Abstract
Surgical instrumentation planning for the correction of scoliosis involves many difficult decisions, especially with the introduction of multi-segmental and other instrumentation technologies. A preliminary study has shown a high variability in planning among a small group of surgeons. The purpose of this paper was to evaluate and analyze the selection of fusion levels and instrumentation choices among a more extended group of scoliosis surgeons. Thirty-two experienced spinal deformity surgeons were asked to provide their preferred posterior instrumentation planning for five patients with adolescent idiopathic scoliosis (AIS) using a graphical worksheet and the usual preoperative X-rays. Overall, the number of implants used ranged from 8 to 30 per patient (mean 16; SD 6): 71% of these were mono-axial screws, 20% multi-axial screws, and 9% hooks. The selected superior and inferior instrumented vertebrae varied up to six levels. The following significant groups of strategies were identified: A- “All Pedicle Screws Constructs” [NA = 103; 66%]; B- “All Hooks constructs” [NB = 5; 3%]; C- “Hybrid Constructs” [NC = 48; 31%]. A top-to-bottom attachment sequence was selected in 49% of all cases, a bottom-up in 46%, and an alternate order in 4%. A large variability in preoperative instrumentation strategy exists in AIS within an experienced group of orthopedic spine surgeons. The impact of such choices on the resulting correction is questioned and will need to be determined with adequate clinical, biomechanical, and computer simulation prospective studies.
Keywords: Scoliosis, Spine, Surgical instrumentation, Preoperative planning
Introduction
Modern instrumentation systems for the surgical correction of adolescent idiopathic scoliosis (AIS) have influenced the principles of management in spinal deformities. These multi-rod, screw, and hook systems are designed to provide selective and three-dimensional (3D) correction of scoliotic deformities with a minimal number of fusion levels while still allowing the best possible correction [11, 20, 25, 26, 39]. Over the past decade, these changes have led to a re-evaluation of many rules, such as those for the selection of proximal and distal fusion levels and for the type and number of implants. The introduction of new surgical techniques such as the rod rotation maneuver [9], cantilever and in situ rod contouring [7], or more recently the direct apical vertebral derotation (DAVD) technique [17] were also influenced by these changes. As a result, the surgical decision-making process has considerably increased in complexity, with many on-going controversies and debates over the choice of fusion levels [22, 23, 27], the proper guidelines for surgical correction [15, 29, 30, 37], the use of thoracic pedicle screws and their insertion techniques [6, 12, 18, 19, 36] as well as the risk of complications [32, 38] and the cost issues [20, 28, 35].
Although several clinical publications [11, 13, 16, 20, 25, 26, 35, 39] have attempted to evaluate the effects of different surgical strategies by reporting the experience of individual surgeons or centers, there is no clear consensus on an objective decision-making process and on the ideal treatment for an optimal correction. The surgical approach, the selection of fusion levels and the choice of the instrumentation system and the various types of constructs rely ultimately on the expertise of the surgeon, on his past experience, on his interpretation of the various rules and guidelines published on the subject and on basic biomechanical principles.
To date, few studies have analyzed the variability in the selection of instrumentation and fusion levels in AIS [22, 23, 27]. Lenke et al. [23] have shown an average of five proximal and four distal fusion levels chosen by 28 reviewers for seven patients with AIS. More recently, we have reported a large variability in instrumentation strategies within a small group of five spine surgeons for five AIS subjects [3]. In this study, the selected superior and inferior instrumented vertebrae varied up to six and five levels, respectively, and the number of instrumented spinal segments varied significantly from 7 to 15 vertebrae. However, the small number of surgeons involved did not allow a proper statistical analysis in search for identifiable patterns or strategies of instrumentation and correction. Therefore, the purpose of this paper was to extend our preliminary study by recruiting a larger sample of spinal deformity surgeons, in order to further analyze the selection of fusion levels and instrumentation choices in AIS. Our working hypothesis was that a large variability would still be present but that patterns of surgical strategies would be identified within a more representative sample.
Materials and methods
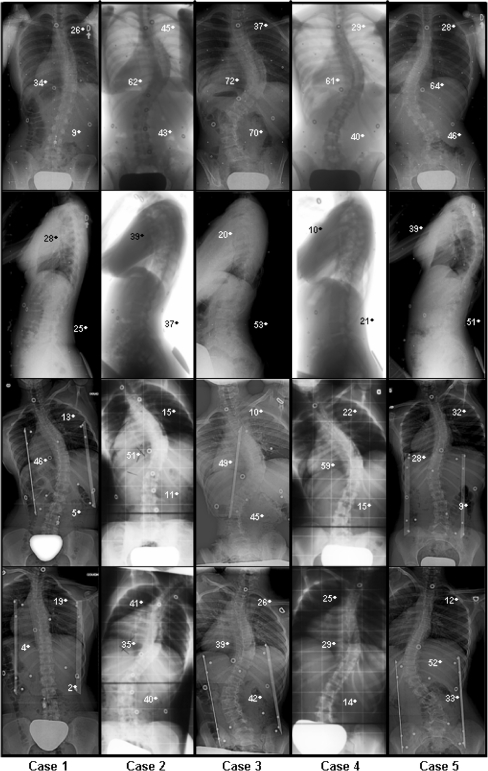
Five adolescent females with AIS having undergone posterior surgical instrumentation and fusion over the past 5 years were selected for evaluation. The choice of these patients was made to reflect a variety of typical curve patterns encountered in AIS (Table 1). Their age at surgery ranged from 13 to 18 years (mean 15; SD 2). The curve patterns display the following typical scoliotic deformities: one right thoracic curve (Cobb: 34°) with a significant trunk shift, three right thoracic and left lumbar curves (Cobb T/L: 62°/43°, 72°/70°, and 61°/40°) and one left thoracolumbar curve (Cobb: 64°). Their radiographs are shown in Fig. 1.
Table 1.
Demographic data and preoperative curve characteristics of the five patients
| Patient numbers | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age at surgery (years) | 18 | 16 | 15 | 13 | 13 |
| Lenke type | 1A | 2B | 3C | 3B | 5C |
| Proximal thoracic Cobb angle | 26° | 45° | 37° | 29° | 28° |
| Main thoracic Cobb angle | 34° | 62° | 72° | 61° | 64° |
| Thoracolumbar/lumbar Cobb angle | 9° | 43° | 70° | 40° | 46° |
| Kyphosis | 28° | 39° | 20° | 10° | 39° |
| Lordosis | 25° | 37° | 53° | 21° | 51° |
Fig. 1.
Preoperative PA, lateral, and side bending radiographs of the five patients
Thirty-two experienced spine surgeons from the Spinal Deformity Study Group (SDSG), who are also Fellows of the Scoliosis Research Society (SRS), agreed to participate in this study. They were asked to provide their preferred posterior instrumentation planning using the Cotrel–Dubousset Horizon system (Medtronic Sofamor-Danek, Memphis, TN, USA). All surgeons received the same information in a similar fashion. Each of the five cases was presented in a folder as follows: an introduction section first provided the pertinent clinical information such as age, gender, weight, height, Risser grade, and was followed by a section illustrating the standard preoperative radiographs: standing posteroanterior, lateral and left and right supine side bending radiographs. All radiographs were provided with Cobb angle measurements as well as with 3D reconstructions of the spine (frontal, sagittal, and top views) [1, 8]. Table 1 summarizes the clinical data of the patients.
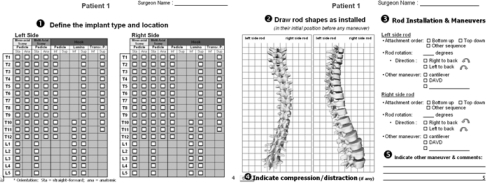
Instrumentation planning by each surgeon was done using a graphical worksheet, as illustrated in Fig. 2. Specifically, each surgeon was required to individually provide the following parameters:
Implant locations (vertebral levels), implant types [transverse process, pedicular and laminar hooks; mono-axial or multi-axial screws (MuAS)] and, if applicable, individual screw trajectories (straight-forward or anatomic),
rod shape in the frontal and sagittal planes, as they would be inserted in their initial position before any rod maneuver,
parameters for each individual rod insertion and maneuvers (attachment order, degree of rod rotation and direction, cantilever correction, amount of in situ bending, or DAVD),
vertebral levels where compression and distraction forces would be applied,
any other maneuver and comments.
Statistical analyses of these variables were performed using Statistica 6.0. (StatSoft Inc., 2001, Tulsa, OK, USA, data analysis software system) and SPSS 13.0. (SPSS Inc., analytical software, Chicago, IL, USA). Difference in the number of fusion levels used between the instrumentation groups was evaluated with an analysis of variance (ANOVA) one-way. The effect of scoliotic curves and surgeons on instrumentation choices (the number of instrumented levels, the number of implants, the selection of proximal and distal levels) were assessed with ANOVA one-factor repeated measures. Statistical significance was set at P < 0.05.
Fig. 2.
Graphical worksheet provided for the survey allowing the specification of the surgeon’s preferred posterior instrumentation configuration
A complementary study was conducted in a subgroup of six surgeons to determine intra-observer variability. On two separate occasions, at 1-year interval, these surgeons were asked to provide the same information using the same protocol for three of the cases (1, 3, and 5).
Results
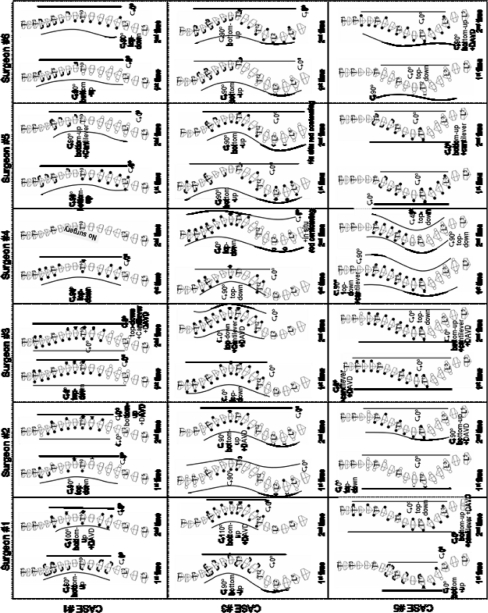
Among a possible 160 planning cases (32 surgeons, each proposing an instrumentation strategy for five patients), a total of 156 different instrumentation strategies were provided (Fig. 3, 4). Four surgeons indicated, in their opinion, that patient 1 was not a suitable candidate for surgical treatment and therefore did not submit an instrumentation strategy in this particular case.
Fig. 3.
Interobserver variability of instrumentation strategies proposed by the 32 surgeons (S1–S32) for patient number 3 (Lenke type 3c)
Fig. 4.
Postoperative radiographs of the patients (real surgery), as well as four representative strategies for each case (UIV upper instrumented vertebra, LIV lowest instrumented vertebra)
For most of the measured parameters related to the implants, rods and primary maneuvers (items 1–3 described in the Materials and methods section), no complete agreement between surgeons could be detected in any of the cases studied.
Overall, an average of 12 different selections of vertebral fusion levels (T2–L2, T3–L2, T4–L3, etc.) were noted per patient (mean 12.2; SD 1.3). The number of instrumented levels per case varied from 7 to 16 vertebrae (mean 11.2; SD 2.1). As shown in Table 2, the variation of the upper instrumented and fused level ranged from 5 to 6 levels. Patients 3 and 5 had the largest distribution with six different upper levels of fusion selected. The smallest variability was for patient 1 where 72% of the surgeons chose either T4 or T5 in equal proportions. The most frequently selected upper instrumented vertebral levels were the upper end vertebra (UEV) and one level above (UEV + 1) for patient 1 (Lenke type 1A), UEV + 1 for patient 4 (Lenke 3B), two levels above (UEV + 2) for patient 3 (Lenke 3C), UEV + 2 and three levels above (UEV + 3) for patient 5 (Lenke 5C) and UEV + 3 for patient 2 (Lenke 2B). The lowest instrumented and fused vertebra ranged from 3 to 6 levels. Patient 3 had the highest agreement with 75% of the surgeons selecting L4. The most frequently selected lowest instrumented vertebra was the lower end vertebra (LEV) and one level above (LEV + 1) for patient 1, one level below (LEV-1) for patients 4 and 5, two levels below (LEV-2) for patient 2, and four levels below (LEV-4) for patient 3.
Table 2.
Selected fusion levels based on end vertebrae
| Patient number | All patients combined | |||||
|---|---|---|---|---|---|---|
| 1 [n1 = 28]a | 2 [n2 = 32] | 3 [n3 = 32] | 4 [n4 = 32] | 5 [n5 = 32] | ||
| Number of instrumented levels | 10.6 ± 1.4 [8–13] | 11.0 ± 1.7 [8–14] | 12.8 ± 1.9 [8–16] | 10.9 ± 1.8 [8–15] | 10.8 ± 1.7 [7–14] | |
| UIV | ΔT2–T6 | ΔT1–T5 | ΔT1–T6 | ΔT2–T6 | ΔT3–T8 | |
| UEV + 5 | 1 (3%) | 3 (9%) | 4 (3%) | |||
| UEV + 4 | 4 (13%) | 5 (16%) | 4 (13%) | 3 (9%) | 16 (10%) | |
| UEV + 3 | 1 (4%) | 10 (31%) | 6 (19%) | 8 (25%) | 9 (28%) | 34 (22%) |
| UEV + 2 | 4 (14%) | 6 (19%) | 10 (31%) | 7 (22%) | 9 (28%) | 36 (23%) |
| UEV + 1 | 10 (36%) | 8 (25%) | 8 (25%) | 10 (31%) | 4 (13%) | 40 (26%) |
| UEV | 10 (36%) | 4 (13%) | 2 (6%) | 4 (13%) | 20 (13%) | |
| UEV −1 | 3 (11%) | 3 (9%) | 6 (4%) | |||
| LIV | ΔL1–L4 | ΔT12–L3 | ΔT12–L5 | ΔL1–L4 | ΔL2–L4 | |
| LEV + 1 | 10 (36%) | 10 (6%) | ||||
| LEV | 10 (36%) | 2 (6%) | 2 (6%) | 5 (16%) | 19 (12%) | |
| LEV −1 | 7 (25%) | 10 (31%) | 2 (6%) | 16 (50%) | 21 (66%) | 56 (36%) |
| LEV −2 | 1 (4%) | 15 (47%) | 0 (0%) | 12 (38%) | 6 (19%) | 34 (22%) |
| LEV −3 | 5 (16%) | 1 (3%) | 2 (6%) | 8 (5%) | ||
| LEV −4 | 2 (6%) | 24 (75%) | 26 (17%) | |||
| LEV −5 | 3 (9%) | 3 (2%) | ||||
UIV upper instrumented vertebra, LIV lowest instrumented vertebra, UEV/LEV upper/lower end vertebra
a Four surgeons indicated that patient 1 was not a suitable candidate for surgical treatment
The selection of the curves to be instrumented was also investigated (Table 3). Those curves [proximal thoracic (PT), main thoracic (MT), and lumbar/thoracolumbar (TL/L)] were defined by the end vertebrae of each curve. Seventy-nine percent of the surgeons proposed to fuse only the MT curve for patient 1. For patient 2, 63% recommended to fuse the PT curve in addition to the MT curve, while a majority (78%) of surgeons suggested avoiding the fusion of the TL/L curve. For patient 3, 37% recommended the fusion of the PT curve and 88% of the TL/L curve. The largest variation was noted for patient 4 where the choice either to fuse or not the PT and TL/L was equally divided between the surgeons (47% suggested to fuse the PT curve and 44% to fuse the TL/L curve). For patient 5, 37% of surgeons fused the PT in addition to the MT curves, and 81% avoided the fusion of the TL/L curve.
Table 3.
Selection of fused curves
| Patient number | |||||
|---|---|---|---|---|---|
| 1 (%) | 2 (%) | 3 (%) | 4 (%) | 5 (%) | |
| MT | 22 (79) | 10 (31) | 2 (6) | 12 (38) | 17 (53) |
| PT + MT | 5 (18) | 15 (47) | 2 (6) | 6 (19) | 9 (28) |
| MT + TL/L | 1 (4) | 2 (6) | 18 (56) | 5 (16) | 3 (9) |
| All curves (PT + MT + TL/L) | 0 (0) | 5 (16) | 10 (31) | 9 (28) | 3 (9) |
PT proximal thoracic, MT main thoracic, TL/L thoracolumbar/lumbar
As shown in Table 4, the number of implants proposed by the surgeons ranged from 8 to 30 per patient (mean 16.4; SD 5.8). The distribution was the following:
On the PT segment: 62% of selected implants were mono-axial screws (MoAS), 15% MuAS, and 21% hooks,
on the MT segment: 71% were MoAS, 19% MuAS, and 10% hooks,
on the TL/L segment: 57% were MoAS, 40% MuAS, and 3% hooks.
The straight-forward screw trajectory was preferred over the anatomical trajectory by 94% of the surgeons.
Table 4.
Summary of selected types of implants
| Patient number | All patients combined | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total number of implants | 14.9 ± 4.6 [8–26] | 15.8 ± 5.4 [8–26] | 19.1 ± 5.1 [10–30] | 16.0 ± 4.6 [8–28] | 16.4 ± 3.4 [11–23] | 16.4 ± 5.8 [8–30] |
| Proximal thoracic curve | ||||||
| Number of implants | 1.2 ± 1.4 [0–4] | 3.0 ± 1.8 [0–6] | 3.8 ± 1.9 [0–8] | 3.9 ± 2.0 [2–10] | 2.8 ± 1.9 [0–7] | 2.9 ± 2.0 [0–10] |
| Nb screws | 1.0 ± 1.4 [0–4] | 2.3 ± 2.1 [0–6] | 2.8 ± 2.5 [0–8] | 3.2 ± 2.6 [0–10] | 2.3 ± 2.1 [0–7] | 2.3 ± 2.3 [0–10] |
| Mono-axial | 61% | 65% | 61% | 65% | 63% | 62% |
| Multi-axial | 21% | 13% | 13% | 16% | 19% | 15% |
| Nb hooks | 0.2 ± 0.7 [0–3] | 0.7 ± 1.2 [0–4] | 1.0 ± 1.5 [0–5] | 0.7 ± 1.1 [0–4] | 0.5 ± 1.1 [0–4] | 0.6 ± 1.2 [0–5] |
| Pedicle | 8% | 9% | 12% | 7% | 9% | 9% |
| Lamina | 0% | 6% | 3% | 2% | 1% | 3% |
| Transverse process | 11% | 7% | 12% | 9% | 8% | 9% |
| Main thoracic curve | ||||||
| Number of implants | 11.3 ± 5.6 [0–20] | 9.1 ± 3.1 [4–14] | 9.2 ± 3.5 [2–14] | 9.4 ± 2.8 [4–14] | 11.6 ± 2.9 [6–16] | 10.1 ± 3.8 [0–20] |
| Nb screws | 9.9 ± 6.3 [0–20] | 8.0 ± 4.4 [0–14] | 8.4 ± 4.4 [0–14] | 8.6 ± 4.0 [0–14] | 10.9 ± 3.8 [3–16] | 9.2 ± 4.7 [0–20] |
| Mono-axial | 68% | 73% | 73% | 73% | 71% | 71% |
| Multi-axial | 19% | 15% | 19% | 18% | 23% | 19% |
| Nb hooks | 1.4 ± 2.3 [0–8] | 1.1 ± 1.9 [0–9] | 0.8 ± 1.3 [0–4] | 0.9 ± 1.8 [0–6] | 0.6 ± 1.4 [0–4] | 0.9 ± 1.8 [0–8] |
| Pedicle | 7% | 6% | 5% | 6% | 3% | 6% |
| Lamina | 2% | 2% | 1% | 2% | 2% | 2% |
| Transverse process | 2% | 3% | 1% | 2% | 1% | 2% |
| Thoracolumbar/lumbar curve | ||||||
| Number of implants | 0.5 ± 0.9 [0–2] | 3.7 ± 1.7 [2–8] | 6.2 ± 2.4 [0–10] | 2.7 ± 1.4 [0–6] | 2.0 ± 1.1 [0–4] | 3.0 ± 2.5 [0–10] |
| Nb screws | 0.4 ± 0.8 [0–2] | 3.5 ± 1.9 [0–8] | 6.1 ± 2.4 [0–10] | 2.6 ± 1.5 [0–6] | 2.0 ± 1.1 [0–4] | 2.9 ± 2.5 [0–10] |
| Mono-axial | 25% | 69% | 53% | 56% | 55% | 57% |
| Multi-axial | 63% | 27% | 46% | 39% | 44% | 40% |
| Nb hooks | 0.1 ± 0.4 [0–2] | 0.2 ± 0.6 [0–3] | 0.1 ± 0.4 [0–2] | 0.1 ± 0.5 [0–2] | 0.0 ± 0.2 [0–1] | 0.1 ± 0.4 [0–3] |
| Lamina | 13% | 4% | 1% | 5% | 2% | 3% |
Analysis of the global instrumentation strategies revealed ten different classes of design constructs distributed in three major groups (Table 5):
- “All Pedicle Screws Constructs” [NA = 103; 66%];
- 35% “All MoAS,”
- 9% “All MuAS,”
- 9% “MoAS thoracic–MuAS lumbar,”
- 7% “MuAS on upper fusion levels (UFL) and lowest fusion levels (LFL) only,”
- 6% others.
“All Hooks Constructs” [NB = 5; 3%];
- “Hybrid (hooks and lumbar screws) Constructs)” [NC = 48; 31%];
- 12% “Hooks thoracic–MoAS lumbar,”
- 8% “Hook on UFL only,”
- 4% “Hooks thoracic–MuAS lumbar,”
- 6% others.
Table 5.
Number of surgeons (and percentage) for the different classes of instrumentation strategies
| Patient number | |||||
|---|---|---|---|---|---|
| 1 (%) | 2 (%) | 3 (%) | 4 (%) | 5 (%) | |
| A- All pedicle screws constructs | 17 (61) | 20 (63) | 20 (63) | 21 (66) | 25 (78) |
| All MoAS | 9 (32) | 12 (38) | 9 (28) | 11 (34) | 13 (41) |
| A2 All MuAS | 3 (11) | 3 (9) | 2 (6) | 3 (9) | 3 (9) |
| A3 MoAS thoracic–MuAS lumbar | 2 (7) | 1 (3) | 3 (9) | 4 (13) | 4 (13) |
| A4 MuAS on UFL and LFL only | 2 (7) | 2 (6) | 2 (6) | 2 (6) | 3 (9) |
| A5 Miscellaneous | 1 (4) | 2 (6) | 4 (13) | 1 (3) | 2 (6) |
| B- All hooks constructs | 1 (4) | 2 (6) | 0 (0) | 2 (6) | 0 (0) |
| C- Hybrid constructs | 10 (36) | 10 (31) | 12 (38) | 9 (28) | 7 (22) |
| C1 Hooks thoracic–MoAS lumbar | 5 (18) | 4 (13) | 4 (13) | 2 (6) | 3 (9) |
| C2 Hook on UFL only | 3 (11) | 3 (9) | 4 (13) | 3 (9) | 0 (0) |
| C3 Hooks thoracic–MuAS lumbar | 1 (4) | 0 (0) | 1 (3) | 3 (9) | 2 (6) |
| C4 Miscellaneous | 1 (4) | 3 (9) | 3 (9) | 1 (3) | 2 (6) |
MoAS mono-axial screws, MuAS multi-axial screws, UFL upper fusion levels, LFL lowest fusion levels, AV apical vertebra
Half of the surgeons used similar classes of design constructs (A1, A2, C1, etc.) for all five cases, while the remainder had more adaptable strategies for each case. No significant difference was identified regarding the extent of the selected fusion levels between the “All Pedicle Screws Constructs” and the “Hybrid Constructs” groups (P = 0.252).
For all patients combined, a top-to-bottom attachment sequence was selected in 49% of all cases, a bottom-up in 46% and an alternate order in 4%. For the intra-operative reduction maneuver, 35% of the surgeons selected a 90° rod rotation, 5% a rod translation technique, 40% a DAVD, and 21% a cantilever. The scoliotic curve type had a significant effect on the number of instrumented levels (P < 0.001), the number of implants (P < 0.001), the UFL (P < 0.001), and the LFL (P < 0.001). The effect of surgeons was significant on the number of instrumented levels (P < 0.001), the number of implants (P < 0.001), and the UFL (P < 0.001). However, no significant effect of surgeons was revealed on the LFL (P > 0.05).
The compression and distraction forces on vertebral levels as well as the other maneuvers and comments (items 4 and 5) suggested by the surgeons were not considered in the results because not enough surgeons have registered these items or the answers were only qualitative.
For the intra-observer study, no complete agreement could be detected between the two preoperative planning (instrumentation design construct, proximal and distal levels, rod shapes as well as rod maneuver) performed by the same surgeons for each patient (Fig. 5). One surgeon (no. 4) initially proposed an instrumentation strategy for patient 1, while he decided not to operate the patient on the second occasion. For the selection of vertebral fusion levels, 71% of the surgeons proposed different proximal levels between the two assessments, and 35% proposed different distal vertebral fusion levels. An average of two different levels for the selection of proximal and distal levels was noted. 71% of the surgeons adopted a different instrumentation strategy. One surgeon, for example, has given up the “All Hooks Construct” or “Hybrid Construct” for the “All Pedicle Screws Construct” for all three patients at his second assessment.
Fig. 5.
Intra-observer variability of instrumentation strategies proposed by the six surgeons for the three patients numbers 1, 3, and 5
Discussion
This study attempted to capture the complexity in the decision-making process for the surgical correction of AIS using a posterior instrumentation and fusion of the spine in a large group of experienced spinal surgeons. The results confirm the findings of our preliminary study [3] and demonstrate a large intersurgeon variability for the preoperative selection of instrumentation levels and fusion planning. In addition, different instrumentation and selection of fusion levels strategies were noted according to the curve type pattern. Based on these findings, preoperative planning for AIS appears to be both surgeon- and curve-type dependent, as demonstrated by the ANOVA. Furthermore, it is clearly surgeon-dependant since none of the surgeons selected exactly the same plan for any of the curve types. It is also curve-dependant since half of the surgeons adopted a different strategy depending on the curve type. Finally, this study has documented an intra-surgeon variability indicating that surgeons will not necessarily always adopt the same strategy when exposed to the same case. However one limitation of the intra-observer study is the 1-year interval between the tests; the documented variability may be partially attributed to an evolution of the surgeon’s approach which could be influenced by the increasing use of thoracic pedicle screws [20, 25, 26], or the introduction of new surgical techniques such as the in situ rod contouring [7] or the direct vertebral derotation technique [17].
Two crucial issues are raised by our findings: first, what is the cause of this variability in surgical strategy among spinal surgeons? And more importantly, what are the consequences of this variability on the outcome of treatment? Do screw constructs provide a better correction and outcome than hook or hybrid constructs? What is the effect of a change in one or two levels of instrumentation and fusion levels on the outcome for the patient? Should planning of surgery be curve-type specific? What is the importance of saving fusion levels in the lumbar spine and in the PT spine?
The probable answer to the first question is that the variability can be largely explained not only by changes of strategies caused by recent technological innovations, but also by the historical controversy concerning which curves should be fused. Indeed, appropriate fusion levels have always remained a debated issue in the preoperative planning of AIS, as evidenced by the different rules proposed over time, which have evolved according to the curve classification [14, 22, 30], the end, neutral and stable vertebrae of the curves [40] and for a given instrumentation system concept [10, 21, 31]. For instance, King’s criteria are that the curve should be fused from the neutral to the stable vertebrae when using Harrington instrumentation [14]. On the other hand, with the Cotrel–Dubousset instrumentation, Richards [31] proposed fusing one segment distal to the stable vertebra in most King Type-II curves. Conversely, Lenke [21] suggested fusion levels from the neutral vertebra to the stable vertebra (in true King Type-II curve). For single thoracic curves using pedicle screws, Suk et al. [40] proposed a selective thoracic fusion from one level above the UEV to the distal neutral vertebra. Puno et al. [29] reported better radiographic results as compared with others which did not follow the Lenke classification system [24].
Moreover, we hypothesize that the cause of variability is multi-factorial and can be explained by many other contributing factors:
The known inter- and intra-observer variability [22] in current curve classifications systems which may change the surgeon’s plan for a specific curve type,
previous experience and training of each surgeon,
differing objectives for correction of scoliotic deformities among surgeons [5],
differing interpretations of the importance of published criteria for the planning of surgery in AIS,
inadequate interpretation by a surgeon of the criteria currently proposed in the literature, and
other indefinite causes.
One main limitation of this study is the inability to reproduce a real operating-room situation. The surgeons were asked to provide their posterior instrumentation planning using only the preoperative and supine side bending radiographs, while in a real clinical situation changes in planning can be made according to intra-operative findings or per-operative X-rays. However, our findings represent the proposed strategies that reflect instrumentation objectives.
Do the intra- and intersurgeon variations observed in this study affect the correction and outcome of treatment? The answer to this question remains unknown and in our opinion can only be solved in two complementary ways: first, by large multi-center studies on the outcome of treatment that will compare the long-term outcome of treatment in a large number of subjects and among a large number of surgeons, and second by the use of biomechanical models to compare the simulated results of various surgical strategies on the same patient and curve types [2, 4, 33, 34].
Conclusion
This study confirms that a large variability in instrumentation strategies exists among experienced spine surgeons for patients with AIS. Planning for posterior instrumentation is both surgeon- and curve-type dependent. We hypothesize that this variability may be attributed to different objectives for correction, variability in curve classification, personal surgeon’s preferences based on their previous experience, the flexibility and numerous options allowed by the modern multi-segmental instrumentation systems, as well as the current lack of clearly defined strategies with modern instrumentation systems. To our knowledge, the exact cause of this variability is unclear and the impact of different surgical strategies on the correction should be seriously questioned. Further investigation with multi-center outcome studies and computer simulations is needed [2, 4, 33, 34].
Acknowledgments
This research was assisted by support from the Spinal Deformity Study Group, and funded by the Natural Sciences and Engineering Research Council of Canada, the Canada Research Chair Program, and by an educational/research grant from Medtronic Sofamor Danek. Special thanks to the members of the Spinal Deformity Study Group: Drs. A. King, B. S. Richards, C. E. Johnston, C. DeWald, C. Shaffrey, C. Brown, D. J. Sucato, D. P. Roye, D. W. Polly, E. Dawson, E. Transfeldt, F. J. Schwab, H. Labelle, J. O. Sanders, J. B. Emans, J. Braun, J. Dimar, K. N. Ibrahim, K. Bridwell, L. G. Lenke, M. T. Hresko, M. Diab, P. D. Sponsseler, R. E. McCarthy, S. S. Hu, S. Berven, S. Suk-II, S. D. Glassman, S. M. Mardjetko, T. R. Kuklo, T. Lowe, V. Arlet.
References
- 1.Aubin CE, Descrimes JL, Dansereau J, Skalli W, Lavaste F, Labelle H. Geometrical modeling of the spine and the thorax for the biomechanical analysis of scoliotic deformities using the finite element method (in French) Ann Chir. 1995;49:749–761. [PubMed] [Google Scholar]
- 2.Aubin CE, Goussev V, Petit Y. Biomechanical modeling of segmental instrumentation for surgical correction of 3D spinal deformities using Euler-Bernoulli thin-beam elastic deformation equations. Med Biol Eng Comput. 2004;42:216–221. doi: 10.1007/BF02344634. [DOI] [PubMed] [Google Scholar]
- 3.Aubin CE, Labelle H, Ciolofan OC. Variability of spinal instrumentation configurations in adolescent idiopathic scoliosis. Eur Spine J. 2007;16(1):57–64. doi: 10.1007/s00586-006-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubin CE, Petit Y, Stokes IAF, Poulin F, Gardner-Morse M, Labelle H. Biomechanical modeling of posterior instrumentation of the scoliotic spine. Comput Methods Biomech Biomed Eng. 2003;6:27–32. doi: 10.1080/1025584031000072237. [DOI] [PubMed] [Google Scholar]
- 5.Aubin CE, Robitaille M, Ciolofan OC (2005) What are the goals of surgical correction in adolescent idiopathic scoliosis? In: Proceedings of the 12th international meeting on advanced spinal techniques, Banff, AB, Canada
- 6.Barber JW, Boden SD, Ganey T, Hutton WC. Biomechanical study of lumbar pedicle screws: does convergence affect axial pullout strength? J Spinal Disord. 1998;11:215–220. doi: 10.1097/00002517-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Chang KW. Cantilever bending techniques for the treatment of large and rigid scoliosis. Spine. 2003;28:2452–2458. doi: 10.1097/01.BRS.0000092063.63315.D5. [DOI] [PubMed] [Google Scholar]
- 8.Delorme S, Petit Y, Guise JA, Labelle H, Aubin CE, Dansereau J. Assessment of the 3-d reconstruction and high-resolution geometrical modeling of the human skeletal trunk from 2-D radiographic images. IEEE Trans Biomed Eng. 2003;50:989–998. doi: 10.1109/TBME.2003.814525. [DOI] [PubMed] [Google Scholar]
- 9.Dubousset J, Cotrel Y. Application technique of Cotrel-Dubousset instrumentation for scoliosis deformities. Clin Orthop. 1991;264:103–110. [PubMed] [Google Scholar]
- 10.Harrington PR. Technical details in relation to the successful use of instrumentation in scoliosis. Orthop Clin North Am. 1972;3:49–67. [PubMed] [Google Scholar]
- 11.Helenius I, Remes V, Yrjönen T, Ylikoski M, Schlenzka D, Helenius M, Poussa M. Harrington and Cotrel-Dubousset instrumentation in adolescent idiopathic scoliosis. Long-term functional and radiographic outcomes. J Bone Joint Surg Am. 2003;85:2303–2309. doi: 10.2106/00004623-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Lenke LG, Bridwell KH, Cho YS, Riew KD. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine. 2004;29:333–342. doi: 10.1097/01.BRS.0000109983.12113.9B. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Lenke LG, Kim J, Bridwell KH, Cho SK, Cheh G, Sides B. Comparative analysis of pedicle screw versus hybrid instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine. 2006;31:291–298. doi: 10.1097/01.brs.0000197865.20803.d4. [DOI] [PubMed] [Google Scholar]
- 14.King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:1302–1313. [PubMed] [Google Scholar]
- 15.Krismer M, Bauer R, Sterzinger W. Scoliosis correction by Cotrel-Dubousset instrumentation. The effect of derotation and three-dimensional correction. Spine. 1992;17:263–269. doi: 10.1097/00007632-199208001-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kuklo TR, Potter BK, Polly DW, Lenke LG. Mono-axial versus multiaxial thoracic pedicle screws in the correction of adolescent idiopathic scoliosis. Spine. 2005;15:2113–2120. doi: 10.1097/01.brs.0000179260.73267.f4. [DOI] [PubMed] [Google Scholar]
- 17.Lee SM, Suk S, Chung ER. Direct vertebral rotation: a new technique of three-dimensional deformity correction with segmental pedicle screw fixation in adolescent idiopathic scoliosis. Spine. 2004;29:343–349. doi: 10.1097/01.BRS.0000109991.88149.19. [DOI] [PubMed] [Google Scholar]
- 18.Lehman RA, Kuklo TR. Use of the anatomic trajectory for thoracic pedicle screw salvage after failure/violation using the straight-forward technique: a biomechanical analysis. Spine. 2003;28:2072–2077. doi: 10.1097/01.BRS.0000084628.37133.BA. [DOI] [PubMed] [Google Scholar]
- 19.Lehman RA, Polly DW, Kuklo TR, Cunningham B, Kirk KL, Belmont PJ. Straight-forward versus anatomic trajectory techniques of thoracic pedicle screw fixation: a biomechanical analysis. Spine. 2003;28:2058–2065. doi: 10.1097/01.BRS.0000087743.57439.4F. [DOI] [PubMed] [Google Scholar]
- 20.Lenke LG. Debate: resolved, a 55o right thoracic AIS curve should be treated by posterior spinal fusion and segmental instrumentation using thoracic pedicle screws. J Pediatr Orthop. 2004;24:329–334. doi: 10.1097/00004694-200405000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Lenke LG. Posterior scoliosis correction of King II curves: hooks and rods. In: Haher TR, Merola AA, editors. Surgical techniques of the spine. New York, NY: Theime; 2003. pp. 145–151. [Google Scholar]
- 22.Lenke LG, Betz RR, Bridwell KH, Clements DH, Harms J, Lowe TG, Shufflebarger HL. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80:1097–1106. doi: 10.2106/00004623-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lenke LG, Betz RR, Haher TR, Lapp MA, Merola AA, Harms J, Shufflebarger HL. Multisurgeon assessment of surgical decision-making in adolescent idiopathic scoliosis: curve classification, operative approach, and fusion levels. Spine. 2001;26:2347–2353. doi: 10.1097/00007632-200111010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169–1181. [PubMed] [Google Scholar]
- 25.Liljenqvist UR, Lepsien U, Hackenberg L, Niemeyer T, Halm H. Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur Spine J. 2002;11:336–343. doi: 10.1007/s00586-002-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liljenqvist UR, Halm HF, Link TM. Pedicle screw instrumentation of the thoracic spine in idiopathic scoliosis. Spine. 1997;22:2239–2245. doi: 10.1097/00007632-199710010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Lonstein JA. Decompensation with Cotrel-Dubousset instrumentation: a multi-center study. Orthop Trans. 1992;16:158. [Google Scholar]
- 28.Potter B, Lehman RA, Kuklo TR. Anatomy and biomechanics of thoracic pedicle screw instrumentation. Curr Opin Orthop. 2004;15:133–144. doi: 10.1097/01.bco.0000120511.04726.d6. [DOI] [Google Scholar]
- 29.Puno RM, An KC, Puno RL, Jacob A, Chung SS. Treatment recommendations for idiopathic scoliosis: an assessment of the Lenke classification. Spine. 2003;28:2102–2114. doi: 10.1097/01.BRS.0000088480.08179.35. [DOI] [PubMed] [Google Scholar]
- 30.Qiu G, Zhang J, Wang Y, Xu H, Zhang J, Weng X, Lin J, Zhao Y, Shen J, Yang X, Luk KD, Lu D, Lu WW. A new operative classification of idiopathic scoliosis: a peking union medical college method. Spine. 2005;30:1419–1426. doi: 10.1097/01.brs.0000166531.52232.0c. [DOI] [PubMed] [Google Scholar]
- 31.Richards BS, Birch JG, Herring JA, Johnston CE, Roach JW. Frontal and sagittal plane balance following Cotrel-Dubousset instrumentation for idiopathic scoliosis. Spine. 1989;14:733–737. doi: 10.1097/00007632-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Rinella AS, Lenke LG (2002) Complication associated with thoracic pedicle screws. Sem Spine Surg: Complicat Spine Surgery, Alex Vaccaro (ed) 14:125–135
- 33.Robitaille M, Aubin CE, Labelle H (2006) Effects of alternative instrumentation strategies in adolescent idiopathic scoliosis. In: Proceedings of the 41st annual meeting of the scoliosis research society, Monterey, CA, 14–16 September
- 34.Robitaille M, Aubin CE, Labelle H. Biomechanical assessment of variable instrumentation strategies in adolescent idiopathic scoliosis: preliminary analysis of 3 patients and 6 scenarios. Stud Health Technol Inform. 2006;123:309–314. [PubMed] [Google Scholar]
- 35.Rohmiller MT, Newton PO, Merola A (2004) Does correlation exist between instrumentation type, number of fixation points, and cost in the surgical correction of adolescent idiopathic scoliosis? In: Proceedings of the SRS 39th annual meeting, Buenos Aires, Argentina
- 36.Storer SK, Vitale MG, Hyman JE, Lee FY, Choe JC, Roye DP. Correction of adolescent idiopathic scoliosis using thoracic pedicle screw fixation versus hook constructs. J Pediatr Orthop. 2005;25:415–419. doi: 10.1097/01.mph.0000165134.38120.87. [DOI] [PubMed] [Google Scholar]
- 37.Sufflebarger HL, Clark CE. Fusion levels and hook patterns in thoracic scoliosis with Cotrel-Dubousset instrumentation. Spine. 1990;15:916–920. doi: 10.1097/00007632-199009000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Suk SI, Kim WJ, Lee SM, Chung ER. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine. 2001;26:2049–2057. doi: 10.1097/00007632-200109150-00022. [DOI] [PubMed] [Google Scholar]
- 39.Suk SI, Lee CK, Kim WJ, Chung ER, Park YB. Segmental pedicle screw fixation in the treatment of thoracic idiopathic scoliosis. Spine. 1995;20:1399–1405. doi: 10.1097/00007632-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Suk SI, Lee SM, Chung ER, Kim JH, Kim WJ, Sohn HM. Determination of distal fusion level with segmental pedicle screw fixation in single thoracic idiopathic scoliosis. Spine. 2003;28:484–491. doi: 10.1097/00007632-200303010-00014. [DOI] [PubMed] [Google Scholar]