lateral and medial temporal lobes, posterior cingulate, and mesial frontal and prefrontal regions [12,31], would be activated by both older and young adults. Based on aging studies with other cognitive tasks [29,30,41,42], older adults were also expected to show more extensive and greater magnitude of activation in many of those principal task-specific regions, as well as more prefrontal activation than young adults in both famous name conditions relative to Foils. In addition, we expected the activation to be greater for Recent names compared to Enduring names and Foils in both participant groups [12].
2. Methods
2.1. Participants
Fifteen older (mean age = 70.4, S.D. = 6.40; 10 female, 5 male) and 15 younger participants (mean age = 23.6, S.D. = 3.52; 10 female, 5 male) were recruited from the community to participate in the study. Participants were strongly right-handed (mean laterality quotient = 92.7, range = 84–100) on the Edinburgh Handedness Inventory [43]. Participants were excluded if they reported a history of neurological disease, major psychiatric disturbance, substance abuse, or were taking psychoactive or cardiovascular prescription medications. Informed consent was obtained from participants according to the institutional guidelines established by the Medical College of Wisconsin Human Subjects Review Committee. Participants were compensated for their time. To ensure the safety of the participants, each individual was screened on the phone prior to the scanner session regarding the presence of metal implants, pacemakers, aneurysm clips and other potential safety hazards. For the older participants, a cognitive screening examination preceded the scan session. All participants performed within normal limits on the Mini-Mental State Examination [13] (mean = 29.2, range = 27–30); and the Repeatable Battery for Assessment of Neuropsychological Status [45,46] (mean = 105.1, range = 95–129).
2.2. Imaging task
The task used was designed for fMRI and its development is discussed in detail elsewhere [12]. The task procedure was as follows: a set of 120 names of famous people and non-famous people, selected by pilot testing from a pool of 784 names, was organized into four categories: people who became famous recently, in the 1990s (Recent); enduringly famous people who became famous in the 1950s and are still well known today by both young and old (Enduring); remotely famous people who became famous in the 1950s but are not well known today (Remote), and non-famous people (Foils). Stimuli were presented for 4 s each with randomly interspersed 4 s intervals consisting of a single centrally placed fixation crosshair at an overall 2:1 (names:fixation) ratio. Participants were instructed to make a right index finger (i.e., dominant hand) key press if the name was famous and a right middle finger key press if the name was unfamiliar (all conditions). Stimuli were presented in three imaging runs of 60 trials each (10 stimuli from each of the four name conditions, 20 fixation trials). Twelve seconds of fixation were added to both the beginning and the end of each run. Run order was counterbalanced across subjects so that the specific names were not presented in the same order to each participant. The Remote trials were not included in analysis for this paper because they are not recognized as famous by young adult subjects (by design).
2.3. Functional MRI
Whole-brain, event-related functional MRI was conducted on a commercial 1.5 Tesla scanner (Signa; General Electric Medical Systems, Milwaukee, WI) equipped with a three-axis local gradient head coil and an elliptical endcapped quadrature radiofrequency coil (Medical Advances, Milwaukee, WI). Echoplanar images were collected using a single-shot, blipped, gradient-echo echoplanar pulse sequence (echo time; TE), 40 ms; field of view (FOV), 24 cm; matrix size, 64 × 64. For the three imaging runs, 22 contiguous sagittal 6-mm-thick slices were selected to provide coverage of the entire brain (voxel size = 3.75 mm × 3.75 mm × 6 mm). The interscan interval (repetition time; TR) was 2 s. During each imaging series, 132 sequential echoplanar images were collected. At the beginning of the scan session, high-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired (TE = 5 ms; TR = 24 ms; 40° flip angle; number of excitations (NEX) = 1; slice thickness = 1.2 mm; FOV = 24 cm; resolution = 256 × 192). Foam padding was used to reduce head movement within the coil.
Functional images were generated with Analysis of Functional NeuroImages (AFNI) software [8]. Each image time-series was spatially registered in-plane to reduce the effects of head motion using an iterative linear least squares method. A deconvolution analysis was used to extract a hemodynamic response (impulse response function; IRF) for each of the three types of name stimuli from the time-series. In addition, only correct responses (true positives for famous names and true rejections for unfamiliar names) were incorporated into the estimate of the IRF for each stimulus type. IRFs were modeled for the 2–14 s period post-stimulus onset. Individual anatomical and functional scans were linearly interpolated to 1 mm3 voxels, co-registered, and transformed into standard stereotaxic space [56]. To compensate for normal variation in anatomy across subjects, functional images were blurred using a 4 mm Gaussian full-width half-maximum filter.
2.4. Voxel-wise analysis
The purpose of the voxel-wise analysis was to determine the network activated by the task and to evaluate the spatial extent of the activation in each participant group; this was done independently in each participant group to preserve age group differences, which were hypothesized. The dependent variable in the analysis was the area under the curve of the impulse response function at 4, 6, and 8 s post-stimulus onset. A repeated measures ANOVA was applied to the conditions on a voxel-by-voxel basis. This was followed by pooled variance t-tests for each group to compare each of the conditions in a pair-wise manner (Recent versus Foils, Enduring versus Foils, Recent versus Enduring). A statistical threshold was applied to the data (t(14) = 3.662, p < 0.001). A cluster size threshold of 0.200 ml was applied as an additional procedure for removing false positive activation foci from the brain maps.
2.5. Region of interest (ROI) analysis
A region of interest (ROI) analysis was done as a follow-up to the voxel-wise analysis to evaluate magnitude under the curve of the impulse response function as a direct test of age group and stimulus type by age group differences in the hemodynamic response. To do this, each of the significantly active, functional regions of interest from the voxel-wise comparisons of famous to non-famous stimuli (Recent–Foils, Enduring–Foils) from both participant groups were combined and the unique regions (p < 0.001, volume > 0.200 ml) were retained. For each region, each participant’s IRF for Recent, Enduring, and Foil names was computed. The time-points for 4, 6, and 8 s post-stimulus were summed and used as the dependent variable in separate 2 (age group) × 3 (stimulus condition) repeated measures analysis of variance (ANOVA) computations for each region. Given the repeated measures variable, Greenhouse-Geisser epsilon correction for significance was applied as needed whenever the assumption of sphericity was violated via Mauchley’s test.
3. Results
3.1. Behavioral data
The behavioral data are shown in Table 1. A 2 (group) × 3 (condition) mixed ANOVA for accuracy showed no significant main effect for condition; performance was similar across the three stimulus conditions (F(2,56) = 0.71, p > 0.45). The main effect for group was also not significant (F(1,28) = 1.3, p > 0.25). However, there was a significant interaction of group × condition (F(2,56) = 6.0, p < 0.01). t-Tests showed that older adults correctly identified significantly more Enduring names than young adults, but there was no group difference for the Recent names or Foils.
Table 1.
Percent correct performance and reaction time data for older and younger adult participants by stimulus category (mean ± S.D.)
| Recent | Enduring | Foils | |
|---|---|---|---|
| Accuracy (%) | |||
| Overall | 94.9 (6.9) | 94.5 (6.2) | 93.1 (7.3) |
| Older adults | 92.8 (8.6) | 97.7 (2.9) | 94.6 (6.4) |
| Younger adults | 96.9 (3.9) | 91.3 (6.9) | 91.6 (8.1) |
| t(28) | 1.7 (p > 0.10) | −3.3 (p < 0.01) | −1.1 (p > 0.26) |
| Reaction time (ms) | |||
| Overall | 1195.5 (276.3) | 1042.5 (191.4) | 1586.2 (404.3) |
| Older adults | 1317.1 (286.7) | 1007.1 (186.5) | 1540.1 (388.1) |
| Younger adults | 1073.8 (210.1) | 1077.9 (195.5) | 1632.2 (428.4) |
| t(28) | −2.7 (p < 0.02) | 1.0 (p > 0.30) | 0.6 (p > 0.54) |
Recent: names becoming famous during the 1990s; Enduring: names becoming famous in the 1950s and maintaining fame to the current day; Foils: unfamiliar names.
A 2 (group) × 3 (condition) mixed ANOVA for reaction time showed a significant main effect for condition (F(2,56) = 79.3, p < 0.001). t-Tests showed that responses to Foils were significantly slower than responses to Enduring names (t(29) = −11.3, p < 0.001) or Recent names (t(29) = −6.5, p < 0.001). The main effect for group was not significant (F(1,28) = 0.08, p > 0.78). However, there was a significant interaction of group × condition (F(2,56) = 8.9, p < 0.001). t-Tests showed that older adults were slower than younger adults to recognize the Recent names, while the groups were comparable in reaction time when recognizing Enduring names and Foils.
3.2. Voxel-wise analyses
Significant clusters of activation for the Enduring names versus Foils are reported in Table 2. The functional maps of these clusters are presented in Fig. 1. The network of activation associated with Enduring famous names involved bilateral middle temporal gyrus, anterior cingulate, right insula, posterior cingulate, and left caudate for the older participants. Among the younger participants, the Enduring names activated the posterior cingulate and the right superior frontal gyrus.
Table 2.
Locations of active clusters in the younger and older groups by condition
| Younger
|
Condition | Older
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vol | X | Y | Z | Region | Vol | X | Y | Z | Region | |
| 2237 | 1.2 | −52.2 | 25.5 | Bilateral posterior cingulate gyrus | EN > FO | 3321 | 0.6 | −51.6 | 18.7 | Bilateral posterior cingulate gyrus |
| 322 | 25.7 | 23.1 | 48.0 | Right superior frontal gyrus | 574 | −23.9 | −20.2 | 29.3 | Left caudate body | |
| 489 | −47.7 | −68.2 | 19.4 | Left middle temporal gyrus | ||||||
| 441 | 1.4 | 44.3 | 0.8 | Bilateral anterior cingulate gyrus | ||||||
| 396 | 44.5 | −60.1 | 10.1 | Right middle temporal gyrus | ||||||
| 213 | 28.5 | −17.4 | 26.2 | Right insula | ||||||
| 3548 | −2.2 | −52.0 | 26.3 | Bilateral posterior cingulate gyrus | RE > FO | 10785 | −3.2 | −51.4 | 19.6 | Bilateral posterior cingulate gyrus |
| 1630 | −51.2 | −56.2 | 23.7 | Left superior temporal gyrus | 2543 | −11.8 | 36.4 | 40.6 | Left medial frontal gyrus | |
| 600 | 50.1 | −10.6 | −16.2 | Right middle temporal gyrus | 2158 | −45.6 | −68.8 | 19.8 | Left middle temporal gyrus | |
| 1472 | −27.7 | 9.9 | 51.5 | Left superior frontal gyrus | ||||||
| 944 | −56.3 | −39.2 | −7.9 | Left middle temporal gyrus | ||||||
| 793 | 48.4 | −61.3 | 12.2 | Right middle temporal gyrus | ||||||
| 700 | 15.6 | 6.0 | 17.4 | Right caudate nucleus | ||||||
| 447 | 16.0 | −18.3 | −15.1 | Right hippocampal gyrus | ||||||
| 353 | 25.7 | −40.3 | −18.9 | Right culmen | ||||||
| 300 | −44.6 | 27.8 | 0.1 | Left inferior frontal gyrus | ||||||
| 275 | −24.9 | −24.0 | 26.0 | Left insula | ||||||
| 249 | 22.6 | −25.7 | −20.5 | Right parahippocampus | ||||||
| 236 | −16.0 | 19.5 | 42.0 | Left superior frontal gyrus | ||||||
| 222 | 57.7 | −11.3 | −14.5 | Right inferior temporal gyrus | ||||||
| 208 | 32.4 | −22.8 | −12.4 | Right hippocampus | ||||||
| 206 | 8.8 | −22.3 | 0.0 | Right thalamus | ||||||
| 204 | 42.5 | −42.3 | −20.1 | Right culmen/fusiform | ||||||
| RE > EN | 1944 | −6.5 | −52.3 | 21.7 | Bilateral posterior cingulate gyrus | |||||
| 760 | −44.5 | 18.0 | 1.1 | Left inferior frontal gyrus | ||||||
| 332 | −5.1 | 4.2 | 57.8 | Left medial frontal gyrus | ||||||
| 319 | 3.0 | 44.5 | 27.7 | Right medial frontal gyrus | ||||||
| 271 | 1.6 | −20.3 | −0.7 | Right red nucleus | ||||||
| 238 | −11.6 | −1.8 | 5.6 | Left lentiform nucleus | ||||||
| 232 | −45.6 | 13.7 | 23.8 | Left inferior frontal gyrus | ||||||
| 220 | −54.2 | 7.9 | 3.5 | Left precentral gyrus | ||||||
EN = Enduring famous names; RE = Recent famous names; FO = Foils. Volume (Vol) is microliters. Coordinates are center of mass in mm from the anterior commissure (Talairach and Tournoux, 1988) with positive = right (X), anterior (Y) and superior (Z). There were no significant clusters of activation for the following comparisons: FO > EN, FO > RE, EN > RE.
Fig. 1.
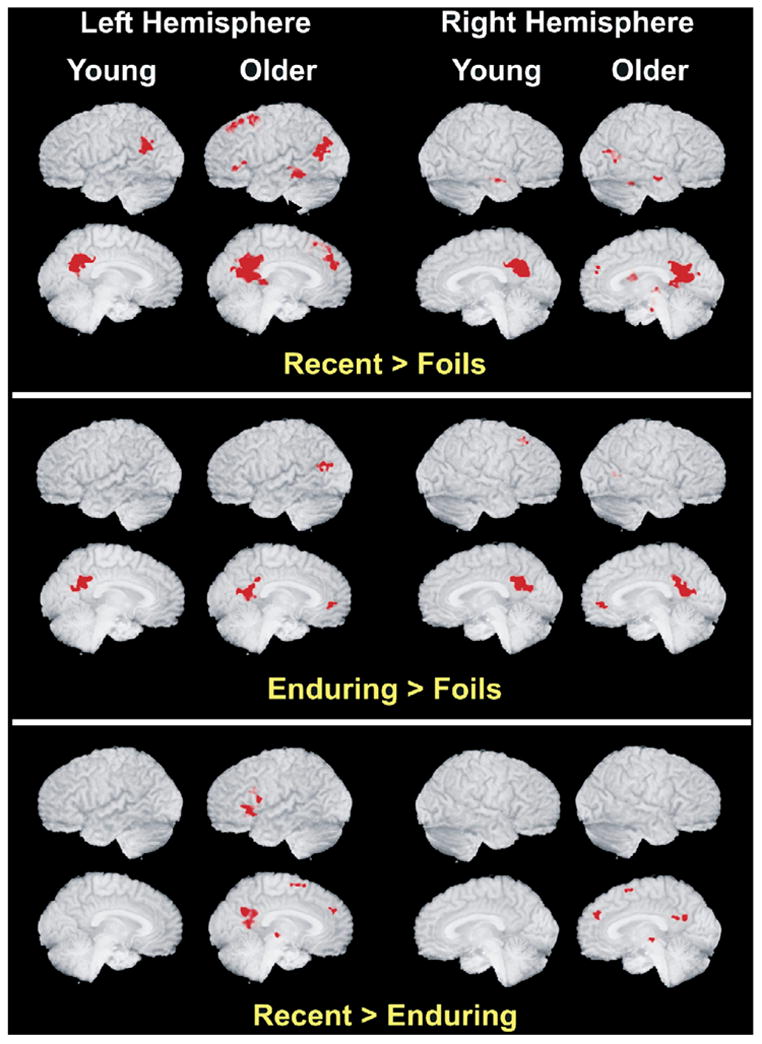
Depicted are the regions of significant activation for the Recent condition (top panel) and the Enduring condition (bottom panel) relative to Foils, separately for older and younger participants. Older adults exhibited more extensive activation and recruited additional brain regions as compared with younger adults. See Table 2 for region locations and coordinates.
Significant clusters of activation for the Recent names versus Foils are reported in Table 2. The functional maps of these clusters are presented in Fig. 1. Activation associated with the Recent names was similar to the Enduring condition with multiple additional frontal regions of activation and an apparent greater overall extent of activation in both age groups. In the younger participants, the Recent names activated a network consisting of the posterior cingulate, the left superior temporal gyrus, and the right middle temporal gyrus. In the older participants, the Recent names activated a larger network, including the posterior cingulate, bilateral middle temporal gyri, left medial frontal gyrus, left superior frontal gyrus, bilateral inferior frontal gyri, right caudate, right parahippocampus, right hippocampus, right culmen and fusiform gyri, left insula and right thalamus.
Subtracting the Enduring from the Recent condition in the younger group produced no significant clusters, but in the older group it resulted in significantly greater activation in the Recent condition (see Table 2). This network was a predominantly frontal lobe circuit consisting of the left inferior frontal gyrus, the bilateral medial frontal gyri, left prefrontal gyrus, posterior cingulate, right red nucleus, and left lentiform nucleus.
3.3. ROI analyses
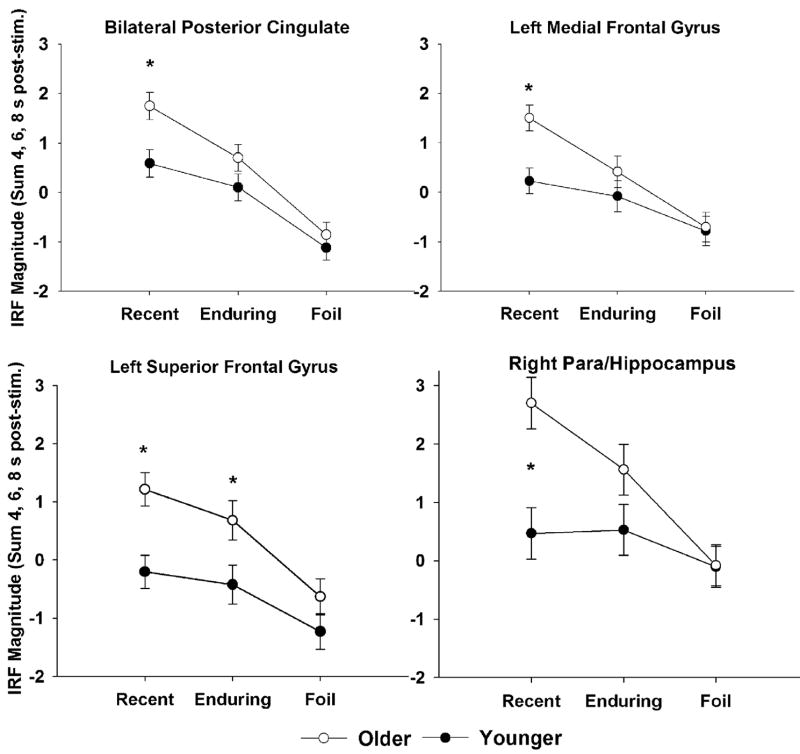
The results of the ROI analyses showed that of the 20 clusters evaluated, 15 exhibited significant group differences. In all cases, activation was greater for older than younger participants and the majority of differences were attributable to Recent names. Each of these clusters is described with statistical results in Table 3 and eight representative clusters are shown with their anatomical localizations and full time-course, group averaged impulse response functions in Fig. 2. As noted in Table 3, only one cluster exhibited departure from sphericity by Mauchley’s test; this cluster was assessed with epsilon adjusted degrees of freedom. Thirteen clusters had significant group by condition interaction effects whereby older adults had greater activation than young adults in famous names versus foils. Two of these clusters ANOVA results are depicted in the right half of Fig. 3. Specifically, all 13 were significantly different between groups for Recent names and seven were significant for Enduring names, but no clusters differed between groups for Foils. Two clusters, the bilateral posterior cingulate and left superior frontal gyrus, had only group main effects, showing overall greater older adult than young adult activation. Importantly, post-hoc contrasts showed that the main effects were due to group differences in response to famous names but not to non-famous names. The ANOVA results for these two regions are depicted in the left panel of Fig. 3.
Table 3.
Combined active clusters showing the significant group and stimulus condition effects
| Region | Vol. | X | Y | Z | ANOVA Result (F(p)) | Significant contrasts (p < .05) and direction |
|---|---|---|---|---|---|---|
| Frontal lobe gyri/regions | ||||||
| Left medial frontal | 2543 | −11.5 | 36.7 | 40.2 | C × G: 4.3 (0.02) | O > Y; Recent |
| Left superior frontal | 1472 | −27.7 | 9.9 | 51.5 | G: 8.2 (0.008) | O > Y; Recent, Enduring |
| Left inferior frontal | 300 | −44.7 | 27.8 | 0.1 | C × G: 5.8 (0.005) | O > Y; Recent |
| Left superior frontal | 236 | −16.0 | 19.5 | 42.0 | G: 6.0 (0.02); C × G: 7.2 (0.002) | O > Y; Recent, Enduring |
| Temporal lobe gyri/regions | ||||||
| Right middle temporal | 1011 | 47.1 | −61.0 | 11.7 | G: 5.4 (0.03); C × G: 3.6 (0.03) | O > Y; Recent, Enduring |
| Left middle temporal | 944 | −56.2 | −39.1 | −7.9 | C × G: 4.1 (0.02) | O > Y; Recent |
| Right para/hippocampus | 447 | 16.0 | −18.3 | −15.3 | G: 7.1 (0.01); C × G: 4.8 (0.01) | O > Y; Recent |
| Right insula | 213 | 28.4 | −17.4 | 26.2 | C × G: 8.8 (0.001)a | O > Y; Recent, Enduring |
| Right hippocampus | 208 | 32.3 | −22.7 | −12.4 | C × G: 3.5 (0.04) | O > Y; Recent |
| Parietal lobe/other regions | ||||||
| Bilateral posterior cingulate | 12461 | −2.5 | −51.3 | 20.5 | G: 5.4 (0.03); C × G: 2.4 (0.10) | O > Y; Recent |
| Right caudate | 700 | 15.6 | 6 | 17.5 | C × G: 11.9 (0.001) | O > Y; Recent, Enduring |
| Left caudate | 691 | −24.2 | −21.5 | 28.4 | C × G: 9.3 (0.001) | O > Y; Recent, Enduring |
| Right culmen | 353 | 25.6 | −40.3 | −19.1 | C × G: 10.4 (0.001) | O > Y; Recent |
| Right thalamus | 206 | 8.9 | −22.3 | −0.1 | G: 5.7 (0.025); C × G: 9.5 (0.001) | O > Y; Recent |
| Right fusiform | 204 | 42.5 | −42.2 | −20.1 | C × G: 6.0 (0.004) | O > Y; Recent, Enduring |
Vol. (volume) is in microliters. Coordinates are center of mass in mm from the anterior commissure (Talairach and Tournoux, 1988) with positive = right (X), anterior (Y) and superior (Z).
Greenhouse-Geisser corrected d.f. (degrees of freedom) for non-sphericity = 1.6, 44.3; no other clusters violated the sphericity assumption. Group (G) main effects d.f.: 1,28; condition (Recent, Enduring, Foil) by group (C × G) d.f.: 2,56.
Fig. 2.
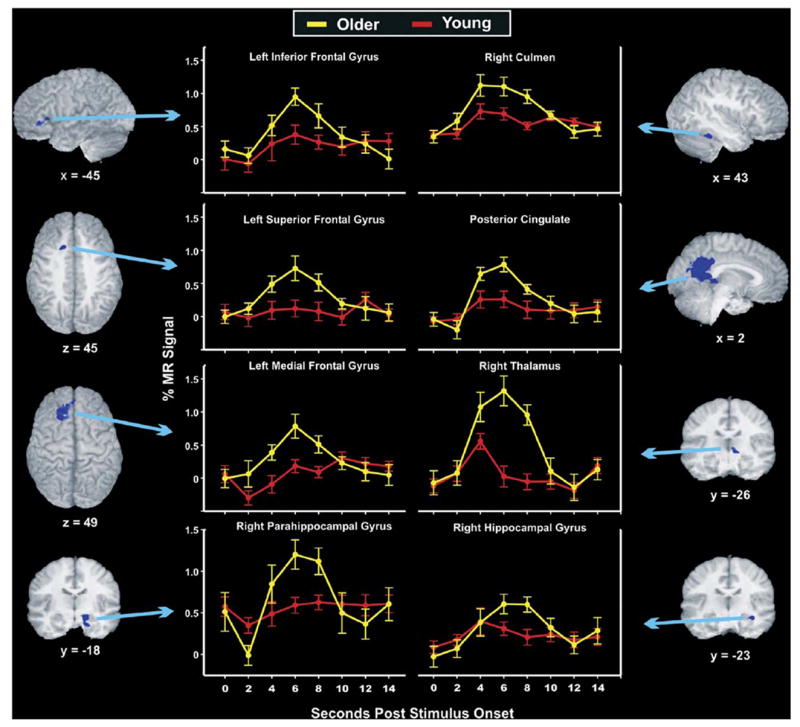
Eight of the 15 regions that showed significant age group differences in the estimates of the hemodynamic response (area under the curve for 4–8 s post-stimulus) are depicted with anatomical localization and full time-course, group averaged impulse response functions for Recent famous names. In all significant analyses, older participants had greater activation than younger participants, which occurred predominantly for the Recent condition as compared with Foils (see Table 3 and Fig. 3).
Fig. 3.
Four representative regions are shown by group and stimulus condition to demonstrate the results of the ROI analysis by ANOVA. The right panel shows two of the 13 clusters with significant group by condition interaction effects where elders had greater activation than younger participants but only for famous names. The left panel shows the two clusters with only group main effects. Importantly, these also show the same pattern; although the interaction was not significant, group differences were limited to famous names. All clusters that had significant effects depicted these same patterns; foil stimuli did not differ by group. Thus, age group effects could not be attributed to baseline activation differences. *p < 0.05.
4. Discussion
Recent neuroimaging research has shown that older adults frequently exhibit recruitment, or greater activation than younger adults, at least under conditions of comparable performance on a variety of cognitive tasks. The purpose of the current study was to test whether recruitment occurs in semantic memory by evaluating age-related differences in neural activation during a famous name recognition task.
Even under comparable performance conditions, a variety of aging studies point to increased regional activation in elders in task-specific areas and in the prefrontal lobes [5,6,11,15–17,29,30,34,38,41,42]. Recruitment theory suggests this increased activation helps to compensate for age-related neural changes. As predicted, the older participants in the present study activated comparable networks to those of younger participants but they were more extensive overall, more extensive within the clusters commonly activated by both groups (e.g., posterior cingulate), and they activated additional regions particularly in prefrontal areas. The ROI analysis also showed that older adults had significantly greater magnitude of activation in seven task-related clusters including the left superior frontal, right middle temporal, right insula, bilateral caudate and right fusiform gyri for the Enduring names, and 15 of 20 clusters for the Recent condition, including four left prefrontal clusters, bilateral temporal, right hippocampal, right insula, bilateral posterior cingulate, right thalamus, and right fusiform regions. Importantly, there were no differences attributable to foil stimuli, thereby eliminating any concerns about non-specific activation increases in elders. Our findings are thus consistent with a number of other recent studies of perceptual-motor, short-term episodic memory and executive functions (e.g., inhibitory control) showing greater activation in elders in both task-related areas and some predominantly left prefrontal regions that may be supplemental to task-dependent areas [5,6,11,15,17,29,30,34,38,41,42]. The current findings thereby extend this literature to semantic memory tasks. Additionally, the presence of multiple prefrontal clusters, particularly in the left hemisphere, with greater activation in older adults is consistent with the existing literature suggesting that the frontal lobes play a central role in age-related cognitive changes [1,21,28,40,57] and in the compensatory activation engaged as a result [5,16,41]. From the present results, this compensation is task-specific and is apparent even when task accuracy is very high.
Importantly, most of the early studies showed recruitment in conjunction with reduced activation in other task-related areas. In contrast, our results and those of several recent studies [11,29,30,41,42] found no areas of reduced activation in elders. The hippocampal complex exhibited greater activation in elders along with several left prefrontal regions. This contrasts with two recent studies that showed prefrontal activation increases were associated with decreases in hippocampal activity [16,19]. The use of an event-related procedure, which allows removal of error trials, may have reduced error-based contributions to the functional maps, resulting in less evidence of age-related activation reductions than some previous studies. Importantly, the removal of error trials can leave fewer trials for analysis, which is typically more an issue for older participants than for young ones. Such losses can lead to increased variability and decreased signal to noise ratio [10]. In the present study however, older adults did not make more errors than younger adults; indeed, they made significantly fewer errors in the Enduring condition. Thus the comparisons made were on an equivalent basis between groups and are not likely to reflect either error or variance differences.
Some have raised caution about using BOLD fMRI because of potential alterations in the hemodynamic response due to the aging process itself or clinical conditions associated with it [9]. However, we have shown that when healthy, unmedicated elders perform cognitively challenging tasks, rather than strictly perceptual or simple motor tasks, the hemodynamic response is identical to that of young adults [42]. Moreover, in such demanding cognitive tasks we have consistently found increased activation in elders, which would not be predicted under conditions of vascular insufficiency or altered BOLD response. Indeed, in the current study, the analysis of individual impulse response functions made it clear that the response parameters are not abnormal in elders. First, ANOVA results showed that all group differences were attributable to famous names; non-famous names did not differ (see Table 3 and Fig. 3). Second, Fig. 2 shows the regions that differed statistically between the groups, showing that the hemodynamic responses of the groups are very comparable except in magnitude, and then only between approximately 4–8 s post-stimulus, when task- and response-related effects are expected. Thus, hemodynamic response differences cannot explain the group differences. These cautions are important, however, in highlighting that the age-related differences we report here may not fully characterize what occurs in the general population of older adults who have more significant health issues than the population from which we sampled. Such studies remain to be performed and might not be adequately served by BOLD fMRI.
Functional neuroimaging studies involving the passive viewing of verbal materials have identified a general semantic memory network that is predominantly left lateralized and includes the left prefrontal, temporal, anterior cingulate, and cerebellar regions [2]. Studies using person-specific stimuli (faces and names) have identified additional regions and implicated a bilateral network including the bilateral anterior and lateral temporal lobe [31], bilateral hippocampus and parahippocampus [12,20,25,31,52], and medial frontal, superior frontal and bilateral posterior cingulate regions [31]. The regions activated by the current task were consistent with these studies (see Tables 2 and 3; Fig. 1) in both age groups, and activation was more extensive and greater in magnitude in older adults than young adults, which was attributable to famous rather than non-famous names. The network of activation associated with Enduring names was less extensive than for Recent names, particularly in older adults who had especially extensive posterior cingulate and prefrontal activation for Recent names. Thus, results make clear that there were age-related differences in regions that were more specific to the general information retrieval aspects of task as well as in the areas specifically associated with person-identity retrieval (e.g., posterior cingulate, prefrontal regions). In addition, there was extensive left pre-frontal activation, particularly in medial and inferior frontal gyri, consistent with various cognitive tasks used to study aging [5,6,11,15–17,29,30,34,38,41,42].
It is not yet definitively known why the Recent names activated more extensive regions than Enduring names in elders. The posterior cingulate shows increased activation with increased familiarity with or exposure to initially unfamiliar faces [27] and it is centrally involved in the retrieval of prior knowledge [7,36]. Further, it plays a role in emotion processing [35], which is of particular importance with famous names because it is increasingly believed that famous names carry both a semantic and an autobiographical (episodic) component [12,58,59]. The autobiographical component might interact with the degree of emotionality or vividness associated with the name [59], which may be reflected in the posterior cingulate and right hippocampal activation during their retrieval. We previously reported right hippocampal activation associated with both Recent and Remote famous names as compared with Foils, and Recent names produced greater activation than Remote names (e.g., Britney Spears > Tab Hunter). It is possible that emotional valence or intensity associated with a famous name might decline over time [12,37]. On the other hand, other factors such as recency and frequency of exposure may also explain the differences observed between these conditions.
Because name recognition performance was comparable between groups but reaction time was slower in older adults for the Recent condition, it is also possible that the greater older adult activation here reflects greater difficulty or effort for retrieval processes [51]. Slowed reaction time is particularly common when task demands are high and under these conditions is associated with increased prefontal activation [26,53]. Indeed, the subtraction of the Enduring from the Recent condition (correct trials only) showed significantly greater activation in the posterior cingulate as well as in multiple frontal regions for older adults in the Recent condition. Yet, following this logic, the greatest activation might be expected to occur for Foil trials, as these received the slowest responses in both groups and for all conditions. But in fact, there were no regions significantly more active for Foil trials than for famous name trials. Perhaps decision making is a better way to conceptualize this issue than task difficulty. The medial frontal gyrus has been associated with cognitive control related to decision uncertainty and response conflict [24,49]. This region showed greater older adult activation for Recent names, which taken with the slowed reaction time for these names, might suggest older adults were less certain or confident in their decisions about Recent names. Elders may have had more recent exposure to Recent names, but these likely have had far fewer total exposures and are potentially less personally meaningful than Enduring names (e.g., Britney Spears versus Frank Sinatra). Young adults would not be expected to have notably greater personal information or exposure to Enduring names than Recent names because their exposure to all these names would have been relatively recent. Factors involving recency and frequency of exposure, valence, arousal, and extent of knowledge about names from these three categories should also be considered and will be explored in future studies.
In conclusion, older adults performed comparably to young adults on a famous name recognition task, but the older adults had significantly greater activation in multiple brain regions, including posterior cingulate, right hippocampus and several left prefrontal regions. The regions activated were consistent with those expected for a famous name task, with more extensive activation and additional regions of left prefrontal activation in elders. Importantly, age differences were particularly attributable to the famous names rather than non-famous names, which assures that age-related activation differences are task-driven rather generalized or non-specific. The age-related results are consistent with studies of other types of memory, as well as executive function tasks recently published, and they suggest that older adult brains use functional recruitment to support task performance. Importantly, this is evident even when task accuracy is high.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01 AG022304), the Medical College of Wisconsin General Clinical Research Center (M01 RR00058), the Advancing a Healthier Wisconsin program, and the W.M. Keck Foundation. We would like to thank Jill Dorflinger, Sally Durgerian, Cathy Elsinger, and Amanda Moths for their assistance.
References
- 1.Arbuckle T, Gold D. Aging, inhibition, and verbosity. J Gerontol: Psychol Sci. 1993;48:225–82. doi: 10.1093/geronj/48.5.p225. [DOI] [PubMed] [Google Scholar]
- 2.Binder JR, Price C. Functional imaging of language processing. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 3.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL, Logan J, Donaldson D, Wheeler M. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12:24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002:17. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 6.Cabeza R, Grady C, Nyberg L, McIntosh A, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 8.Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 9.D’Esposito M, Deouell LY, Gazzaley A. Alteration in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev. 2003;4:863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 10.D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- 11.DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12:2065–71. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- 12.Douville KL, Woodard JL, Seidenberg M, Leveroni CL, Nielson KA, Franczak M, et al. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Gorno Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, et al. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–18. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- 15.Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–62. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grady CL, McIntosh AR, Craik FI. Task-related activity in the prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005:1466–81. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–21. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 18.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 19.Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- 20.Haist F, Bowden GJ, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–45. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- 21.Hasher L, Zacks R. Working memory, comprehension and aging: a review and a new view. Psychol Learn Motiv. 1988;22:193–225. [Google Scholar]
- 22.Hodges JR, Bozeat S, Lambon Ralph MA, Patterson K, Spatt J. The role of conceptual knowledge in object use evidence from semantic dementia. Brain. 2000;123:1913–25. doi: 10.1093/brain/123.9.1913. [DOI] [PubMed] [Google Scholar]
- 23.Huettel S, Singerman J, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–75. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- 24.Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci. 2005;25:3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995;31:99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- 26.Klingberg T, O’Sullivan BT, Roland PE. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb Cortex. 1997;7:465–71. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka H, Omori M, Iidaka T, Murata T, Shimoyama T, Okada T, et al. Neural substrates participating in acquisition of facial familiarity: an fMRI study. Neuroimage. 2003;20:1734–42. doi: 10.1016/s1053-8119(03)00447-6. [DOI] [PubMed] [Google Scholar]
- 28.Kramer A, Humphrey D, Latish J, Logan G, Strayer D. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- 29.Langenecker SA, Nielson KA. Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage. 2003;20:1384–92. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- 30.Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20:878–86. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madden D, Turkington T, Provenzale J, Denny L, Hawk T, Gottlob L, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Map. 1999;7:115–35. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madden DJ, Hoffman JM. Application of positron emission tomography to age-related cognitive changes. In: Krishman KRR, Doraiswamy PM, editors. Brain imaging in clinical psychiatry. New York, NY: Marcel Dekker; 1997. [Google Scholar]
- 34.Madden DJ, Turkington TG, Provenzale JM, Hawk TC, Hoffman JM, Coleman RE. Selective and divided visual attention: age-related changes in regional cerebral blood flow measured by M2150 PET. Hum Brain Map. 1997;5:389–409. doi: 10.1002/(SICI)1097-0193(1997)5:6<389::AID-HBM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 36.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 37.Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–7. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- 39.McDowd J, Oseas-Kreger D. Aging, inhibitory processes, and negative priming. J Gerontol: Psychol Sci. 1991;46:340–5. doi: 10.1093/geronj/46.6.p340. [DOI] [PubMed] [Google Scholar]
- 40.Moscovitch M, Winocur G. Frontal lobes, memory, and aging. In: Grafman J, Holyoak K, Bohler F, editors. Structure and functions of the human prefrontal cortex. Vol. 769. New York: Annals of the New York Academy of Sciences; 1995. pp. 119–50. [DOI] [PubMed] [Google Scholar]
- 41.Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult lifespan. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- 42.Nielson KA, Langenecker SA, Ross TJ, Garavan H, Rao SM, Stein EA. Comparability of functional MRI response in young and old during inhibition. Neuroreport. 2004;15:129–33. doi: 10.1097/01.wnr.0000093293.85057.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 44.Parkin A, Walter B. Recollective experience, normal aging and frontal dysfunction. Psychol Aging. 1992;7:290–8. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- 45.Randolph C. Repeatable battery for the assessment of neuropsychological status. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 46.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 47.Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salt-house TA, editors. Handbook of aging and cognition II. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- 48.Raz N. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–8. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 49.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 50.Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–15. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 51.Salthouse T. The processing speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 52.Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115(Pt 1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- 53.Stuss DT, Toth JP, Franchi D, Alexander MP, Tipper S, Craik FIM. Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia. 1999;37:1005–27. doi: 10.1016/s0028-3932(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–8. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Szentkuti A, Guderian S, Schiltz K, Kaufmann J, Munte TF, Heinze HJ, et al. Quantitative MR analyses of the hippocampus: unspecific metabolic changes in aging. J Neurol. 2004;251:1345–53. doi: 10.1007/s00415-004-0540-y. [DOI] [PubMed] [Google Scholar]
- 56.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 57.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 58.Westmacott R, Black SE, Freedman M, Moscovitch M. The contribution of autobiographical significance to semantic memory: evidence from Alzheimer’s disease, semantic dementia, and amnesia. Neuropsychologia. 2004;42:25–48. doi: 10.1016/s0028-3932(03)00147-7. [DOI] [PubMed] [Google Scholar]
- 59.Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Mem Cogn. 2003;31:761–74. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]