In his article Panjabi gives a concise overview on the current knowledge and understanding of low back and neck pain [12]. He introduces the hypothesis that chronic back pain originates from subfailure injuries of three types of spinal ligamentous structures and their embedded mechanoreceptors: namely the spinal ligaments, the disc annulus and the facet capsules. These injured tissues then send out corrupted transducer signals to the neuromuscular control unit, and as a result corrupted muscle response patterns are generated leading to adverse consequences such as higher stresses, muscle fatigue, further injuries, and inflammation.
While paying less attention to the central learning processes involved in chronic back pain [5, 6, 19], this model focuses mainly on the structural mechanisms of pain generation. We are appreciative about the value of the hypothesis within this structural field and are optimistic about its successful application to the understanding and treatment of many cases of back pain. While we agree with the basic hypothesis and its emphasis on the transducer (mechanosensory) function of ligamentous tissues, we suggest to refine the model in terms of an inclusion of the thoracolumbar fascia (TLF). We present evidence that the TLF is significantly involved in all three levels of the hypothesis concerning spinal ligamentous structures: the transducer function of these tissues, their structural spinal function, and their proneness for subfailure injuries.
Transducer function of the TLF
Several studies revealed the presence of mechanosensory receptors in fascia [14, 20, 21, 24]. In particular for the TLF, the presence of Ruffini’s and Vater-Pacini corpuscles has been demonstrated [27]. The same type of mechanoreceptors were found in the spinal ligamentous structures mentioned by the author [10, 18, 28], and it is likely that these fascial receptors may serve a similar purpose, i.e. that they are involved in a neurosensory transducer function as indicated by investigations involving fascial mechnoreceptor stimulation in cats [14]. Indeed, since most of the spinal ligamentous structures are located relatively close to the axis of spinal movements [7, 9], they are in a less favourable position for the accurate detection of small load or position changes; compared with the TLF which is positioned at the furthest possible distance and therefore at a more advantageous position for this purpose (Fig. 1). It could be objected, that the supraspinous ligament, connecting the tips of the spinous processes, is similarly well positioned. Yet in fact, this can be also seen as further support for our suggestion, since this ligament is formed by a local attachment of the TLF (inferior to the spinous process of L4 the supraspinous ligament even ceases to exist in most bodies, in exchange for a diagonal fibre crossing of the TLF) [8, 13, 25]. Since TLF stiffness can be adjusted by muscular tonus changes, such as from the transversus abdominis [3], this servomechanism provides an additional advantage of this important dorsal tissue: the ability for the fine tuning of its mechanoreceptors over a wide range of spinal movement. Such a tuning mechanism is not known about the spinal ligamentous structures mentioned by Panjabi.
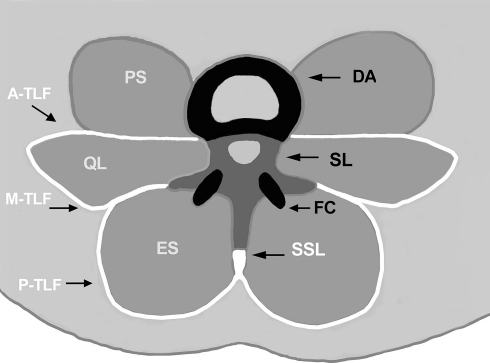
Fig. 1.
The spinal ligamentous structures considered by Panjabi, complemented by inclusion of the thoracolumbar fascia (TLF). Black and dark gray: the spinal ligamentous structures already included in the hypothesis; i.e. disc annulus (DA), facet capsules (FC), and the spinal ligaments (SL) which are connecting the vertebral bodies, arcs and their processes of adjacent vertebrae. White: the TLF, proposed in this letter for inclusion into the hypothesis and consisting of a posterior layer (P-TLF) covering the erector spinae (ES), middle layer (M-TLF) on the posterior side of quadratus lumborum (QL), and anterior layer (A-TLF) on the anterior side of QL and posterior to psoas (PS). The supraspinous ligament (SSL) is formed by the TLF as well. Note: axes of spinal movements usually traverse the central area between DA and both FCs, including their respective outlines. The TLF structures therefore tend to have a larger distance from the axes of spinal movements than the spinal ligamentous structures
Structural function of the TLF
Anatomical studies demonstrate that the different layers of the TLF are a sophisticated integrated system for tension transmission. The alignment of the collagen fibres reveals the direction of the load bearing function and is the basis for the effective load transfer from spine to pelvis, legs and arms [1, 23]. It has been shown that tensional stiffness of the different layers of the TLF influences spinal stability, such as in resisting spinal flexion or extension [2, 3]. This is exemplified by the anteroposterior fibre orientation of the interspinous ligament, which seems to reflect its primary function as a transmitter of TLF tension, rather than merely a direct holding against spinal flexion [25].
Subfailure injuries of the TLF
As fascia—like ligaments—has a much less abundant blood supply than muscles, it can be expected to heal as slowly as ligaments, and may therefore be a more likely facilitator of chronic back pain than injured muscles. Creep deformation in response to sustained lumbar flexion has been described for the spinal ligamentous structures included in the hypothesis [11]. Since the TLF has a lower stiffness than spinal ligaments [22] and is additionally positioned further away from the axis of spinal flexion, it can be expected to be exposed to at least similar deformation challenges [26]. Similarly, subfailure injuries due to strenuous tissue extensions can be expected to occur in the TLF as commonly or even more often than in spinal ligaments. Indeed, this consideration is corroborated by the frequent presence of clusters of myofibroblasts—cells commonly associated with a tissue repair function—in muscular fasciae such as the fascia lata, plantar fascia and TLF [15, 16].
We therefore suggest that the TLF should be included as an additional element—besides the spinal ligamentous structures considered by the author—in the new explanatory model for chronic back pain proposed in the article. Although fascia has been neglected in back pain research during the last decades, this inclusion will provide a basis for further research and new therapeutic directions in application of the new hypothesis. Indeed, this promise can be readily illustrated by two examples: recent findings based on tissue sections from 32 adult bodies revealed a higher average density of myofibroblasts in the lumbar TLF (compared with other fasciae, such as the fascia lata or plantar fascia), indicative of a high presence of subfailure injuries [17]. And a histological examination of the TLF in 24 chronic back pain patients reported a striking absence of mechanoreceptors normally found in this tissue [4]. Both findings fit well into the suggested hypothesis of Panjabi, given our suggested inclusion of the TLF.
Footnotes
This Letter to the Editor refers to the article http://www.dx.doi.org/10.1007/s00586-005-0925-3.
References
- 1.Barker PJ, Briggs CA. Attachments of the posterior layer of lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Barker PJ, Briggs CA, Bogeski G. Tensile transmission across the lumbar fasciae in unembalmed cadavers. Effects of tension to various muscular attachments. Spine. 2004;29:129–138. doi: 10.1097/01.BRS.0000107005.62513.32. [DOI] [PubMed] [Google Scholar]
- 3.Barker PJ, Guggenheimer KT, Gskovic I, et al. Effects of tensioning the lumbar fasciae on segmental stiffness during flexion and extension. Spine. 2006;31:397–405. doi: 10.1097/01.brs.0000195869.18844.56. [DOI] [PubMed] [Google Scholar]
- 4.Bednar DA, Orr FW, Simon GT. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain. A microscopic study. Spine. 1995;20:1161–1164. doi: 10.1097/00007632-199505150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;41(Suppl):66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- 6.Flor H, Diers M, Birbaumer N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci Lett. 2004;361:147–150. doi: 10.1016/j.neulet.2003.12.064. [DOI] [PubMed] [Google Scholar]
- 7.Holt CA, Evans SL, Dillon D, et al. Three-dimenional measurement of intervertebral kinematics in vitro using optical motion analysis. Proc Inst Mech Eng [H] 2005;219:393–399. doi: 10.1243/095441105X34374. [DOI] [PubMed] [Google Scholar]
- 8.Johnson GM, Zhang M. Regional differences within the human supraspinous and interspinous ligaments: a sheet plastination study. Eur Spine J. 2002;11:382–388. doi: 10.1007/s00586-001-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kettler A, Marin F, Sattelmayer G, et al. Finite helical axes of motion are a useful tool to describe the three-dimensional in vitro kinematics of the intact, injured and stabilised spine. Eur Spine J. 2004;13:553–559. doi: 10.1007/s00586-004-0710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima Y, Maeda T, Arai R, et al. Nerve supply to the posterior longitudinal ligament and the intervertebral disc of the rat vertebral column as studied by acetylcholinesterase histochemistry. II. Regional differences in the distribution of the nerve fibres and their origins. J Anat. 1990;169:247–255. [PMC free article] [PubMed] [Google Scholar]
- 11.Little JS, Khalsa PS. Human lumbar spine creep during cyclic and static flexion: creep rate, biomechanics, and facet joint capsule strain. Ann Biomed Eng. 2005;33:391–401. doi: 10.1007/s10439-005-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–676. doi: 10.1007/s00586-005-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prestar FJ. Morphology and function of the interspinal ligaments and the supraspinal ligament of the lumbar portion of the spine. Morphol Med. 1982;2:53–58. [PubMed] [Google Scholar]
- 14.Sakada S. Mechanoreceptors in fascia, periosteum and peridontal ligament. Bull Tokyo Med Dent Univ. 1974;21(Suppl):11–13. [PubMed] [Google Scholar]
- 15.Schleip R, Naylor IL, Ursu D, et al. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med Hypotheses. 2006;66:66–71. doi: 10.1016/j.mehy.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Schleip R, Klingler W, Lehmann-Horn F (2004) Active contraction of the thoracolumbar fascia—indications of a new factor in low back pain with implications for manual therapy. In: Vleeming A, Mooney V, Hodges P (eds) Proceedings of the 5th interdisciplinary world congress on low back and pelvic pain, Melbourne, pp 319–321
- 17.Schleip R, Klingler W (2006) Is fascia able to contract and relax in a smooth muscle-like manner and thereby influence musculoskeletal dynamics? In: Remvig L, Beyer L, Vacek J (eds) Proceedings of the 1st FIMM international academy conference, Leipzig, pp 19–20
- 18.Sekine M, Yamashita T, Takebayashi T, et al. Mechanosensitive afferent units in the lumbar posterior longitudinal ligament. Spine. 2001;26:1516–1521. doi: 10.1097/00007632-200107150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Soyka D, Haase C, Lindner V, et al. Forgotten pain. Schmerz. 1996;10:36–39. doi: 10.1007/s004820050018. [DOI] [PubMed] [Google Scholar]
- 20.Stillwell DL. Regional variations in the innervation of deep fasciae and aponeuroses. Anat Rec. 1957;127:635–648. doi: 10.1002/ar.1091270402. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Ito T. Histochemical demonstration of adrenergic fibers in the fascia periosteum and retinaculum. Clin Orthop Relat Res. 1977;126:276–281. [PubMed] [Google Scholar]
- 22.Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine. 1987;12:501–508. doi: 10.1097/00007632-198706000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Spine. 1995;20:753–758. doi: 10.1097/00007632-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Vshivtseva VV, Lesova LD. Features of the innervation of fascia and their microcirculatory bed in the rat. Arkh Anat Gistol Embriol. 1985;88:16–22. [PubMed] [Google Scholar]
- 25.Willard FH. The muscular, ligamentous and neural structure of the low back and its relation to back pain. In: Vleeming A, editor. Movement, stability and low back pain. The essential role of the pelvis. New York: Churchill Livingstone; 1997. pp. 3–35. [Google Scholar]
- 26.Yahia LH, Pigeon P, DesRosiers EA. Viscoelastic properties of the human lumbodorsal fascia. J Biomed Eng. 1993;15:425–429. doi: 10.1016/0141-5425(93)90081-9. [DOI] [PubMed] [Google Scholar]
- 27.Yahia L, Rhalmi S, Newman N, Isler M. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–197. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T, Cavanaugh JM, el-Bohy AA, et al. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg Am. 1990;72:865–870. [PubMed] [Google Scholar]