Abstract
A central role of the hippocampus is to consolidate conscious forms of learning and memory, while performance on implicit tasks appears to depend upon other structures. Recently, considerable debate has emerged about whether hippocampal-dependent tasks necessarily entail task awareness. In the contextual cueing task, repetition facilitation is implicit, but impaired in patients with amnesia. Whether the hippocampus alone or other MTL structures are required is unclear. Event-related functional magnetic resonance imaging revealed hippocampal activity that differentiates novel from repeated arrays. This pattern of results was observed without recognition of the repeating arrays. This finding provides support for the claim that the hippocampus is involved in processes outside the domain of conscious learning and memory.
Hippocampal differentiation without recognition: An fMRI analysis of the contextual cueing task
It has long been understood that damage to the hippocampus and medial temporal lobe structures (MTL) in humans diminishes or eliminates the capacity to form new long-term episodic (autobiographical) and semantic (knowledge) memory (Milner 1972; Takashima et al. 2006). Together, episodic and semantic memory are termed declarative memory (Cohen et al. 1985) because they both require conscious or deliberative access. Conversely, numerous studies demonstrate implicit forms of learning and memory that are not substantially affected by damage to the hippocampus or MTL (Keane et al. 1995; Stark and Squire 2000). Implicit forms of learning and memory are demonstrated by experience-dependent changes in task performance and do not require conscious recollection. Examples of implicit forms of learning and memory include perceptual priming or facilitation (repetition leads to greater accuracy and shorter response latency), procedural or skill learning, and simple forms of classical conditioning. The most common interpretation of this evidence is that distinct systems mediate declarative and implicit forms of learning and memory (Squire and Zola 1996; Cohen et al. 1997). Accordingly, the hippocampus would be critically involved in learning and memory if, and only if, conscious awareness of the contingencies occurs (Clark and Squire 1998; Reed and Squire 1999; Manns and Squire 2001; Smith et al. 2006).
While there is no meaningful dispute that the hippocampus is required for conscious learning and memory formation, it may serve a broader function. Several studies have been recently published suggesting that the hippocampus is also implicated in certain implicit tasks (Chun and Phelps 1999; Ryan et al. 2000; Greene et al. 2006). However, for some of these tasks there is controversy about whether they are indeed implicit tasks (Smith and Squire 2005; Smith et al. 2006; Greene 2007), while for others there is controversy as to whether the task depends upon the hippocampus (Manns and Squire 2001).
In the contextual cueing task (Chun and Jiang 1998), context-dependent target search is impaired in MTL amnesics but does not depend upon recognition (Chun and Phelps 1999). An array of distractors provides a unique context that determines the target (rotated “T”) location (see Fig. 1). Normal memory participants show repetition facilitation even though they do not recognize repeated arrays; amnesic patients with MTL damage do not show repetition facilitation (Chun and Phelps 1999). Both normal memory participants and amnesic patients show a practice-related reduction in reaction time (RT), but only normal memory patients show additional RT facilitation for repeated arrays. Normal memory participants show near chance performance on a subsequent recognition task, indicating that conscious memory processes cannot account for the repetition facilitation effects. This finding demonstrates repetition facilitation, which is impaired in amnesia but is not attributable to declarative memory.
Figure 1.
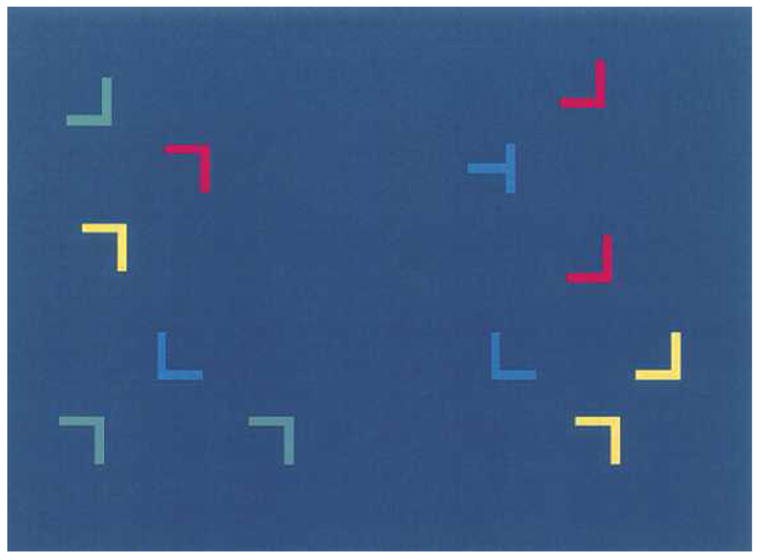
Typical stimulus array. The task was to locate the rotated “T” from among the rotated L distractors. The position and color of the distractors serves as a context that determines the location of the target.
One problem with the interpretation of this finding is ambiguity over whether the hippocampus proper or other temporal lobe structures are mediating repetition facilitation in normal memory participants. The extent of hippocampal damage was assessed in the patient group, but not the extent of concomitant damage to other temporal lobe regions (Chun and Phelps 1999), so the observed impairment could be due to the loss of extra-hippocampal structures. More recently the contextual cueing task produced differential outcomes for hippocampus-only amnesics (CA fields and dentate gyrus only) compared with temporal-lobe amnesics (broad damage to include most of the MTL, virtually all of the hippocampus, as well as other temporal-lobe regions). The temporal-lobe amnesics showed no repetition facilitation, while the hippocampus-only amnesics did show near normal repetition facilitation (Manns and Squire 2001). This suggests that the ability to implicitly apprehend specific cue-context relations may depend on extra-hippocampal structures and not the hippocampus proper. Thus, the existing evidence does not rule out the hypothesis that the hippocampus is required only for declarative tasks. Problematically, while the temporal-lobe amnesics had nearly complete hippocampal loss, the hippocampus-only amnesics averaged only ~32% loss of hippocampal tissue with none greater than a 50% loss (Manns and Squire 2001). Among the hippocampus-only amnesics, if the damaged tissue included critical pathways, the remaining hippocampal volume could be functionally ineffective (e.g., Gold and Squire 2005). On the other hand, hippocampal damage can spare task-critical pathways (e.g., Mayanagi et al. 2001), in which case the remaining hippocampal tissue could have mediated performance in these patients. Whether contextual cueing is a hippocampal-dependent task is, therefore, unresolved.
One approach that may clarify ambiguous neuropsychological findings is to use functional imaging to elucidate the role of the hippocampus during performance of the contextual cueing task in memory-normal participants. If repetition facilitation on the contextual cueing task depends upon extra-hippocampal temporal lobe structures, we should observe distinct patterns of hemodynamic activity associated with novel and repeated items within temporal-lobe regions but not within the hippocampus proper. If repetition facilitation on the contextual cueing task requires the hippocampus, we should observe distinct patterns of hemodynamic activity for novel and repeated items within the hippocampus proper, which may or may not include extra-hippocampal temporal-lobe regions.
We conducted an event-related fMRI study using the contextual cueing task with 26 participants (Sex: 19 f, 7 m; Age: range = 18–38, mean = 21.4, SD = 4.2). The behavioral methods followed those published elsewhere (Chun and Jiang 1998; Chun and Phelps 1999) for normal memory participants, with the exceptions that we used visual instead of auditory feedback and incorporated a random interstimulus interval (ISI). In each of 20 blocks, 12 repeated and 12 novel arrays were presented. Arrays consisted of one target (“T” intersection rotated 90° or 270°) and 11 distractors (“L” intersection rotated either 0°, 90°, 180°, or 270°). Prior to the scanning session, verbal instructions were given, and participants had one block of training as practice. Items presented during practice were not repeated during the experimental scan session. The task was to locate the target and indicate discovery using the right or left key of a button box to indicate the direction of the tail of the “T” target. Each trial consisted of an array displayed for 3 sec followed by 1 sec of visual feedback. Accuracy and RT were recorded for each trial and only correct responses were considered for further analysis. During each block of trials, 12 fixation screens were randomly intermixed with the 24 arrays—12 novel and 12 repeated—which introduced a random ISI necessary for deconvolution analysis of event-related designs. Immediately after scanning, participants were given a recognition test: The 12 repeated arrays were sequenced randomly with 12 novel arrays, and participants were asked for each array if they recognized the array from the initial trials. The recognition task was not done in the scanner because there were an insufficient number of trials for deconvolution.
The RT data for the contextual cueing task were analyzed with a two-way repeated measures ANOVA. The independent variables were (1) Array Type (novel or repeated), and (2) Block (1–20). For the recognition test, accuracy was assessed with a single-sample t-test to determine whether accuracy differed significantly from chance (0.50).
For the fMRI analysis, we grouped the 20 blocks into five imaging runs, each containing 96 trials and 48 fixations, with an interscan interval (TR) of 2 sec. The scanning apparatus and methods for both functional and anatomical data acquisition were identical to those we have published elsewhere (Greene et al. 2006). Functional images were generated with AFNI software (Cox 1996). Each image time series was time-shifted and then spatially registered to reduce the effects of head motion. To provide adequate trials for deconvolution, behavioral blocks (1–20) were imaged in sequences of four at a time, yielding five fMRI runs. The deconvolution analysis included regressors for array type (novel vs. repeated), run (1–5) and RT (continuous) to extract hemodynamic responses for the 14-sec period post-stimulus onset. RT was included as a regressor in the analyses to identify the extent to which changes in hippocampal hemodynamic response could be attributed to differences in dwell time for novel and repeated items. Any hemodynamic effect is evident either as a main effect of RT, a main effect of stimulus type (orthogonal to RT), or as an interaction of RT by stimulus type. Area under the curve (AUC) was calculated by summing the hemodynamic responses at all timepoints.
Individual images were transformed into standard stereotaxic space (Talairach and Tournoux 1988) and blurred using a 4-mm FWHM Gaussian filter. GLM analyses were conducted for (1) the main effect of accuracy, (2) the main effect of RT, and (3) the interaction of accuracy and RT. The region of interest (ROI) was anatomically defined as the hippocampus (Binder et al. 2005). The cluster threshold was 50 μL within the ROI and 200 μL for whole-brain analyses. Minimum cluster thresholds were established using Monte Carlo simulations with voxelwise P < 0.005 and groupwise α = 0.05 (Cox 1996).
Our behavioral results replicate those found elsewhere for normal-memory participants (Chun and Phelps 1999; Manns and Squire 2001). Participants’ accuracy for determining the direction of the targets was nearly perfect (mean = 97.51% correct). Figure 2 shows the RT data. The main effect of array type (novel versus repeated) was significant (F(1,16) = 12.19, P < 0.01), indicating repetition facilitation (target location for repeated arrays was faster than for novel arrays). The main effect of block (1–20) was significant (F(19,304) = 7.43, P < 0.01), indicating a practice effect. The interaction of array type and block did not reach significance (F(19,304) = 1.24, n.s.), indicating that facilitation did not significantly increase as the number of exposures to repeated items increased. Importantly, the observed repetition facilitation did not depend upon recognition. On the recognition test, we found that participants recognized the arrays at chance (binomial chance = 0.50; Mean = 0.48; t(15) = −0.69, n.s.). To further address the possibility that explicit recognition could influence target-location RT, we assessed decision time on the recognition test for novel versus repeated arrays and found no significant difference (novel RT = 3026.4 ± 145.1 msec; repeated RT = 3039.7 ± 143.2 msec; t <1). That recognition took more than twice as long as target location (see Fig. 2) further underscores that recognition is unlikely to have contaminated implicit performance.
Figure 2.
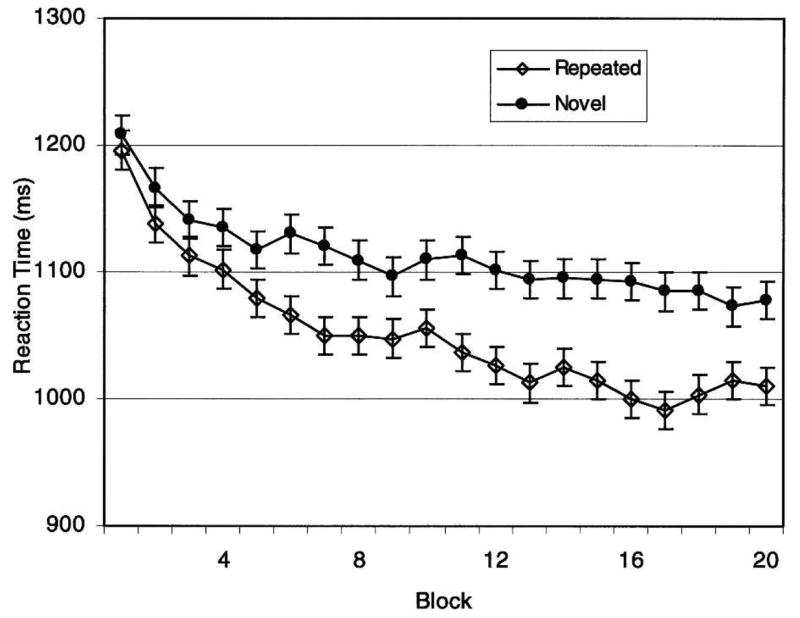
Reaction time data for repeated versus novel arrays. Means ± SEM computed for each of 20 blocks.
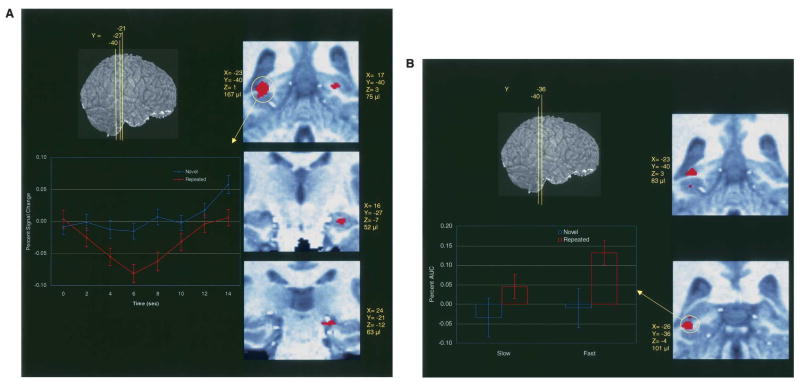
Consistent with our predictions, we found hemodynamic activation in the hippocampal ROIs that were differentially sensitive to array type. Two principle findings are of interest. First, we found a main effect of array type, such that hippocampal activation was less for repeated than for novel arrays (see Fig. 3A). The observed functional deactivation for repeated arrays is consistent with findings wherein repeated encoding results in decreased hippocampal activity (e.g., Zeineh et al. 2003). A second area of activation showed a significant RT by array type interaction (see Fig. 3B), such that for repeated arrays, hippocampal activation increased as RT decreased; that is, hippocampal activity was observed corresponding to repetition facilitation. This activation is comparable to findings from the explicit memory literature demonstrating greater hippocampal activity during successful retrieval (Davachi and Wagner 2002; Strange et al. 2005; Greene et al. 2006). Thus, the hippocampus differentiates repeated from novel arrays in the absence of conscious recognition. While a comparable division of labor between encoding and retrieval is frequently observed in explicit hippocampal tasks, the respective hippocampal subregions involved vary considerably by task and there is little consensus about how to interpret these differences (Gabrieli et al. 1997; Dolan and Fletcher 1999; Greicius et al. 2003; Eldridge et al. 2005). However, in this task, involvement of posterior hippocampal regions for both activations may be due to the spatial nature of the task (e.g., Maguire et al. 2003; Goel et al. 2004). Anatomical connections between the posterior hippocampus, posterior parahippocampal cortices, and parietal systems (Munoz and Insausti 2005) may constitute a network for the acquisition and expression of spatial relational learning (Manns and Eichenbaum 2006; van Asselen et al. 2006).
Figure 3.
The hippocampus differentiates novel from repeated arrays. (A) Main effect of array type: activity is decreased for repeated items. At left is the Impulse Response Function (IRF; mean percent signal change ± SEM) for the largest of the activations (circled in yellow) in the left posterior hippocampus. The IRF shows a decrease in functional activity for repeated items relative to novel items and baseline. Maps of hemodynamic activity are shown on the right. Coordinates (Talairach and Tournoux 1988) and volumes are provided adjacent to each activation. The top left image provides the index (Y-coordinate) for the positions of three coronal slices for four distinct clusters. (B) The interaction of array type by reaction time: only repeated items show activation that is inversely correlated with reaction time. Faster reactions correspond to greater activity. At the left is an interaction plot (the area under the curve for the IRF) for the larger of the two activations. For graphing purposes, the continuous RT variable was split along its median. The functional activity shows that repeated arrays at faster RTs show increased hemodynamic activity compared with novel arrays and repeated arrays at slower RTs, which do not differ significantly from zero. Maps of hemodynamic activity are shown on the right.
Importantly, the hippocampal activations are not attributable to differential dwell time for novel and repeated arrays: (1) there was no main effect of RT within the hippocampus; (2) the main effect of array type was orthogonal to RT; and (3) in the interaction, only repeated arrays showed greater activation and only at faster RTs.
Whole-brain tables for factors in the general linear model (GLM) are shown in Table 1. Note that several temporal lobe regions to include the MTL are involved in both the main effect of array type and in the interaction of array type by RT. While the present experiment was designed to test the involvement of the hippocampus proper in the contextual cueing task, most treatments of declarative memory assert that neither the hippocampus nor surrounding MTL regions are involved in implicit tasks (Squire and Zola 1996; Squire et al. 2004); on the other hand, processes involved in familiarity may involve certain MTL regions, but not the hippocampus (for review, see Eichenbaum et al. 2007).
Table 1.
Whole brain activations
| Region | Hemisphere | Volume (uL) | x | y | z |
|---|---|---|---|---|---|
| Main effect of stimulus type | |||||
| Frontal | |||||
| Middle Frontal Gyrus | L | 2266 | −35.1 | −7.2 | 46.8 |
| Medial Frontal Gyrus | L | 1139 | −5.6 | 3.5 | 51.9 |
| Medial Frontal Gyrus | R | 570 | 8.9 | −9 | 53.2 |
| Inferior Frontal Gyrus | L | 492 | −43.5 | 7.6 | 20.5 |
| Insula | L | 443 | −42.2 | −28.4 | 18.7 |
| Precentral Gyrus | R | 369 | 19.9 | −22.6 | 59.9 |
| Insula | L | 344 | −33.3 | −19.5 | 16 |
| Superior Frontal Gyrus | L | 242 | −15.7 | 26.1 | 42.3 |
| Cingulate | |||||
| Cingulate Gyrus | L | 222 | −6.1 | 16.9 | 30.1 |
| Parietal | |||||
| Postcentral Gyrus | L | 942 | −34 | −28.8 | 46.5 |
| Temporal | |||||
| Superior Temporal Gyrus | L | 244 | −48.9 | −25.7 | −2.1 |
| Middle Temporal Gyrus | L | 229 | −44.6 | −68.1 | 13.7 |
| Middle Temporal Gyrus | L | 219 | −39.9 | −68 | 25.5 |
| Parahippocampal Gyrus | L | 455 | −21.7 | −40.9 | −4.4 |
| Parahippocampal Gyrus | R | 348 | 16.5 | −43.3 | 4.2 |
| Occipital | |||||
| Middle Occipital Gyrus | L | 284 | −42.6 | −69.4 | 4.9 |
| Subcortical | |||||
| Culmen | L | 615 | −8.7 | −44.1 | −4.7 |
| Caudate | L | 337 | −9 | 6.3 | 6.8 |
| Thalamus | R | 285 | 10.6 | −18.5 | −0.1 |
| Lentiform Nucleus | L | 226 | −29 | −19.8 | 0.4 |
| Interaction of RT by stimulus type | |||||
|
| |||||
| Frontal | |||||
| Insula | L | 447 | −35.7 | −15.5 | 15.9 |
| Medial Frontal Gyrus | L | 438 | −6.5 | 3.9 | 52.3 |
| Insula | L | 303 | −42.8 | −28.4 | 18.7 |
| Precentral Gyrus | L | 289 | −37.1 | −9.5 | 54.6 |
| Medial Frontal Gyrus | R | 269 | 8.7 | −10.1 | 52.9 |
| Superior Frontal Gyrus | L | 231 | −15.3 | 25.3 | 42.2 |
| Middle Frontal Gyrus | L | 219 | −29.7 | −12.8 | 45.4 |
| Temporal | |||||
| Parahippocampal Gyrus | L | 230 | −23.8 | −39.1 | −5.7 |
| Subcortical | |||||
| Culmen | L | 372 | −9.4 | −44.8 | −6.7 |
| Main effect of RT | |||||
|
| |||||
| Frontal | |||||
| Medial Frontal Gyrus | R | 10349 | 22.7 | −0.3 | 45.5 |
| Medial Frontal Gyrus | L | 7975 | −22.3 | −1.9 | 43.6 |
| Insula | R | 2969 | 37.3 | 13.5 | 1.5 |
| Insula | L | 1273 | −32.5 | 13.3 | 4.6 |
| Middle Frontal Gyrus | R | 1204 | 30.8 | 34.5 | 24.7 |
| Parietal | |||||
| Inferior Parietal Lobule | L | 21159 | −24.5 | −59.7 | 38.4 |
| Inferior Parietal Lobule | R | 17415 | 21.9 | −58.7 | 43.1 |
| Occipital | |||||
| Middle Occipital Gyrus | L | 9019 | −35.1 | −62.8 | −10.2 |
| Cuneus | L | 1355 | −6.5 | −69 | 6.3 |
| Cuneus | L | 694 | −1.9 | −89.7 | 7.1 |
| Middle Occipital Gyrus | R | 478 | 38.5 | −68.5 | −7.8 |
| Middle Occipital Gyrus | R | 273 | 31.3 | −81.6 | 16.9 |
| Middle Occipital Gyrus | R | 248 | 43.8 | −55.3 | −5.7 |
| Subcortical | |||||
| Culmen | R | 696 | 19.8 | −55.5 | −9.7 |
Region is defined as center of mass. Coordinates represent distance in millimeters from anterior commissure: x right(+)/left(−); y anterior(+)/posterior(−); z superior(+)/inferior(−). Individual voxel probability < 0.005, minimum cluster size > 200 μL.
The purpose of this experiment was to provide converging evidence that the hippocampus is involved in implicit contextual learning. We found that the hippocampal hemodynamic response differentiated novel from repeated items despite the fact that participants could not differentiate the repeated items in a recognition task. Our findings are consistent with neuropsychological findings suggesting that the hippocampus plays a potentially important role in this implicit task (Chun and Phelps 1999). To our knowledge, no study has found task-differential hippocampal activation in a perceptual priming task.
This finding also helps establish an important and necessary bridge between amnesic studies (which are limited by small samples and substantial variation in focus and extent of damage) and functional imaging studies (limited to showing that an area is involved in a particular process, but not necessarily critical for its performance). Where possible, converging evidence between neuropsychological and functional imaging methodologies provides stronger evidence than either can provide alone.
According to the declarative memory model, context-dependent tasks, such as contextual cueing, involve the hippocampus because the contingencies are sufficiently complex that they require reorganization that may only take place as a deliberate act of memory manipulation (Clark and Squire 1998; Reed and Squire 1999; Manns and Squire 2001). By this account, context-dependent learning is a subset of declarative memory, which necessarily requires the hippocampus. Conversely, it has been argued that episodic and semantic memory are specific instances of context-dependent learning (Ryan et al. 2000; Greene et al. 2006; Manns and Eichenbaum 2006; Greene 2007). Accordingly, declarative memory is encoded as associations to context and the necessary context for recognition and recall is provided by retrieval cues (Nyberg et al. 1996; Rudy et al. 2002). A third hypothesis is that the hippocampus is involved in binding nonpre-disposed associations. That is, some types of association formation require synaptic connections where existing connectivity is inadequate. Some of these instances occur where the contextual associations require a complex network (Chun and Phelps 1999; Reed and Squire 1999; Ryan et al. 2000; Manns and Squire 2001; Davachi and Wagner 2002; Greene et al. 2006) and some involve multiple cortical systems that span distant regions of cortex (McEchron and Disterhoft 1997; Davachi and Wagner 2002; Eichenbaum 2006). According to this view, the large proportion of observed hippocampal-dependent tasks that are declarative occurs because declarative memory is generally both context dependent and involves numerous cortical regions (Eichenbaum and Cohen 2001).
The present results, taken together with neuropsychological findings (Chun and Phelps 1999), provide converging evidence to suggest hippocampal involvement in a context-dependent task that does not rely on declarative memory. One possibility is that declarative memory is a subset of context-dependent learning and memory, and accordingly, contextual learning is closer to the central role of the hippocampus. Problematically, learning tasks that one might argue are contextual in nature may be independent of the hippocampus (Knowlton and Squire 1996). Other tasks such as trace conditioning are hippocampal dependent but have no greater claim to context dependency than comparable nonhippocampal-dependent conditioning tasks (McEchron and Disterhoft 1997). Whereas the present findings tend to argue against declarative memory as the core function of the hippocampus, future research is needed to determine the relative merit of binding and context-driven models.
Acknowledgments
This study was supported in part by a grant from the National Institute of Aging (R01 AG022304) to S.R., and the National Center for Research Resources, National Institutes of Health (M01-RR00058) to the Medical College of Wisconsin General Research Center, and the W.M. Keck Foundation.
References
- Binder JR, Bellgowan PS, Hammeke TA, Possing ET, Frost JA. A comparison of two fMRI protocols for eliciting hippocampal activation. Epilepsia. 2005;46:1061–1070. doi: 10.1111/j.1528-1167.2005.62004.x. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognit Psychol. 1998;36:28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H, Deacedo BS, Corkin S. Different memory systems underlying acquisition of procedural and declarative knowledge. Ann N Y Acad Sci. 1985;444:54–71. doi: 10.1111/j.1749-6632.1985.tb37579.x. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software foranalysis and visualization of functional megnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PF. Encoding and retrieval in human medial temporal lobes: An empirical investigation using functional magnetic resonance imaging (fMRI) Hippocampus. 1999;9:25–34. doi: 10.1002/(SICI)1098-1063(1999)9:1<25::AID-HIPO3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Remembering: Functional organization of the declarative memory system. Curr Biol. 2006;16:R643–R645. doi: 10.1016/j.cub.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; New York: 2001. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. J Cogn Neurosci. 2004;16:654–664. doi: 10.1162/089892904323057362. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AJ. Human hippocampal-dependent tasks: Is awareness necessary or sufficient? Hippocampus. 2007;17:429–433. doi: 10.1002/hipo.20296. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger C, Rao SM. An fMRI analysis of the human hippocampus: Inference, context and task awareness. J Cogn Neurosci. 2006;18:1156–1173. doi: 10.1162/jocn.2006.18.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Keane MM, Gabrieli JD, Mapstone HC, Johnson KA, Corkin S. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal-lobe lesions. Brain. 1995;118:1129–1148. doi: 10.1093/brain/118.5.1129. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. Artificial grammar learning depends on implicit acquisition of both abstract and exemplar-specific information. J Exp Psychol Learn Mem Cogn. 1996;22:169–181. doi: 10.1037//0278-7393.22.1.169. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N. Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus. 2003;13:250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Perceptual learning, awareness, and the hippocampus. Hippocampus. 2001;11:776–782. doi: 10.1002/hipo.1093. [DOI] [PubMed] [Google Scholar]
- Mayanagi Y, Watanabe E, Nagahori Y, Nankai M. Psychiatric and neuropsychological problems in epilepsy surgery: Analysis of 100 cases that underwent surgery. Epilepsia. 2001;42(Suppl 6):19–23. [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Houle S, Nilsson LG, Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired transverse patterning in human amnesia is a special case of impaired memory for two-choice discrimination tasks. Behav Neurosci. 1999;113:3–9. doi: 10.1037//0735-7044.113.1.3. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Declarative memory, awareness, and transitive inference. J Neurosci. 2005;25:10138–10146. doi: 10.1523/JNEUROSCI.2731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Hopkins RO, Squire LR. Experience-dependent eye movements, awareness, and hippocampus-dependent memory. J Neurosci. 2006;26:11304–11312. doi: 10.1523/JNEUROSCI.3071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Recognition memory and familiarity judgments in severe amnesia: No evidence for a contribution of repetition priming. Behav Neurosci. 2000;114:459–467. doi: 10.1037//0735-7044.114.3.459. [DOI] [PubMed] [Google Scholar]
- Strange BA, Duggins A, Penny W, Dolan RJ, Friston KJ. Information theory, novelty and hippocampal responses: Unpredicted or unpredictable? Neural Netw. 2005;18:225–230. doi: 10.1016/j.neunet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G. Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proc Natl Acad Sci. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- van Asselen M, Kessels RP, Neggers SF, Kappelle LJ, Frijns CJ, Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44:1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]