Abstract
Hepatitis C virus (HCV) is hyperendemic among injection drug users (IDUs). However, few scientifically proven interventions to prevent secondary transmission of HCV from infected IDUs to others exist. This report describes the design, feasibility, and baseline characteristics of participants enrolled in the Study to Reduce Intravenous Exposure (STRIVE). STRIVE was a multisite, randomized-control trial to test a behavioral intervention developed to reduce distribution of used injection equipment (needles, cookers, cottons, and rinse water) and increase health-care utilization among antibody HCV (anti-HCV) positive IDUs. STRIVE enrolled anti-HCV positive IDU in Baltimore, New York City, and Seattle; participants completed behavioral assessments and venipuncture for HIV, HCV-RNA, and liver function tests (LFTs) and were randomized to attend either a six-session, small-group, peer-mentoring intervention workshop or a time-matched, attention-control condition. Follow-up visits were conducted at 3 and 6 months. At baseline, of the 630 HCV-positive IDUs enrolled (mean age of 26 years, 60% white, 76% male), 55% reported distributive needle sharing, whereas 74, 69, and 69% reported sharing cookers, cottons, and rinse water, respectively. Health-care access was low, with 41% reporting an emergency room as their main source of medical care. Among those enrolled, 66% (418/630) were randomized: 53% (222/418) and 47% (196/418) to the intervention and control conditions, respectively. Follow-up rates were 70 and 73% for the 3- and 6-month visits, respectively. As distributive sharing of used injection equipment was common while reports of receiving HCV care were low, these findings indicate an urgent need for HCV-related interventions with IDUs and demonstrate the acceptability and feasibility to do so.
Keywords: Behavioral intervention, Health-care utilization, hepatitis C virus, Injection drug user, Randomized trial
INTRODUCTION
With an estimated 4 million persons currently infected, hepatitis C virus (HCV) is the most commonly transmitted blood-borne infection in the United States and the leading cause of liver disease, cirrhosis, and hepatocellular carcinoma.1,2 The majority of HCV infections in the US (>60%) are among injection drug users (IDUs),3,4 and HCV is often the first blood-borne infection acquired by new initiates to injection drug use.5,6 High HCV prevalence among IDUs is largely attributed to increased exposure to HCV infected blood during sharing of syringes and injection paraphernalia.5,7,8 Additionally, parenteral HCV transmission is estimated to be approximately 10 times more efficient than HIV,9 accounting for the high incidence and prevalence of HCV among IDUs even in areas where HIV incidence is low.10
HCV treatment options involve the combined use of pegylated interferon and ribavirin, the efficacy of which depends on strict adherence to a rigorous medication regimen.1 In 2002, the NIH Consensus Statement detailing the treatment guidelines for HCV therapy noted the challenges inherent in treating chronic HCV among users of alcohol and illicit drugs but nevertheless highlighted the need to offer therapy to HCV-positive IDUs. Despite a shift toward recommending HCV treatment for IDUs, studies show that most IDUs are, in fact, not offered appropriate referrals or treatment for HCV.11,12
The high burden of HCV and low levels of treatment among IDUs provide compelling public health and clinical reasons for implementing intervention programs for both anti-HCV positive IDUs and IDUs at risk for HCV. In the absence of a vaccine to prevent HCV infection, effective prevention programs must rely on changing behavior. Most programs such as counseling and testing13,14 or structural interventions such as syringe exchange and methadone maintenance programs15–17 have focused on primary prevention for those at risk. However, their efficacy in addressing secondary prevention by curbing the spread of HCV from infected IDUs to others has been inconclusive. A comprehensive approach to reduce HCV among IDUs involves identifying effective ways to reduce the distribution of used syringes and injection equipment among those with HCV while also improving access to HCV treatment in order to reduce the reservoir of HCV in the IDU community. We are unaware of any study to evaluate the effectiveness of a behavioral intervention to curb the HCV epidemic that specifically addresses these two issues among HCV-positive IDUs.
STRIVE (Study to Reduce Intra-Venous Exposures) evaluated the efficacy of a peer-mentoring intervention designed to reduce distributive syringe- and injection paraphernalia-sharing behaviors among HCV-positive IDUs and promote uptake of HCV health care and treatment. Given that this is the first trial to work with young, HCV-positive IDUs, a detailed description of this approach is warranted. This paper describes the methods, intervention content, and feasibility of recruiting, screening, and enrolling anti-HCV positive IDUs into STRIVE. We also present baseline demographic, behavioral, and health-care utilization characteristics of the study sample.
METHODS
Study Design
STRIVE was a two-arm, randomized behavioral intervention trial conducted in three US cities: Baltimore, MD; New York City, NY; and Seattle, WA by investigators also implementing the Collaborative Injection Drug Users Studies-III/Drug User Intervention Trial (CIDUS-III/DUIT), a behavioral intervention for HIV- and HCV-antibody (anti-HCV) negative IDUs.18 The STRIVE intervention curriculum was pilot-tested for comprehension, acceptability, and logistics with HCV-positive IDUs before implementation. All study activities were approved by institutional review boards at participating sites, and all participants provided written informed consent to take part in the trial. Individuals were remunerated for study participation according to site-specific guidelines. A schematic of the study design and participant flow is presented in Figure 1.
FIGURE 1.
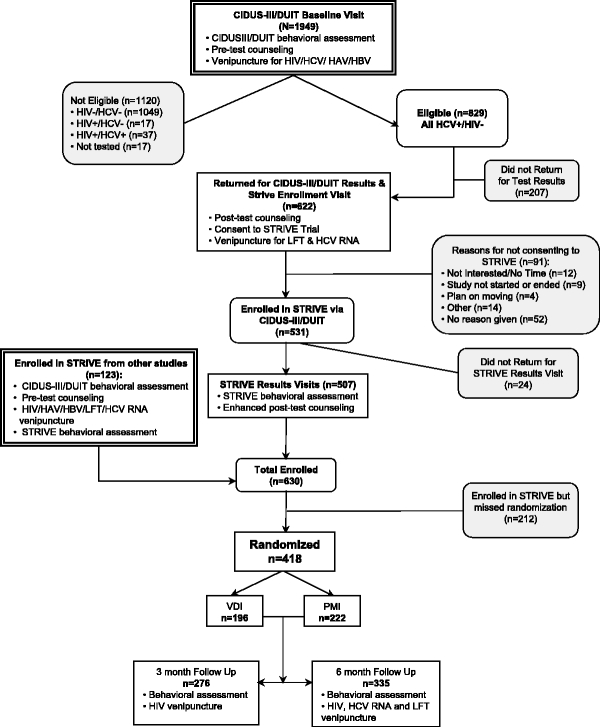
Participant flow for STRIVE. PMI=Peer Mentoring Intervention; VDI=Video and Discussion Intervention.
Recruitment and Eligibility
STRIVE participants were recruited between April 2002 and May 2004 from two sources. First, those who took part in the baseline visit for the CIDUS-III/DUIT study but were ineligible due to anti-HCV positive serostatus were invited to participate in STRIVE. The CIDUS-III/DUIT study has been described in detail elsewhere.18 Briefly, participants for the CIDUS-III/DUIT study were recruited via street outreach, from community-based sources, and a modified respondent-driven sampling approach. Second, anti-HCV positive IDUs who were ineligible for other studies being conducted at the Seattle and New York City sites were referred to STRIVE at those sites; no similar studies were conducted at the Baltimore site during this time.
Irrespective of recruitment source, individuals were eligible for STRIVE if they were between 18 and 35 years old, reported injection drug use during the 6 months preceding screening, planned on living in the area for the next 12 months, provided documentation of their anti-HCV positive and HIV-negative serostatus, were willing to provide a blood sample for liver function and HCV-RNA testing, and were able to comprehend English well enough to complete English-only assessments and to participate in group sessions. We carefully considered whether to enroll HIV-infected persons into this study but elected not to do so because HIV prevalence in the target population was low across the three study sites (3–10%) and thus, any differential effect of the intervention by HIV status would not be detected. Therefore, all persons found to be HIV-infected were referred to appropriate medical care and social services according to the CIDUS-III/DUIT study protocol.
CIDUS-III/DUIT Baseline Visit
All participants referred to STRIVE from CIDUS-III/DUIT took part in that study’s baseline activities, including a behavioral assessment, pretest counseling, and venipuncture. The CIDUS-III/DUIT baseline behavioral assessment was completed using audio computer-assisted self interview (ACASI) and collected information on sociodemographics, injection and sexual practices, psychosocial characteristics including self-esteem, self-efficacy for risk reduction practices, psychological distress, and knowledge, attitudes, and perceived norms about HIV- and HCV-related risk behaviors. Next, all participants received standard-of-care, client-centered pretest counseling and were offered referrals for free hepatitis A and B (HA/HB) vaccinations if they had not already completed the full course of vaccination.
After counseling, participants underwent venipuncture for HIV/HCV antibody testing. Testing for HCV antibodies was performed using an enzyme immunoassay (EIA) test (Abbott Laboratories EIA 2.0 [Chicago, IL] or Ortho Diagnostic Systems EIA 2.0 [Raritan, NJ] in Seattle and Ortho Diagnostic Systems EIA 3.0 [Raritan, NJ] in Baltimore and New York City). Reactive specimens were retested in duplicate using EIA and determined to be anti-HCV positive if either or both specimens were reactive. Recombinant Immunoblot Assay (RIBA, Chiron Corporation, Emeryville, CA) was used to evaluate samples without definitive results on the immunoassay only at the Seattle site.19 HIV antibody testing was performed using standard ELISA screening and Western blot for confirmation. Participants were scheduled to return in 1–2 weeks to receive their test results. Participants referred from other studies due to their positive anti-HCV status completed the CIDUS-III/DUIT baseline assessment, were tested for HIV (if not previously tested by the referring study), and offered HA/HB vaccinations.
CIDUS-III/DUIT Results and STRIVE Enrollment Visit
At the CIDUS-III/DUIT results visit, participants first received their HIV/HCV test results and posttest counseling. Any participant testing HIV positive was provided with referrals for medical care. Participants eligible for STRIVE were informed of the study and were invited to enroll in the intervention trial. All eligible participants provided written informed consent to participate in STRIVE intervention activities and to share behavioral and biological data obtained at the CIDUS-III/DUIT baseline visit. A second venipuncture for LFT and HCV-RNA was conducted at this visit to determine the proportion of IDUs who met the criteria for HCV therapy as indicated by alanine aminotransferase levels or the presence of HCV-RNA. LFT testing was conducted locally, and serum samples were batched and shipped to the Baltimore site for HCV-RNA testing using the COBAS AMPLICOR™ HCV test version 2.0 (Roche Molecular Systems, Branchburg, NJ). All participants were then scheduled for a second visit in 1–2 weeks to receive LFT results.
STRIVE Results Visit
At this visit, participants first completed a STRIVE-specific baseline ACASI assessment and then received enhanced posttest counseling. The STRIVE survey ascertained information not previously obtained during the CIDUS-III/DUIT baseline assessment; this included more detailed items on alcohol use, health-care utilization, patient–doctor interactions, HCV-treatment readiness, depression, and utilization of alcohol and drug treatment. In response to the confusion around HCV-related medical care expressed by participants during the pilot phase, we enhanced STRIVE’s counseling protocol to provide LFT results, additional referrals, and assistance in accessing health-care services. Counselors were trained to stress that the best way to interpret LFT results was in consultation with a health-care provider who could provide more comprehensive information and care. Participants were then scheduled to return on a prespecified date to be randomized and to begin their first small-group session.
To ensure that behaviors reported at baseline reflected recent behavior before randomization, we required individuals to be randomized no later than 15 weeks after their CIDUS-III/DUIT baseline activities occurred. Participants whose CIDUS-III/DUIT baseline data were collected more than 15 weeks before randomization were rescreened to ensure eligibility and repeated both the CIDUS-III/DUIT and STRIVE baseline procedures. In these instances, only the latter set of baseline data were retained. Any individual who missed two randomization appointments or completed two screening and baseline visits without being randomized was deemed ineligible for the trial.
Randomization
If at least 10 participants were present on the prescheduled intervention launch date and time, participants were randomly assigned to either the intervention or the control arm, thus constituting an intervention cohort. A password-protected computer program, based on a block randomization scheme to ensure an equal balance of males and females in both trial arms, was used by the project coordinator at each study site to randomly assign participants to either the intervention or the control arm. Randomization was conducted in a private area immediately before the first group session to prevent attrition bias between the actual randomizing of participants and the beginning of the group session. For the first session, both intervention and control groups were conducted simultaneously in different rooms. However, the remaining sessions were scheduled on different days for logistical purposes.
When five to nine participants presented on randomization day, the entire group was randomized into only one of the study arms. This group randomization scheme was created at the data coordinating center at the Johns Hopkins University site using a random numbers table generator. The scheme consisted of a series of sequentially numbered, sealed envelopes containing a randomization assignment to either condition; these envelopes were maintained in a locked file cabinet in the project coordinators’ office. When a group randomization was required, the project coordinator would open one envelope and notify the facilitators which intervention condition would be delivered to the group. This protocol was instituted to avoid convening cohorts where the number of participants within each intervention arm was low, thereby limiting the feasibility of conducting the group exercises and discussions.
Trial Conditions
Participants received either a six-session peer mentoring intervention (PMI) or a video and discussion intervention (VDI), an attention-control condition. The conditions differed only in content, not logistics. All sessions were 2 hours long, held twice a week in a private room at the research site. Except for session 1, where two additional facilitators were required during an individual randomization, the remaining sessions were led by the same two-trained facilitators who followed a scripted PMI or VDI manual to minimize potential for cross-contamination. Participants in both arms were equally remunerated for every session attended and were offered with an additional reimbursement if they attended all six sessions. Participants also received small souvenirs (i.e., water bottle, key chains) with study logos to enhance study identification and group cohesion. Refreshments were provided at every session.
Experimental Condition: PMI
A peer-mentoring approach was chosen for the experimental condition because it combined key aspects of cognitive behavioral20–22 and social learning23 theories to empower participants to enact new prosocial identities and subsequently sustain positive behaviors within their networks. Based on this theoretical framework, the PMI involved two distinct components addressing both individual and contextual factors known to influence risky IDU behaviors. The first component, reviewed during the first four sessions, utilized a cognitive-behavioral, skills-building approach to increase knowledge about HCV and provide skills for reducing distributive injection behaviors and alcohol use, for managing their HCV health care, and for effectively conducting educational outreach to other IDUs about HCV infection. The second component involved providing participants with communication techniques to facilitate HCV-related peer mentoring between sessions and conducting peer mentoring in community-based settings.
We hypothesized that this second approach would reduce distributive injection risk by: (1) providing opportunities to rehearse new peer-mentoring skills, (2) allowing participants to model their new skills in environments where they could reassociate risky cues with safer behaviors, (3) providing participants with new prosocial identities in their community to reinforce their motivation to practice safer behaviors, and (4) increasing their comfort and familiarity with NEPs and drug treatment programs—structural approaches to facilitate sustained risk reduction behaviors. Intervention material was delivered through a variety of fun, interactive activities such as games, facilitated discussions, viewing videos, role plays, and interactive demonstrations that did not require high levels of literacy. To raise awareness about HCV and explain liver pathology, participants viewed videos developed by the Hepatitis Foundation International.
Attention-control Condition: VDI
Participants in the control condition viewed a video series focusing on the lives of young IDUs living in an urban setting and then participated in a facilitator-guided discussion. To minimize cross-contamination between conditions, facilitators used a scripted manual to guide discussion around family, education, self-respect, relationships, violence, parenting, and employment to prevent discussion about injection-related topics. Any participant who sought information on HCV-related risk behavior or health-care issues was referred to a resource table located in the meeting room.
Training and Quality Assurance Procedures
All facilitators were trained during a 2-day training session held at the Baltimore site before the beginning of field operations. The training session was led by investigators responsible for the development of the intervention manuals and covered intervention manual content, group leadership techniques, and facilitation skills. The main focus of the training was on the importance of maintaining “intervention fidelity” by adhering to the manual outlines, instructions, and scripts, promoting active listening, effective communication, group safety, and managing emotional reactions. Facilitators were also trained on ways to create a safe environment in order to facilitate open and honest discussion among the participants by reinforcing confidentiality, normalizing all emotions, allowing differences of opinion, and facilitating nonjudgmental discussions among the participants.
Three levels of quality assurance for the experimental and control conditions were undertaken to ensure that the intervention sessions were delivered as conceptualized and comparably conducted by facilitators across sites. First, project coordinators at each site who were trained on intervention content and delivery monitored sessions by observing them in progress. Group sessions in both conditions were initially observed more frequently and at least once per cohort as the study progressed. This ensured that minor corrections were given to the facilitators immediately. Second, a committee comprised of one monitor from each site observed the same two PMI sessions at each site to assess the consistency of delivery between sites. Finally, every session in both conditions was audiotaped. One taped session from each condition per cohort was selected at random and reviewed by two trained monitors from external sites (no site reviewed its own tapes) who evaluated delivery of intervention content and fidelity to the scripted manuals against a standardized checklist. Reviews and feedback to facilitators were provided in real time, but facilitators were blind to the review schedule. Results from this quality assurance protocol indicate that for the 10% of all session tapes reviewed across trial conditions, the facilitators delivered both PMI and VDI content with fidelity to the respective manuals 96% of the time.
Follow-up Visits
All individuals randomized into a cohort were scheduled for follow-up at exactly 3 and 6 months after the date of the sixth session of the cohort they participated in, irrespective of session attendance after randomization. A window period of 1 week before and 2 weeks after the ideal follow-up date was established for the 3-month follow-up visit. The window period for the 6-month follow-up visit was 1.5 months before the 6-month date and extended to the end of the study period in order to maximize follow-up yield. Participants were contacted 1–2 weeks prior to their scheduled follow-up visit via regular mail, phone, or e-mail, depending on what type of contact information was provided and what form of contact the participant felt was the best way to be reached by.
At both follow-up visits, participants completed an ACASI-administered behavioral assessment and provided a blood sample for HIV antibody testing at 3 months and for HIV antibody, HCV-RNA, and LFT testing at 6 months. As follow-up interviews assessed behaviors during the past 3 months, a minimum of 3 months was required between each of the follow-up assessment visits to avoid measuring the same behavioral events on two assessments. Follow-up results visits were scheduled approximately 2 weeks after the 3- and 6-month follow-up visits in order to provide test results and appropriate posttest counseling. Participants at all three sites were remunerated for each follow-up visit attended, and in Baltimore and New York City, participants were compensated for the results visit they attended.
Statistical Analyses
Separate analyses were conducted to identify associations between key sociodemographic, injection drug use, and health-care utilization measures by randomization status and trial arm assignment. Pearson chi-square tests were used to compare categorical variables, and ANOVA was used to compare mean values for continuous variables.
RESULTS
Trial Characteristics
Between April 2002 and May of 2004, a total of 1,949 individuals completed the baseline portion of the CIDUS-III/DUIT baseline study in Baltimore, New York City, and Seattle (Figure 1). Based on the additional anti-HCV positive and HIV-negative serostatus requirements for STRIVE, 43% (829/1949) of CIDUS-III/DUIT participants were eligible for STRIVE. As STRIVE enrollment occurred at the CIDUS-III/DUIT results visit, those participants who returned for this visit (75% or 622/829) were invited to join STRIVE; of these, 85% (531/622) provided written informed consent to participate in the trial, and 95% (507/531) returned to complete the STRIVE behavioral baseline assessment. An additional 123 individuals were referred to STRIVE from other studies where they tested anti-HCV positive. In total, 66% (418/630) were randomized to either one of the two intervention conditions; there were 212 participants who did not return for randomization despite multiple efforts to contact and follow up these participants. Among those who did return, 53% (222/418) were randomized to the experimental condition and 47% (196/418) to the attention-control condition.
Overall, 45 cohorts were conducted: 20 involving individual randomization and 25 involving group randomization. Cohort size varied by type of randomization with a mean of 12 participants in individually randomized groups and a mean of 7 participants in group-randomized cohorts. While session attendance for individuals attending all six sessions was similar between intervention arms, it varied by site, with Seattle reporting the highest session attendance (72 and 73% for the PMI and the VDI, respectively) followed by New York City (69 and 62%) and then Baltimore (46 and 40%).
Follow-up rates of 66% (n = 276) and 80% (n = 335) were obtained for the three- and six-month visits, respectively, after accounting for loss to follow-up from incarceration (n = 11), having moved away from the study site (n = 8), enrollment in a residential drug treatment program (n = 3), or death (n = 1). There were no significant differences for key sociodemographic and behavioral characteristics between participants who returned for the 3-month follow-up visit and those who did not. Similar comparisons between participants who completed the 6-month follow-up visit and those who did not complete indicated that those who returned were more likely to have health insurance, report symptoms associated with HCV, and report a regular source of medical care (data not shown).
Baseline Characteristics by Randomization Status and Trial Condition
At baseline, the average age of participants was 26.2 years (SD = 3.9 years), with randomized participants slightly older than nonrandomized participants (p < 0.001) (Table 1). Approximately 75% of this sample was male, and there was no association between gender and randomization status. However, randomized participants were more likely to be Hispanic/Latino (p < 0.001) and have ever been incarcerated (p = 0.002). Homelessness among this sample was considerable, with 44% of participants reporting no housing; while this may have impacted initial participation in the intervention trial, there was no association between homelessness and randomization status. There were no statistically significant differences with regard to drug use by randomization status, with 60% of the sample reporting alcohol use on a daily or weekly basis and heroin as the drug of choice. However, sharing of injection equipment did vary slightly, although not significantly, by randomization status, with more nonrandomized individuals reporting distributive needle sharing (60 vs. 52%; p = 0.051) and sharing cookers (79 vs. 71%; p = 0.051) compared to randomized participants. There was no significant association between sharing other injection paraphernalia, such as cottons or rinse water, or backloading and randomization status. Recent involvement in drug treatment (such as methadone maintenance, inpatient or outpatient programs) or alcohol treatment programs was reported by 63 and 39% of participants, respectively, with no difference by randomization status.
TABLE 1.
Baseline sociodemographic, drug use, and health-care characteristics of STRIVE participants by randomization status
| Characteristic | Total % (n) | Not randomized % (n) | Randomized % (n) | p-value |
|---|---|---|---|---|
| Total n | 630 | 212 | 418 | |
| Site | ||||
| Baltimore | 50 (313) | 56 (118) | 47 (195) | 0.100 |
| New York | 27 (168) | 23 (49) | 29 (119) | |
| Seattle | 24 (149) | 21 (45) | 25 (104) | |
| Age; mean (SD) | 26.2 (3.9) | 25.4 (3.7) | 26.6 (3.9) | <0.001 |
| Gender | ||||
| Male | 76 (477) | 77 (161) | 76 (316) | 0.925 |
| Female | 24 (147) | 23 (49) | 24 (98) | |
| Race/Ethnicity | ||||
| Black or African-American | 5 (33) | 2 (5) | 7 (28) | 0.015 |
| Hispanic/Latino | 24 (153) | 20 (42) | 27 (111) | |
| White | 60 (380) | 68 (143) | 57 (237) | |
| Other (Asian, American- Indian, Mixed, Other) | 10 (64) | 10 (22) | 10 (42) | |
| Highest level of education completed | ||||
| Less than high school | 45 (281) | 46 (96) | 45 (185) | 0.768 |
| High school graduate or higher | 55 (342) | 54 (113) | 55 (229) | |
| Source of regular income during last 6 months | ||||
| Legal source | 67 (418) | 66 (136) | 68 (282) | 0.656 |
| Illegal source | 25 (156) | 26 (53) | 25 (103) | |
| Other | 7 (46) | 9 (18) | 7 (28) | |
| Homeless during last 6 month months | ||||
| No | 55 (345) | 55 (116) | 55 (229) | 0.478 |
| Yes | 44 (276) | 44 (92) | 44 (184) | |
| Ever spent time in jail, prison, or juvenile detention | ||||
| No | 18 (110) | 11 (23) | 21 (87) | 0.002 |
| Yes | 82 (514) | 89 (187) | 79 (327) | |
| Frequency of alcohol use in last 3 months | ||||
| None | 31 (195) | 34 (70) | 30 (125) | 0.718 |
| Daily (everyday) | 9 (56) | 8 (16) | 10 (40) | |
| Weekly (1–6 days/week) | 31 (194) | 30 (62) | 32 (132) | |
| Monthly (1–3 days/month) | 28 (176) | 29 (60) | 28 (116 | |
| Drug injected most often during last 3 months | ||||
| Heroin alone | 60 (363) | 59 (119) | 61 (244) | 0.486 |
| Heroin and cocaine | 30 (179) | 32 (65) | 29 (114) | |
| Crack/Cocaine | 6 (37) | 5 (9) | 7 (28) | |
| Other | 4 (23) | 5 (9) | 4 (14) | |
| Distributive needle sharing in last 3 months | ||||
| Did not share an injection needle with someone else | 46 (247) | 40 (73) | 49 (174) | 0.051 |
| Shared an injection needle | 55 (296) | 60 (111) | 52 (185) | |
| Shared a cooker in the last 3 months | ||||
| No | 26 (157) | 21 (43) | 29 (114) | 0.051 |
| Yes | 74 (445) | 79 (160) | 71 (285) | |
| Shared cottons in the last 3 months | ||||
| No | 32 (190) | 27 (55) | 34 (135) | 0.092 |
| Yes | 68 (412) | 73 (148) | 66 (264) | |
| Shared rinse water in the last 3 months | ||||
| No | 32 (189) | 27 (54) | 34 (135) | 0.073 |
| Yes | 68 (411) | 73 (148) | 66 (263) | |
| Backloaded in the last 3 months | ||||
| No | 35 (169) | 30 (48) | 38 (121) | 0.083 |
| Yes | 65 (312) | 70 (113) | 62 (199) | |
| Received treatment for alcohol use in last 6 months | ||||
| No | 61 (146) | 61 (53) | 60 (93) | 0.936 |
| Yes | 39 (95) | 39 (34) | 40 (61) | |
| Participated in drug treatment in last 3 months | ||||
| No | 37 (205) | 39 (74) | 35 (131) | 0.336 |
| Yes | 63 (355) | 61 (114) | 65 (241) | |
| Length of time known to be HCV positive | ||||
| Less than 1 year | 47 (263) | 46 (84) | 48 (179) | 0.726 |
| One or more years | 53 (294) | 54 (98) | 52 (196) | |
| Received first positive HCV test from | ||||
| Research study | 54 (325) | 52 (105) | 55 (220) | 0.848 |
| Needle exchange/drug treatment program | 16 (98) | 17 (35) | 16 (63) | |
| Private doctor/hospital/health department | 19 (112) | 20 (40) | 18 (72) | |
| Jail, prison, juvenile detention center | 11 (67) | 10 (21) | 12 (46) | |
| Health-insurance status | ||||
| Not insured | 31 (194) | 34 (71) | 29 (123) | 0.347 |
| Insured | 30 (190) | 26 (56) | 32 (134) | |
| Don’t know | 12 (77) | 14 (30) | 11 (47) | |
| Refused to answer | 27 (169) | 26 (55) | 27 (114) | |
| Main source for medical care | ||||
| Emergency room | 41 (235) | 41 (80) | 40 (155) | 0.444 |
| Private doctor | 20 (118) | 18 (35) | 22 (83) | |
| Health department or other clinic | 27 (156) | 26 (50) | 28 (106) | |
| Nowhere | 12 (71) | 15 (29) | 11 (42) | |
Nearly half (48%) had learned of their anti-HCV positive status within the calendar year preceding the baseline visit. Syringe exchange and drug treatment programs or hospital and health departments were additional sources of HCV testing for almost one-third of the sample. Forty percent cited obtaining most of their medical care from an emergency room. Thirty percent of participants reported having health insurance, 31% reported having no insurance, and 39% were either unsure or refused to report their health-insurance status.
Baseline sample characteristics were also examined to ensure balance between those randomized to either the intervention or attention-control condition (Table 2). A comparison of key sociodemographic, alcohol and injection drug use behaviors, and health-care utilization factors revealed no significant differences between those randomized to the intervention versus attention-control condition.
TABLE 2.
Baseline sociodemographic, drug use and health care characteristics of STRIVE participants by trial arm
| Characteristic | Total % (n) | PMI % (n) | VDI % (n) | p-value |
|---|---|---|---|---|
| Total n | 418 | 222 | 196 | |
| Site | ||||
| Baltimore | 47 (195) | 50 (111) | 43 (84) | 0.343 |
| New York | 29 (119) | 27 (59) | 31 (60) | |
| Seattle | 25 (104) | 23 (52) | 27 (52) | |
| Mean age (SD) | 26.6 (3.9) | 26.7 (3.9) | 26.4 (4.0) | 0.434 |
| Gender | ||||
| Male | 76 (316) | 76 (167) | 76 (149) | 0.971 |
| Female | 24 (98) | 24 (52) | 24 (46) | |
| Race/Ethnicity | ||||
| Black or African-American | 7 (28) | 7 (15) | 7 (13) | 0.843 |
| Hispanic/Latino | 27 (111) | 25 (56) | 28 (55) | |
| White | 57 (237) | 57 (126) | 57 (111) | |
| Other (Asian, American- Indian, Mixed, Other) | 10 (42) | 11 (25) | 9 (17) | |
| Highest level of education completed | ||||
| Less than high school | 45 (185) | 46 (101) | 43 (84) | 0.534 |
| High school graduate or higher | 55 (229) | 54 (118) | 57 (111) | |
| Source of regular income during last 6 months | ||||
| Legal source | 68 (282) | 69 (151) | 68 (131) | 0.547 |
| Illegal source | 25 (103) | 23 (51) | 27 (52) | |
| Other | 7 (28) | 8 (17) | 6 (11) | |
| Homeless during last 6 month months | ||||
| No | 55 (229) | 58 (128) | 52 (101) | 0.244 |
| Yes | 44 (184) | 42 (91) | 48 (93) | |
| Ever spent time in jail, prison, or juvenile detention | ||||
| No | 21 (87) | 22 (49) | 20 (38) | 0.472 |
| Yes | 79 (327) | 78 (170) | 81 (157) | |
| Frequency of alcohol use in last 3 months | ||||
| None | 30 (125) | 31 (68) | 29 (57) | 0.874 |
| Daily (everyday) | 10 (40) | 11 (23) | 9 (17) | |
| Weekly (1–6 days/week) | 32 (132) | 31 (67) | 34 (65) | |
| Monthly (1–3 days/month) | 28 (116 | 28 (61) | 28 (55) | |
| Drug injected most often during last 3 months | ||||
| Heroin alone | 61 (244) | 61 (130) | 61 (114) | 0.982 |
| Heroin and cocaine | 29 (114) | 29 (61) | 28 (53) | |
| Crack/Cocaine | 7 (28) | 7 (14) | 7 (14) | |
| Other | 4 (14) | 3 (7) | 4 (7) | |
| Distributive needle sharing in last 3 months | ||||
| Did not share an injection needle with someone else | 49 (174) | 47 (89) | 50 (85) | 0.654 |
| Shared an injection needle | 52 (185) | 53 (99) | 50 (86) | |
| Shared a cooker in the last 3 months | ||||
| No | 29 (114) | 29 (62) | 28 (52) | 0.751 |
| Yes | 71 (285) | 71 (150) | 72 (135) | |
| Shared cottons in the last 3 months | ||||
| No | 34 (135) | 35 (75) | 32 (60) | 0.488 |
| Yes | 66 (264) | 65 (137) | 68 (127) | |
| Shared rinse water in the last 3 months | ||||
| No | 34 (135) | 34 (71) | 34 (64) | 0.961 |
| Yes | 66 (263) | 66 (139) | 66 (124) | |
| Backloaded in the last 3 months | ||||
| No | 38 (121) | 37 (61) | 39 (60) | 0.683 |
| Yes | 62 (199) | 63 (105) | 61 (94) | |
| Received treatment for alcohol use in last 6 months | ||||
| No | 60 (93) | 61 (44) | 60 (49) | 0.864 |
| Yes | 40 (61) | 39 (28) | 40 (33) | |
| Participated in drug treatment in last 3 months | ||||
| No | 35 (131) | 37 (73) | 34 (58) | 0.576 |
| Yes | 65 (241) | 63 (127) | 66 (114) | |
| Length of time known to be HCV positive | ||||
| Less than 1 year | 48 (179) | 44 (87) | 52 (92) | 0.145 |
| One or more years | 52 (196) | 56 (110) | 48 (86) | |
| Received first positive HCV test from | ||||
| Research study | 55 (220) | 53 (111) | 55 (109) | 0.863 |
| Needle exchange/drug treatment program | 16 (63) | 17 (35) | 15 (28) | |
| Private doctor/hospital/health department | 18 (72) | 19 (39) | 17 (33) | |
| Jail, prison, juvenile detention center | 12 (46) | 12 (25) | 11 (21) | |
| Health-insurance status | ||||
| Not insured | 29 (123) | 30 (66) | 29 (57) | 0.203 |
| Insured | 32 (134) | 28( 63) | 36 (71) | |
| Don’t know | 11 (47) | 11 (24) | 12 (23) | |
| Refused to answer | 27 (114) | 31 (69) | 23 (45) | |
| Main source for medical care | ||||
| Emergency room | 40 (155) | 38 (77) | 42 (78) | 0.335 |
| Private doctor | 22 (83) | 19 (39) | 24 (44) | |
| Health department or other clinic | 28 (106) | 29 (59) | 25 (47) | |
| Nowhere | 11 (42) | 13 (26) | 9 (16) | |
DISCUSSION
The methods used to recruit, randomize, and retain anti-HCV positive IDUs for this behavioral intervention trial indicate that this study was acceptable to participants and feasible to conduct. One-half of the study participants were recently diagnosed with HCV infection, and few reported receiving health care for their HCV infection, underscoring the need for intervention efforts and indicating that the appropriate target population had been reached. Some attrition occurred before randomization and was associated with demographic characteristics but not HCV transmission risk behaviors. Regarding randomization, baseline sociodemographic and behavioral characteristics were balanced between participants in the two trial conditions, indicating that the randomization scheme was successful and that these two groups were also balanced with respect to unmeasured characteristics.24 Thus, the trial participants were representative of the recruited population in terms of risk behaviors that the intervention intended to change.
A key feature of this study is the recruitment of individuals who recently learned of their HCV status. Prior research indicates that IDUs who become infected with HCV, especially during the first few years after onset of injection drug use, may continue to engage in high-risk syringe and equipment sharing because they may be unaware of their anti-HCV positive serostatus.25 In fact, our data revealed that over half of the IDUs in STRIVE were engaging in distributive needle sharing, and three quarters reported injection equipment sharing with peers and partners, a significant risk factor for HCV transmission.5,8 Consequently, the ability to reach out to young, HCV-positive IDUs and to provide accurate information about the transmission of HCV represents a crucial step in potentially stemming the spread of HCV among IDUs.
Also of note is the high level of homelessness and incarceration reported in this sample. While this high degree of HCV among homeless IDUs is similar to that reported in other studies of IDUs,26–28 it does indicate that HCV interventions will need to account for environmental and behavioral factors associated with homelessness such as lack of proper hygiene, poor nutrition, and high levels of risky IDU behavior. The high prevalence of lifetime incarceration among STRIVE participants is not surprising given that previous studies have also shown higher rates of HCV and other blood-borne viruses among previously incarcerated individuals.29–31
Finally, as shown in other research studies among IDUs, access to and utilization of health-care services are poor among this population.32 It is important to note that a significant portion of this sample either did not have (31%) or were uncertain (12%) about their health-insurance coverage. As limited access to health-care services may delay HCV testing, this is significant to note because HCV therapy may be more effective if initiated early after infection.33,34
To date, the majority of interventions for young IDUs have concentrated on primary or secondary HIV prevention with an ancillary focus on preventing HCV infection. Given the overwhelming burden of HCV infection among IDUs, the lack of an effective vaccine against HCV, and a treatment regimen that is not completely effective and in many cases not offered to IDUs,35 the need for comprehensive and effective HCV prevention programs is compelling. The STRIVE study represents the first major attempt to meet these needs. This overview of STRIVE provides detailed information on the key logistics involved in conducting a behavioral intervention for a hard-to-reach population of HCV-positive young IDUs, who may be less willing to seek mainstream support services or have access to health-care services. A major strength of this intervention trial was its integrated approach to the prevention of secondary HCV transmission, specifically in that it allows participants to address both behavioral (i.e., alcohol consumption, drug use, needle sharing, etc.) and health-care concerns (i.e., improved access to HCV monitoring and treatment, fostering better communication with providers, etc.) within a harm reduction framework.
In addition to the strengths noted above, all assessments were conducted using ACASI, where participants were able to complete all baseline and follow-up surveys on their own. As such, we expect less socially desirable responding with respect to unsafe injection practices compared to interviewer administered surveys.36–38 Finally, the intervention trial not only provided HCV-positive individuals with an opportunity to openly discuss their serostatus but to also gain correct information about the nature of HCV disease, skills to interact with health-care providers, appropriate disease management skills, and communication techniques to deal with peers and partners.
Some limitations of this study design should be noted. First, despite concerted efforts to recruit an ethnically representative sample, the study may not be generalizable to minority IDUs as 60% of participants were white and only 24 and 5% self-identified as Hispanic/Latino and African-American, respectively. Consequently, it may be difficult to conduct subgroup analyses to determine whether intervention effects differed by racial/ethnic background. Second, the six-session design of this study may have limited the involvement of individuals who had jobs or other commitments and were not able to attend prescheduled group sessions. For women, in particular, the inability to procure appropriate child care may have prevented study participation and may be one reason for the low enrollment (24%) of women in this study. Finally, although the nature of the study was explained to all potential participants, the stigma associated with revealing one’s HCV-positive serostatus in a group setting may have discouraged some individuals from participating in the trial.
In response to the large number of IDUs living with HCV, there is an urgent need for the development, testing, and implementation of intervention programs that not only address the risky behaviors associated with injection drug use but also the barriers to health-care seeking and utilization. Although the logistical details of the STRIVE study were complex, these strict procedures were necessary in order to ensure the integrity of the randomized trial design and measure the efficacy of this intervention study. Given our ability to retain up to 70% of participants for all six sessions of the intervention, we are confident that, if found to be effective, this intervention would be acceptable among young IDUs and could be implemented by trained staff at community-based organizations serving IDUs.
Acknowledgements
The authors gratefully acknowledge the investigators, staff, and participants of the STRIVE project for their total commitment to the success of this project. Additionally, we thank David Purcell from the U.S. Centers for Disease Control for consulting on study design and the investigators of the DUIT study for generously sharing the DUIT study protocol and instruments.
The STRIVE group includes the following individuals:
Steffanie A. Strathdee, Elizabeth T. Golub, David Thomas, Marie Bailey-Kloch, Yvette Bowser, Peter O’Driscoll, Janet Reeves, Marcella Sapun, Dale Netski, McCay Moiforay, Fleesie Hubbard, Coralee Meslin, Karen Yen-Hobelmann, Marie Bailey-Kloch, Eddie Poole, David Hudson, Gina Gant, Eric Hendren from Johns Hopkins University, Baltimore, MD; Mary Latka, Farzana Kapadia, David Vlahov, Danielle Ompad, Micaela Coady, Sebastian Bonner, Joanna Cruz, Sandra DelVecchio, Dirk Jackson, Gregory Malave, Joan Monserrate, Clarisse Miller O’Shea, Manny Yonko from The New York Academy of Medicine, New York, NY; Holly Hagan, Jennifer V. Campbell, Eileen Hough, Hanne Thiede, Rong Lee, Susan Nelson, Jef St. De Lore, Kimberly Houk, Sarah Brooks, Carrie Shriver, Jeanette Frazier, Jean Pass, Paul Swenson from Seattle-King County Public Health, Seattle, WA; and Richard S. Garfein from the University of Califormia at San Diego, San Diego, CA.
Funding for this project was provided by the National Institute on Drug Abuse through grant DA14499. Trial registration: Study to Reduce Intravenous Exposures, NCT00391482, http://clinicaltrials.gov/ct/show/NCT00391482?order=1.
References
- 1.NIH consensus statement on management of hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed]
- 2.Williams I. Epidemiology of hepatitis C in the United States. Am J Med. 1999;107:2S–9S. [DOI] [PubMed]
- 3.Yen T, Keeffe EB, Ahmed A. The epidemiology of hepatitis C virus infection. J Clin Gastroenterol. 2003;36:47–53. [DOI] [PubMed]
- 4.Alter MJ, Mast EE, Moyer LA, Margolis HS. Hepatitis C. Infect Dis Clin North Am. 1998;12:13–26. [DOI] [PubMed]
- 5.Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. [DOI] [PubMed]
- 6.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Human Retrovirol. 1998;18(Suppl 1):S11–S19. [DOI] [PubMed]
- 7.Thorpe L, Ouellet L, Hershow R, Bailey S, Williams I, Monerosso E. The multiperson use of non-syringe injection equipment and risk of hepatitis c infection in a cohort of young adult injection drug users in Chicago, 1997–1999. Ann Epidemiol. 2000;10:472–473. [DOI] [PubMed]
- 8.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. [DOI] [PMC free article] [PubMed]
- 9.Gerberding JL. Management of occupational exposures to blood-borne viruses. N Engl J Med. 1995;332:444–451. [DOI] [PubMed]
- 10.Hagan H, Des Jarlais DC. HIV and HCV infection among injecting drug users. Mt Sinai J Med. 2000;67:423–428. [PubMed]
- 11.Stoove MA, Gifford SM, Dore GJ. The impact of injecting drug use status on hepatitis C-related referral and treatment. Drug Alcohol Depend. 2005;77:81–86. [DOI] [PubMed]
- 12.Stephenson J. Former addicts face barriers to treatment for HCV. JAMA. 2001;285:1003–1005. [DOI] [PubMed]
- 13.Strauss SM, Astone JM, Jarlais DD, Hagan H. A comparison of HCV antibody testing in drug-free and methadone maintenance treatment programs in the United States. Drug Alcohol Depend. 2004;73:227–236. [DOI] [PubMed]
- 14.Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clin Infect Dis. 2002;35:783–788. [DOI] [PubMed]
- 15.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149:203–213. [DOI] [PubMed]
- 16.Crofts N, Nigro L, Oman K, Stevenson E, Sherman J. Methadone maintenance and hepatitis C virus infection among injecting drug users. Addiction. 1997;92:999–1005. [DOI] [PubMed]
- 17.Strauss SM, Astone JM, Hagan H, Des J. The content and comprehensiveness of hepatitis C education in methadone maintenance and drug-free treatment units. J Urban Health. 2004;81:38–47. [DOI] [PMC free article] [PubMed]
- 18.Garfein RS, Latka M, Hagan H, et al. CIDUS-III/Drug Users Intervention Trial (DUIT): A Randomized, Controlled Trial of a Peer-Education Behavioral Intervention to Prevent HIV and Hepatitis C Virus Infection Among 15–30 Year Old Injection Drug Users. Paper Presented at: National HIV Prevention Conference; June 12, 2005; Atlanta, GA.
- 19.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Morb Mortal Wkly Rep. 2003;52:1–13. [PubMed]
- 20.Fishbein M, Ajzen F. Belief, Attitude and Behavior: An Introduction to Theory and Research. Reading, MA: Addison-Wesley; 1975.
- 21.Montano DE, Kasprzyk D, Taplin SH. The Theory of Reasoned Action and the Theory of Planned Behavior. In: Glanz K, Lewis FM, Rimer BK, eds. Health Behavior and Health Education. San Francisco, CA: Jossey-Bass Publishers; 1997.
- 22.Bandura A. Self-efficacy mechanism in human agency. Am Psychol. 1986;40:359–373.
- 23.Bandura A. A Social-Cognitive Approach to the Exercise of Control over AIDS Infection. In: Albee GW, Schneider SF, eds. Primary Prevention of AIDS: Psychological Approaches. Newbury Park, CA: Sage Publications; 1991.
- 24.Rothman KJ, Greenland S. Modern Epidemiology, 2nd edition. Philadelphia, PA: Lippincott-Raven; 1998.
- 25.Hagan H, Campbell JV, Thiede H, et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep. 2006;121:710–719. [DOI] [PMC free article] [PubMed]
- 26.Rosenblum A, Nuttbrock L, McQuistion HL, Magura S, Joseph H. Hepatitis C and substance use in a sample of homeless people in New York City. J Addict Dis. 2001;20:15–25. [DOI] [PubMed]
- 27.Klinkenberg WD, Caslyn RJ, Morse GA, et al. Prevalence of human immunodeficiency virus, hepatitis B, and hepatitis C among homeless persons with co-occurring severe mental illness and substance use disorders. Compr Psychiatry. 2003;44:293–302. [DOI] [PubMed]
- 28.Stein JA, Nyamathi A. Correlates of hepatitis C virus infection in homeless men: a latent variable approach. Drug Alcohol Depend. 2004;75:89–95. [DOI] [PubMed]
- 29.Macalino GE, Vlahov D, Sanford-Colby S, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–1223. [DOI] [PMC free article] [PubMed]
- 30.Reindollar RW. Hepatitis C and the correctional population. Am J Med. 1999;107:100S–103S. [DOI] [PubMed]
- 31.Samuel MC, Doherty PM, Bulterys M, Jenison SA. Association between heroin use, needle sharing and tattoos received in prison with hepatitis B and C positivity among street-recruited injecting drug users in New Mexico, USA. Epidemiol Infect. 2001;127:475–484. [DOI] [PMC free article] [PubMed]
- 32.Gebo KA, Diener-West M, Moore RD. Hospitalization rates differ by hepatitis C status in an urban HIV cohort. J Acquir Immune Defic Syndr. 2003;34:165–173. [DOI] [PubMed]
- 33.Wiegand J, Buggisch P, Boecher W, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. [DOI] [PubMed]
- 34.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. [DOI] [PubMed]
- 35.Hagan H, Latka MH, Campbell JV, et al. Eligibility for treatment of hepatitis C virus infection among young injection drug users in 3 US cities. Clin Infect Dis. 2006;42:669–672. [DOI] [PubMed]
- 36.Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280:867–873. [DOI] [PubMed]
- 37.Des Jarlais DC, Paone D, Milliken J, et al. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. Lancet. 1999;353:1657–1661. [DOI] [PubMed]
- 38.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152:99–106. [DOI] [PubMed]


