Abstract
Several studies have suggested that the pelvis is involved in the etiology or pathogenesis of adolescent idiopathic scoliosis (AIS). The purpose of this retrospective, cross-sectional radiographic study is to identify any correlation between the transverse plane rotational position of the pelvis in stance and operative-size idiopathic or congenital scoliosis deformities, using Scheuermann’s kyphosis and isthmic spondylolisthesis patients for comparison. The hypothesis tested was that the direction of transverse pelvic rotation is the same as that for a thoracic scoliosis. As a group, AIS patients had a significant transverse plane pelvic rotation in the same direction as the thoracic curve. When subdivided into the six Lenke curve patterns, this was true for the groups with a major thoracic curve: thoracic (1), double thoracic (2) and double curve patterns (3). It was not true for patterns with a major thoracolumbar/lumbar curve: single thoracolumbar/lumbar (5) and double thoracic-thoracolumbar/lumbar (6). Nor was it true for triple (4) curves. The Lenke 1 and 2 major thoracic curves without compensatory thoracolumbar/lumbar curves did not have the predicted pelvic rotation. All congenital scoliosis patients studied had main thoracic curves and significant transverse plane pelvic rotation in the same direction as the thoracic curve. There was no transverse plane pelvic rotation in the Scheuermann’s kyphosis or isthmic spondylolisthesis patients. We interpret these findings as consistent with a compensatory rotation of the pelvis in the same direction as the main thoracic curve in most patients with a compensatory thoracolumbar/lumbar curve as well as in patients with main thoracic congenital scoliosis.
Keywords: Adolescent idiopathic scoliosis, Transverse plane, Spinal deformity, Pelvic rotation
Introduction
Our three-dimensional study of adolescent idiopathic scoliosis (AIS) [1, 18] and the observations of others [19] led us to visualize the AIS deformity as one, two or three imperfect geometrical torsions [2]. Based on these studies, surgical techniques were developed to provide corrective loads principally in the transverse plane [4]. As this work progressed, we noted anecdotally that on standing preoperative posterior–anterior radiographs of AIS patients the right ilium often appeared to be wider than the left, suggesting a clockwise pelvic rotation position. On postoperative radiographs, particularly in patients with multiple curves, this asymmetry occasionally appeared to be increased.
More recently we have become aware of the theory of Karski that a right hip abduction contracture may be important in the pathogenesis of AIS [25, 26]. In reviewing the coronal plane radiographic illustrations included with his work, we noted that the right ilium often appeared to be wider than the left.
To assist study of the transverse plane rotational position of the pelvis, we developed a method of quantification utilizing clinically available coronal plane posterior–anterior radiographs [28].
The purpose of this study was to determine the transverse plane pelvic rotational position in stance in a population of presurgical AIS patients. Our hypothesis was that preoperatively the pelvis is significantly rotated in the same direction as the thoracic curve and thus in the opposite direction of the thoracolumbar/lumbar curve. For comparison, patients with congenital scoliosis as well as those with deformity in the sagittal plane only (Scheuermann’s kyphosis and isthmic spondylolisthesis) were studied.
Materials and methods
This study was approved by the Kansas University Medical Center Human Subjects Committee. All measurements were made on clinically obtained, coronal plane posterior to anterior exposure spine radiographs taken on 91-cm (36-in.) film at a 183-cm (72-in.) tube to film distance. Patients were positioned so that a line connecting their heels would be parallel to the X-ray cassette.
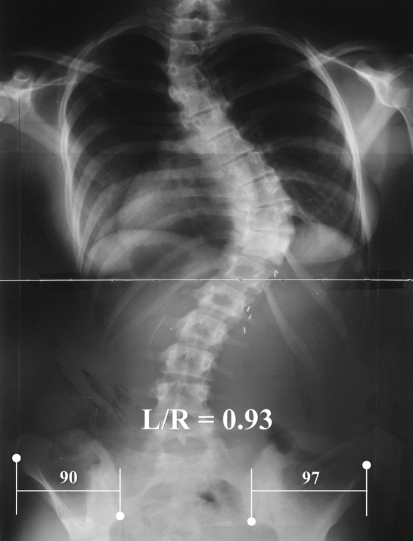
The transverse plane pelvic position was determined by a method we have previously described [28]. Briefly, in experimentation with a sawbones model of an adult female we found that rotation in the transverse plane of up to 20° was accurately reflected by a ratio of the iliac widths. Of a number of landmark possibilities studied, the best were found to be the inferior ilium at the sacroiliac joint medially and the anterior superior iliac spine laterally. These landmarks are usually visible on an exposure that includes the iliac crest for the purpose of viewing the Risser sign. For each side the linear distance between uprights through these points was measured and expressed as a left/right (L/R) ratio (Fig. 1). Because the iliac shadow becomes wider on the side to which the anterior aspect of the pelvis is rotated, the ratio is less than one when the pelvis is rotated to the right and greater than one when rotated to the left, when viewed posterior to anterior. We did not convert this ratio to degrees rotation because accurate conversion requires measurements not available on a coronal plane radiograph. The degrees of rotation observed were small, usually less than 5° and always less than 10°.
Fig. 1.
Standing position posterior–anterior radiograph of a patient with a right thoracic scoliosis illustrating the location of the hemi-pelvis landmarks, inferior ilium at the sacroiliac joint and the anterior superior iliac spine; and the lines necessary to measure the left and right hemi-pelvis coronal plane widths
The International Standards Organization Cartesian coordinate convention for expressing three planar positions was adopted [23]. According to this convention, vertebral body rotation to the right is in the clockwise direction and to the left is counterclockwise. Likewise, rotation of the anterior aspect of the pelvis to the right is in the clockwise direction and to the left is counterclockwise.
The most common idiopathic scoliosis curve patterns involve right/clockwise thoracic vertebra rotation and left/counterclockwise thoracolumbar/lumbar vertebra rotation. For this reason, all measurements were oriented for these directions. For patients with left thoracic, right thoracolumbar/lumbar, or both curves, the reciprocal L/R ratio was used, obtained by dividing the right by the left iliac width measurement.
Scoliosis curves were measured using the Cobb technique [17]. Idiopathic scoliosis curve patterns were defined using the Lenke classification [29]. In addition the Lenke 1A and 2A categories were analyzed again after those curves were divided into short or long thoracic curves. Short thoracic curves, labeled 1A1 and 2A1, had L4 tilt of <5° in the direction of the thoracic curve. Long thoracic curves, labeled 1A2 and 2A2, had L4 tilt of ≥5° in the direction of the thoracic curve. Thus, the 1A2 curve was the same as the King/Moe IV curve [27]. Our reason for doing this was based on the observation that the A2 thoracolumbar/lumbar compensatory curves are partial or fractional and place the pelvis at or near the apex of the compensatory curve. As a result, it is plausible that the transverse plane pelvic rotation would occur in the opposite, counterclockwise direction to the hypothesized clockwise direction. The congenital scoliosis curves were classified by the main curve apex level.
Statistical analysis
Descriptive statistics were used to characterize the study group. Intra- and inter-observer agreement (or reliability) of the pelvis L/R ratio was determined with the Intra-class Correlation Coefficients (ICC) [34]. The largest Cobb angle and age at surgery in each of the nine Lenke groups were compared with the Kruskal–Wallis test to determine if there was a difference between groups. Due to a small sample size in some groups, selected pair-wise comparisons were carried out on a priori basis. The L/R pelvis ratio was compared to one, or neutral. The one-sample t-test for a mean was used for this purpose for all normally distributed groups. The only non-parametric test needed was for Lenke group 2A1. For this group the Wilcoxon signed-rank test was used to compare the median L/R pelvis ratio to 1. The simple t-test for a mean was used to determine if the mean after subtracting 1 was significantly different from zero. Pearson’s correlation coefficient test was used to determine if there was a correlation between the largest Cobb angle and L/R ratio in each of the Lenke groups. Alpha was set at P < 0.05.
Study groups
All patients with adolescent idiopathic scoliosis (AIS), congenital scoliosis, spondylolisthesis or Scheuermann’s kyphosis receiving their index spine surgery by one surgeon at one hospital using one instrumentation system (either posterior or anterior) from January 1989 through December 2002 were eligible for the study. The AIS and congenital study group inclusive ages were 10 through 20 years. Because of small numbers the Scheuermann’s kyphosis and isthmic spondylolisthesis study groups were not age limited.
Two hundred forty patients with AIS met the inclusion criteria. One patient had had an innominate osteotomy for developmental dysplasia of the hip and was excluded from further analysis, leaving 239 patients eligible for study. Preoperative X-rays allowing measurement of the transverse plane pelvic rotation were available for 188 patients (79%), of whom 160 were female and 28 male. Their average age was 14.6 years (±2.35), and the average size of their largest Cobb was 61° (±13°). Their breakdown by Lenke curve type is shown in Table 1.
Table 1.
Demographic and curve characteristics of the adolescent idiopathic scoliosis (AIS) patients
| Curve classification | n | Age | Apex | Cobb | |||
|---|---|---|---|---|---|---|---|
| Lenke | Curve(s) | Years (SD)a | Direction | High Thoracic × (SD) | Thoracic × (SD) | TL/L × (SD) | |
| 1 | Thoracic | 75 | 14.6 ± 2.6 | T 69R/6L | NA | 58° ( ± 8.3)* ** *** | NA |
| 2 | Dbl Thor | 45 | 14.2 ± 2.0 | T 45R/0L | 43° (±8.4) | 65° ( ± 10.1)* | NA |
| 3 | Double | 11 | 14.0 ± 3.1 | T 10R/1L | NA | 70° (±13.1)** | 64° (±15.4) |
| 4 | Triple | 7 | 17.0 ± 2.5 | T 7R/0L | 48° (±14.6) | 86° (±34.2)*** | 66° (±25.3) |
| 5 | TL/L | 35 | 14.5 ± 1.8 | TL/L 29L/6R | NA | NA | 53° (±8.7) |
| 6 | T-TL/L | 15 | 14.9 ± 2.1 | TL/L 11L/4R | NA | 52° (±11.4) | 65° (±9.5) |
Dbl Thor Double thoracic, TL/L Thoracolumbar/lumbar
* P = 0.0002
** P = 0.0013
*** P = 0.0064
aThere were no significant age differences
Twenty-one patients with congenital scoliosis met the inclusion criteria and were eligible for study. However, 8 had kyphoscoliosis and 1 a thoracolumbar curve, leaving 12 with major thoracic scoliosis. Preoperative X-rays allowing measurement of the transverse plane pelvic rotation were available for ten (88%), of whom seven were female and three male. Their average age was 13.7 years (±2.17). Their major thoracic curve, apex right in 6 and left in 4, averaged 59° (±16°). Five had a major high thoracic curve, averaging 36° (±12°) and 7 a major thoracolumbar/lumbar curve, averaging 46° (±22°).
Ten patients with Scheuermann’s kyphosis met the inclusion criteria and were eligible for study. Preoperative X-rays allowing measurement of the transverse plane pelvic rotation was available for eight (80%). Of the 8, 5 were female and 3 were male. Their average age was 22.5 years (±6.12).
Eighteen patients with isthmic spondylolisthesis met the inclusion criteria. One patient with thoracic idiopathic scoliosis operated the same day as her spondylolisthesis operation was excluded, leaving 17 patients eligible for study. Pre-operative X-rays allowing measurement of the transverse plane pelvic rotation were available for 7 (41%), 5 of whom were female and 2 male. Their average age was 28.8 years (±11.20).
Results
The intra-observer (JG) agreement for the L/R ratio measurement was 0.97 for 197 comparisons. The inter-observer (JG and DB) agreement for 48 AIS comparisons was 0.88. The transverse plane position for the adolescent idiopathic scoliosis group is shown in Table 2. For the group as a whole the hypothesis was true with a L/R ratio of 0.95, significantly less than 1 at P < 0.0001. The hypothesis was also true for the Lenke groups where the thoracic curve was the largest of the curves: groups 1 (thoracic), P < 0.0001; 2 (double thoracic), P = 0.02; and 3 (double), P = 0.0317. It was not true for those curves patterns with a single major thoracolumbar/lumbar curve: group 5 (thoracolumbar/lumbar) or a double curve with a larger thoracolumbar/lumbar curve group 6 (thoracic-thoracolumbar/lumbar). It was also not true for the triple curves where, even though the numbers were small, the trend was in the direction opposite than hypothesized.
Table 2.
Left/right hemi-pelvis ratios of the idiopathic scoliosis patients as a group and by curve pattern
| Lenke | Curve(s) | n | L/R ratio | P* | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| 1 through 6 | All | 188 | 0.95 | ±0.13 | <0.0001 |
| 1 | Thoracic | 75 | 0.93 | ±0.12 | <0.0001 |
| 2 | Dbl Thor | 45 | 0.96 | ±0.12 | 0.0175 |
| 3 | Double | 11 | 0.91 | ±0.11 | 0.0317 |
| 4 | Triple | 7 | 1.07 | ±0.25 | NS |
| 5 | TL/L | 35 | 0.99 | ±0.13 | NS |
| 6 | T-TL/L | 15 | 0.96 | ±0.12 | NS |
A ratio less than 1 in this and the following tables indicates clockwise pelvic rotation, the same direction as a right thoracic apex (scoliosis)
* P (Mean compared to 1)
The Thoracic (1) and Double Thoracic (2) curve groups were separated on the basis of the lumbar modifier (Table 3). The hypothesis was true only for the Lenke 1A and 1B groups.
Table 3.
Left/right hemi-pelvis ratios of the thoracic and double thoracic curve pattern groups subdivided by the Lenke lumbar curve modifier
| Lenke | Curve | Lumbar | n | L/R ratio | P* | |
|---|---|---|---|---|---|---|
| Modifier | Mean | SD | ||||
| 1 | Thoracic | A | 46 | 0.92 | ±0.13 | <0.0001 |
| B | 14 | 0.92 | ±0.12 | 0.036 | ||
| C | 15 | 0.95 | ±0.1 | NS | ||
| 2 | Dbl Thor | A | 35 | 0.96 | ±0.13 | NS |
| B | 6 | 0.94 | ±0.07 | NS | ||
| C | 4 | 0.98 | ±0.08 | NS | ||
* P Mean compared to 1
The thoracic and double thoracic curves with an A lumbar modifier were separated into those with <5° L4 tilt (A1) and those with ≥5° L4 tilt (A2) toward the thoracic curve convexity (Table 4). The 1A1 and 2A1 curves behaved as the hypothesis predicted, with pelvic rotation in the direction of the main thoracic curve. Interestingly, the 1A2 and 2A2 curves did not show any significant pelvic rotation, either toward or opposite to the thoracic rotation.
Table 4.
Left/right hemi-pelvis ratios of the thoracic and double thoracic lumbar modifier A groups subdivided by L4 tilt of <5° toward the thoracic apex (A1) and L4 tilt of ≥5° toward the thoracic apex (A2)
| Lenke | Curve | Lumbar | n | L/R ratio | P* | |
|---|---|---|---|---|---|---|
| Modifier | Mean | SD | ||||
| 1 | Thoracic | A1** | 31 | 0.89 | ±0.11 | <0.0001 |
| A2*** | 15 | 0.97 | ±0.14 | NS | ||
| 2 | Dbl Thor | A1** | 21 | 0.92a | NA | 0.0154 |
| A2*** | 14 | 0.99 | ±0.15 | NS | ||
* P Mean compared to 1
** A1 = L4 tilt of <5° toward the thoracic apex
*** A2 = L4 tilt of ≥5° toward the thoracic apex
aMedian (Wilcoxin Rank Sum)
The transverse plane position for the congenital scoliosis group as well as the comparison Scheuermann’s kyphosis and spondylolisthesis groups is shown in Table 5. The hypothesis was proven for the congenital scoliosis group. For neither the Scheuermann’s kyphosis nor the spondylolisthesis groups was the transverse plane pelvic rotation different than zero.
Table 5.
Left/right hemi-pelvis ratios of the congenital scoliosis, Scheuermann’s kyphosis and spondylolisthesis groups
| n | L/R ratio | P* | ||
|---|---|---|---|---|
| Mean | SD | |||
| Congenital Scoliosis | 10 | 0.77 | 0.11 | <0.0001 |
| Scheuermann’s | 8 | 1.08 | 0.05 | NS |
| Spondylolisthesis | 7 | 1.01 | 0.04 | NS |
* P (Mean compared to 1)
There were no correlations between the major Cobb and the pelvis L/R ratio for any of the scoliosis study groups. However, for the AIS 1A1 thoracic sub-group it was suggestive, cc 0.3259, P = 0.0736. To further explore the possible relationships between the coronal and transverse plane angular spine deformities and the transverse plane pelvic position, additional analyses were performed on this 1A1 sub group. Apex transverse plane vertebra rotation of both the main thoracic and the compensatory thoracolumbar/lumbar curves was measured using the Perdriolle method [33]. For 6 of the 31 patients this measurement was not available for the thoracic curve, leaving 25 patients. In addition, the compensatory thoracolumbar/lumbar scoliosis was measured. Finally, the thoracic angle of trunk inclination (ATI) [3], which was routinely performed clinically, was used as an additional measure of transverse plane deformity.
The thoracic apex rotation was 27° (±7.5°), the thoracic ATI 17° (±4.7°), the compensatory thoracolumbar/lumbar Cobb 35° (±7.1°), and the thoracolumbar/lumbar apex rotation 6° (±5.6°).
The correlations between the coronal and transverse plane measures of the 1A1 thoracic sub-group are shown in Table 6. There was significant correlation between the major thoracic and the compensatory thoracolumbar/lumbar Cobbs (P < 0.0001), but not between their transverse plane apex vertebral rotations. The pelvis L/R ratio correlated significantly with the thoracolumbar/lumbar apex vertebral rotation (P = 0.0105), but not with the thoracic apex vertebral rotation or trunk rotation and not with either the thoracolumbar or thoracic Cobb. The thoracic Cobb significantly correlated with both thoracic apex vertebral rotation (P = 0.0191) and the thoracic ATI (P = 0.0011). These two were significantly correlated (P = 0.0298). Finally, the thoracic apical vertebral rotation and the thoracolumbar/lumbar Cobb were significantly correlated (P = 0.038).
Table 6.
Correlations between the coronal and transverse plane measures of the 1A1 subgroup (n = 25)
| Pelvis left/right ratio | Thoracic Cobb | Thoracic rotationa | Thoracic ATIb | TL/Lc Cobb | TL/Lc rotationa | |
|---|---|---|---|---|---|---|
| Pelvis left/right ratio | 1 | 0.24328 | 0.23368 | 0.18621 | 0.16187 | 0.50252 |
| P value | 0.2413 | 0.2609 | 0.3728 | 0.4395 | 0.0105 | |
| Thoracic Cobb | 1 | 0.46517 | 0.61364 | 0.78961 | 0.16413 | |
| P value | 0.0191 | 0.0011 | <0.0001 | 0.4331 | ||
| Thoracic rotationa | 1 | 0.43484 | 0.41713 | −0.28158 | ||
| P value | 0.0298 | 0.038 | 0.1727 | |||
| Thoracic ATIb | 1 | 0.3512 | −0.02276 | |||
| P value | 0.0852 | 0.914 | ||||
| TL/Lc Cobb | 1 | 0.31311 | ||||
| P value | 0.1275 | |||||
| TL/Lc Rotation a | 1 | |||||
| P value |
aApex vertebral rotation
bAngle of trunk inclination (ATI)
cThoracolumbar/lumbar
Discussion
The etiology or etiologies and pathogenesis of adolescent idiopathic scoliosis are unknown. The possibility that the pelvis or hips are involved has been raised in several studies [5, 25, 26, 34, 36].
Our findings support the hypothesis that transverse plane pelvic angular position is involved in the deformity of major thoracic AIS curves. Initially it appeared to us that this clockwise pelvic rotation might be involved in the thoracic curves’ pathogenesis [22]. However, this theory seems unlikely given the lack of predictable pelvic rotation position for major thoracic curves without compensatory thoracolumbar/lumbar curves. And, the similar rotation direction of congenital major thoracic curves and the pelvis suggests that the transverse plane pelvic rotation position is compensatory. This view is also supported by the relatively small magnitude of pelvic rotation in comparison to the major thoracic apex vertebra transverse plane rotation. Finally, the absence of significant transverse plane pelvic rotation in either of the patient groups with spine deformity limited to the sagittal plane further suggests that the transverse plane pelvic rotation is related to the thoracic scoliosis.
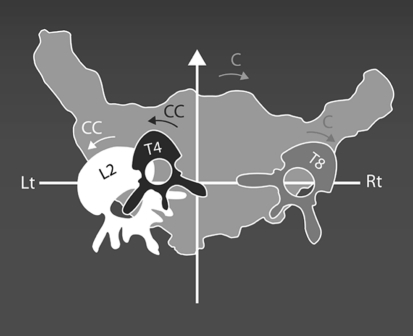
Compensatory coronal plane scoliosis curves are well recognized. And sagittal plane compensation including the pelvis has received recent study [38]. Our focus is on the transverse plane and, based on our studies, we are suggesting that the transverse plane pelvis position accompanying major thoracic curves is the fourth transverse plane compensation. This concept is well visualized from the top, as illustrated in Fig. 2. The major thoracic curve is rotated in the clockwise direction and is compensated for with counterclockwise high thoracic and thoracolumbar/lumbar rotations. The clockwise pelvis rotation is the next compensation. Regardless of the plane, compensatory curves and positions probably serve to help preserve balance.
Fig. 2.
Top view drawing to illustrate the compensations above and below a typical T8 right apex thoracic adolescent idiopathic scoliosis
To our knowledge our study is the first to quantify transverse plane pelvic rotation position in a full-length study. There is a preliminary mini-paper in which pelvic rotation, as quantified from landmarks on a coronal plane radiograph, was correlated to the apex of the compensatory thoracolumbar/lumbar curve [34]. This is complementary to our finding of a correlation between the transverse plane pelvic rotation and the thoracolumbar/lumbar compensatory curve apex vertebra rotation. Otherwise our experimental designs were too different to allow further comparison.
Our results show that the association of clockwise thoracic and clockwise pelvic rotation for major thoracic curves is disturbed by a structural thoracolumbar/lumbar curve, as in Lenke 4 and 6 curves, and by a fractional thoracolumbar/lumbar curve, when the L4 vertebral body is tilted in the frontal plane toward the thoracic curve apex (Lenke 1A2 and 2A2 in this study). These findings suggest that many different forces are competing to maintain trunk compensation.
Many asymmetries have been associated with the asymmetry of idiopathic scoliosis. The anecdotal observation of right hip relative or real abduction contracture by Karski [25, 26] has recently been supported in a preliminary study [13]. In their study 67% of the AIS patients studied had asymmetry of hip adduction of ≥5°, and, when present, it was on the right in 96%. However, a comparable control population has not been studied. It is entirely possible that such a small adduction asymmetry is a residual of the in utero left side lie, which encourages left hip adduction and right hip abduction [20].
Experimentally, a pelvic wing deformity has been noted in a series of pinealectomized chicks that developed scoliosis [12]. However, this scoliosis was always at the thoracolumbar junction, and the thoracolumbar junction and the lumbar spine appeared to be deeply seated between the iliac wings. The relevance of this finding to the clinical situation may be questioned.
Patients with adolescent idiopathic scoliosis have been found to have significantly larger femoral neck-shaft angles than normal subjects [36]. This symmetrical increase in femoral neck-shaft angles suggests a systemic effect. One possibility is a neuropathic effect, which could be related to mild cerebellar tonsil protrusion [11] or to a functional tethering of the spinal cord [16]. Other possibilities include decreased stress on the developing femur from decreased body weight [32], decreased activity [30], osteoporosis [14] and malnutrition [39].
In addition to being longer, the AIS patients’ neck-shaft angles were asymmetrical, not as a group but by curve pattern [36]. In patients with right thoracic scoliosis the left neck-shaft angle was greater than the right. This is consistent with a relative right hip abduction contracture, which has been implicated in the pathology of idiopathic scoliosis, as has been noted. In AIS patients with left lumbar or double major scoliosis the femoral neck-shaft angle was greater on the right than the left. This seems consistent with the increase in vertical height of the ilium found on the side of the thoracolumbar/lumbar curve concavity in two studies [8, 37].
Many significant skeletal asymmetries have been identified in patients with idiopathic scoliosis. Some correlate with the spinal deformity. These include iliac height and thoracolumbar/lumbar Cobb [8], upper arm length asymmetry and thoracic vertebral rotation [9], and ilio-femoral length asymmetry and sacral alar height asymmetry [10]. Some asymmetries do not correlate with the spinal deformity. These include right total leg and right tibia relative lengthening with lower spine scoliosis [8] and increased right tibial torsion in thoracic curves [7]. In addition to asymmetry, variations from normal growth have been identified by several workers and recently summarized [15]. Many of these asymmetries are also present in patients with congenital scoliosis [6].
An appealing, unifying explanation of these many anthropometric variations from normal is the concept of developmental instability proposed by Goldberg et al. [21]. In this paradigm scoliosis is viewed as a loss of symmetry “when the developmental program coded in the genome fails to run properly”, due to the timing and severity of any number of stress factors. This theory accommodates “factors common to all etiologies”. Females are at increased risk for at least two reasons: they have more directional asymmetry than males [24] and their trunk muscle strength per lean body weight actually decreases from their juvenile to adolescent years, whereas in males it increases [40].
The major weakness of our study is that we do not know if the pelvic iliac wing L/R ratio is positional, as we suppose, or intra-pelvic. To know this would require an axial imaging study. If done supine, as it is almost always, any intra-pelvic rotation could likely be determined but compensatory positioning change would be lost. Although the transverse plane pelvic rotation did not correlate with the thoracic Cobb, there were overlapping correlations from the thoracic spine to the pelvis. Compensations in one plane are not necessarily reflected in a second plane; witness the correlated coronal plane Cobbs and the uncorrelated transverse plane apex rotation in the Lenke 1A1 thoracic subgroup. Finally, the Lenke classification may not separate adolescent curves into homogenous curve patterns [31], an observation we confirmed for the 1A group.
Conclusions
The method of measuring static transverse pelvic rotation was reliable. In patients with main single thoracic, double thoracic and double adolescent idiopathic scoliosis curves large enough to warrant surgery there was significant static pelvic transverse plane rotation in the same direction as the thoracic curve. The same was true for patients with main thoracic congenital scoliosis. There was no transverse plane pelvic rotation in patients with sagittal plane deformities. For adolescent idiopathic scoliosis patients with major thoracolumbar/lumbar curves, as well as triple curves, there was not a predictable correlation between pelvic rotation and curve direction. These findings support the hypothesis that static transverse plane pelvic rotation is one of the compensatory curves in major thoracic scoliosis curve patterns.
References
- 1.Asher MA, Cook LT. The transverse plane evolution of the most common adolescent idiopathic scoliosis deformities. A cross-sectional study of 181 patients. Spine. 1995;20:1386–1391. doi: 10.1097/00007632-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Asher MA, Burton DC. A concept of idiopathic scoliosis deformities as imperfect torsion(s) Clin Orthop. 1999;364:11–25. doi: 10.1097/00003086-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bunnell WP. An objective criterion for scoliosis screening. J Bone Joint Surg Am. 1984;66:1381–1387. [PubMed] [Google Scholar]
- 4.Burton DC, Asher MA, Lai SM. The selection of fusion levels using torsional correction techniques in the surgical treatment of idiopathic scoliosis. Spine. 1999;24:1728–1739. doi: 10.1097/00007632-199908150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Burwell RG, Cole AA, Cook TA, Grivas TA, Kiel AW, Moulton A, Thirlwall AS, Upadhyay SS, Webb JK, Wemyss-Holden SA, Whitwell DJ, Wojcik AS, Wythers DJ. Pathogenesis of idiopathic scoliosis. The Nottingham concept. Acta Orthop Belg. 1992;58(Suppl 1):33–58. [PubMed] [Google Scholar]
- 6.Burwell RG, Dangerfield PH, Vernon CL (1977) Anthropometry and scoliosis. In: Zorab PA (ed) Scoliosis. In: 5th Symposium. Academic, London, pp 123–163
- 7.Burwell RG, Kirby AS, Cole AA, Moulton A, Pratt RK, Webb JK. Torsion in lower limb bones of patients with adolescent scoliosis (AIS) treated surgically. In: Sevastik JA, Diab KM, editors. Research into spinal deformities. Amsterdam: IOS Press; 1997. pp. 123–126. [Google Scholar]
- 8.Burwell RG, Aujla RK, Freeman BJC, Dangerfield PH, Cole AA, Kirby AS, Pratt RK, Webb JK, Moulton A. Patterns of extra-spinal left–right skeletal asymmetries in adolescent girls with lower spine scoliosis: relative lengthening of the ilium on the curve concavity and of right lower limb segments. In: Uyttendaele D, Dangerfield PH, editors. Research into spinal deformities 5. Amsterdam: IOS Press; 2006. pp. 57–65. [PubMed] [Google Scholar]
- 9.Burwell RG, Freeman BJC, Dangerfield PH, Aujla RK, Cole AA, Kirby AS, Pratt PK, Webb JK, Moulton A. Left–right upper arm length asymmetry associated with apical vertebral rotation in subjects with thoracic scoliosis: anomaly of bilateral symmetry affecting vertebral, costal and upper arm physes? In: Uyttendaele D, Dangerfield PH, editors. Research into spinal deformities 5. Amsterdam: IOS Press; 2006. pp. 66–71. [PubMed] [Google Scholar]
- 10.Burwell RG, Aujla RK, Freeman BJC, Dangerfield PH, Cole AA, Kirby AS, Pratt RK, Webb JK, Moulton A. Patterns of extra-spinal left–right skeletal asymmetries and proximo-distal disproportion in adolescent girls with lower spine scoliosis: Ilio-femoral length asymmetry and bilateral tibial/foot length disproportion. In: Uyttendaele D, Dangerfield PH, editors. Research into spinal deformities 5. Amsterdam: IOS Press; 2006. pp. 101–108. [PubMed] [Google Scholar]
- 11.Cheng JCY, Chau WW, Guo X, Chan YL. Redefining the magnetic resonance imaging reference for the cerebellar tonsil: a study of 170 adolescents with normal versus idiopathic scoliosis. Spine. 2003;28:815–818. doi: 10.1097/00007632-200304150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Cheung KMC, Wang T, Hu G, Leong JCY. Primary thoracolumbar scoliosis in pinealectomized chickens. Spine. 2003;28:2499–2504. doi: 10.1097/01.BRS.0000092366.30032.97. [DOI] [PubMed] [Google Scholar]
- 13.Cheung KMC, Chooi YS. Hip adduction differences in adolescent idiopathic scoliosis: a cause or effect of scoliosis? J Orthop Surg (Hong Kong) 2005;9(Suppl):80. [Google Scholar]
- 14.Cheung CSK, Lee WTK, Tse YK, Lee KM, Guo X, Qin L, Cheng JCY. Generalized osteopenia in adolescent idiopathic scoliosis-association with abnormal pubertal growth, bone turnover, and calcium intake? Spine. 2006;31:330–338. doi: 10.1097/01.brs.0000197410.92525.10. [DOI] [PubMed] [Google Scholar]
- 15.Cole AA, Burwell RG, Dangerfield PH, Grivas TB, Webb JK, Moulton A. Anthropometry. In: Burwell RG, Dangerfield PH, Lowe TG, Margulies JY, editors. Etiology of adolescent idiopathic scoliosis. Philadelphia: Hanley & Belfus; 2000. pp. 411–421. [Google Scholar]
- 16.Chu WC, Lam WW, Chan YL, Ng BK, Lam TP, Lee KM, Guo X, Cheng JC. Relative shortening and functional tethering of spinal cord in adolescent idiopathic scoliosis? Study with multiplanar reformat magnetic resonance imaging and somatosensory evoked potential. Spine. 2006;31:E19–E25. doi: 10.1097/01.brs.0000193892.20764.51. [DOI] [PubMed] [Google Scholar]
- 17.Cobb JR. Outline for the study of scoliosis. In: Edwards JE, editor. Instructional course lectures, Ann Arbor: American Academy of Orthopaedic Surgeons; 1948. pp. 261–276. [Google Scholar]
- 18.DeSmet AA, Tarlton MA, Berridge AS, Asher MA. The top view of analysis of scoliosis progression. Radiology. 1983;147:369–372. doi: 10.1148/radiology.147.2.6340156. [DOI] [PubMed] [Google Scholar]
- 19.Dubousset J. Three-dimensional analysis of the scoliosis deformity. In: Weinstein SL, editor. The pediatric spine. New York: Raven; 1994. pp. 479–496. [Google Scholar]
- 20.Dunn PM. Congenital postural deformities. Br Med Bull. 1976;32:71–76. doi: 10.1093/oxfordjournals.bmb.a071327. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg CJ, Fogarty EE, Moore DP, Dowling FE. Scoliosis and developmental theory: adolescent idiopathic scoliosis. Spine. 1997;22:2228–2238. doi: 10.1097/00007632-199710010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Gum J (2006) Transverse plane pelvic rotation in spinal deformity: measurement reliability and relationship to diagnosis and deformity. http://hdl.handle.net/2271/163
- 23.ISO 2631-1978 (1978) Guide to Evaluation of Human Exposure to Whole Body Vibration
- 24.Jantz RL, Brehme H. Directional and fluctuating asymmetry in the palmar interdigital ridge-counts. Anthropol Anz. 1993;51:59–67. [PubMed] [Google Scholar]
- 25.Karski T. The etiology of the so-called idiopathic scoliosis. The new rehabilitation treatment. Prophylaxis. Lublin: FOLIUM; 2002. [PubMed] [Google Scholar]
- 26.Karski T. Biomechanical explanation of etiology of the so-called idiopathic scoliosis. Two etiopathological groups—important for treatment and neo-prophylaxis. Pan Arab J Ortho Trauma. 2005;9(1):123–135. [Google Scholar]
- 27.King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:1302–1313. [PubMed] [Google Scholar]
- 28.Lucas B, Asher M, McIff T, Lark R, Burton D. Estimation of transverse plane pelvic rotation using a posterior–anterior radiograph. Spine. 2005;30:E20–E27. doi: 10.1097/01.brs.0000175181.28730.ab. [DOI] [PubMed] [Google Scholar]
- 29.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis. A new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169–1181. [PubMed] [Google Scholar]
- 30.McMaster M, Lee AJ, Burwell RG. Physical activities of patients with adolescent idiopathic scoliosis (AIS) compared with a control group: implications for etiology and possible prevention. J Bone Joint Surg Br. 2006;88:225. doi: 10.2106/JBJS.F.00794. [DOI] [Google Scholar]
- 31.Miyanji F, Newton PO, Perry A. Van Valin S, Pawelek J (2006) Analysis of the Lenke 1A classification: defining 2 sub-types based on L4 tilt. In: Scoliosis Research Society 41st Annual Meeting Program. SRS, Monterey, E-Poster #33, p 206
- 32.Normelli H, Sevastik J, Ljung G, Aaro S, Jönsson-Söderström AM. Anthropometric data relating to normal and scoliotic Scandinavian girls. Spine. 1985;10:123–126. doi: 10.1097/00007632-198503000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Perdriolle R, Vidal J. Thoracic idiopathic scoliosis curve evolution and prognosis. Spine. 1996;17:513–517. doi: 10.1097/00007632-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Rigo M. Pelvis asymmetry in idiopathic scoliosis. Evidence of whole torsional body deformity? In: Sevastik JA, Diab KM, editors. Research into spinal deformities. Amsterdam: IOS Press; 1997. pp. 63–65. [Google Scholar]
- 35.Ross B. Fundamentals of biostatistics. New York: Duxbury Press; 1995. pp. 518–519. [Google Scholar]
- 36.Saji J, Upadhyay SS, Leong JCY. Increased femoral neck-shaft angles in adolescent idiopathic scoliosis. Spine. 1995;20:303–311. doi: 10.1097/00007632-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Schwender JD, Denis F. Coronal plane imbalance in adolescent idiopathic scoliosis with left lumbar curves exceeding 40°. Spine. 2000;25:2358–2363. doi: 10.1097/00007632-200009150-00015. [DOI] [PubMed] [Google Scholar]
- 38.Skalli W, Zeller RD, Miladi L, Bourcereau G, Savidan M, Lavaste F, Dubousset J. Importance of pelvic compensation in posture and motion after posterior spinal fusion using CD instrumentation for idiopathic scoliosis. Spine. 2006;31:E359–E366. doi: 10.1097/01.brs.0000219402.01636.87. [DOI] [PubMed] [Google Scholar]
- 39.Smith F, Latchford G, Hall R, Millner P, Dickson R. Indications of disordered eating behavior in adolescent patients with idiopathic scoliosis. J Bone Joint Surg Br. 2002;84:392–394. doi: 10.1302/0301-620X.84B3.12619. [DOI] [PubMed] [Google Scholar]
- 40.Sunnegårdh J, Bratteby L-E, Nordesjö L-O, Nordgren B. Isometric and isokinetic muscle strength, anthropometry and physical activity in 8 and 13 year old Swedish children. Eur J Appl Physiol. 1988;58:291–297. doi: 10.1007/BF00417265. [DOI] [PubMed] [Google Scholar]