Abstract
Adolescent idiopathic scoliosis girls are known to display standing imbalance. In addition to a motor deficit problem, the axial torsion of the spine and trunk torsion could reflect an imbalance around the vertical axis. Unlike the excursion of the center of pressure (COP), the forces and moments were rarely addressed to characterize the quiet standing balance. Nonetheless, one dynamical parameter, called free moment (TV), representing the vertical torque on the feet can reflect the oscillation around the vertical axis associated to the standing imbalance. The objectives of this study were to test if the free moment variability can be utilized to characterize standing balance in a group of able-bodied and non-treated scoliotic girls and to determine if it was associated with that of the COP among each group of subjects tested. Forty-six adolescent girls with half of them presenting an adolescent idiopathic scoliosis were tested during quiet standing balance. Standing balance was assessed with the subjects standing upright and bare feet on a force plate. RMS and range of COP excursions and free moment were calculated.The scoliotic group displayed higher variability in COP excursion by about 24% than the able-bodied girls. Similarly, the TV RMS (P = 0.00136) and range (P = 0.00197) were statistically higher by about 42% in the scoliotic group. The variability of TV was associated with that of the COP in both groups. In the medio-lateral direction, the significant correlations between the RMS and range of the free moment and those of the COP were about 0.7 for the able-bodied group and 0.5 for the medio-lateral COP range for the scoliotic group girls. Along the antero-posterior axis, the only statistically significant correlations were observed for the scoliotic group. The free moment variability about the COP measured during quiet standing can be suggestive of an asymmetry control of the trunk around the vertical axis during standing balance. Its variability was more pronounced in scoliotic girls and was associated with the antero-posterior COP variability reflecting both biomechanical and motor control deficits. Free moment calculation could be a supplement insight into the standing balance of scoliotic subjects.
Keywords: Scoliosis, Standing balance, Free moment
Introduction
Adolescent idiopathic scoliosis girls are known to display standing imbalance [36]. In addition to a motor deficit problem, the spinal deformity is also responsible for posture or body misalignment in the horizontal plane [13, 27, 29]. Both the type of curve [18] and body posture attitude [32] was associated with standing imbalance in scoliotic girls. In standing, it is argued that, postural sway control can be achieved by the whole body moving in a single block [17] or through individual body segment movements to stabilize the joints [1, 40]. This applies not only to the sagittal and frontal plane oscillatory motions but also to transversal plane rotations occurring at the hips or subtalar joint. Nevertheless, the axial torsion of the spine and trunk torsion could also reflect a stability imbalance around the vertical axis.
Quiet standing is often characterized by the center of pressure (COP) excursion. It is calculated from the ground reaction forces and moments at the feet. The COP is in fact the point of application of the ground reaction forces acting on the feet. Its excursion and its temporal, frequential, random and structural characteristics are used to describe standing imbalance [4]. But it is not obvious to get from the COP an insight of the instability around the longitudinal axis of the body. As well as the COP displacements are derived from force measurements, another dynamical parameter, called the free moment, TV, can be calculated. The free moment represents the torque on the feet about the vertical axis at the COP [8, 9, 20, 33]. Since moments are expressed as a function of angular accelerations, it is expected that variability in one express the variability in the other. It is assumed that there is a rotational variability around the vertical axis in quiet standing just as there is a migration of the COP. This body rotational oscillation around the vertical axis could be associated with a form of instability or could be indicative of the muscular contribution in maintaining quiet standing balance in the horizontal plane [24].
In non-scoliosis individuals, the free moment, TV, was described by Nigg [33] in running shoe research. The pattern of the free moment versus time was observed to illustrate the foot ab/adduction during stance phase. Later on, Holden and Cavanagh [20] were able to demonstrate that an increase in foot pronation was associated with a significant increase of the free moment during running. During standing, Bleuse et al. [9] demonstrated that vertical moment about the COP was a useful parameter for quantifying anticipatory postural adjustments associated with unilateral arm raising. They concluded that it contributed to the generation of slow arm movement when stabilization of the center of mass was not necessarily. To the best of our knowledge, only, Kramers-de Quervain et al. [23] observed during walking the free moment in adolescent idiopathic scoliosis girls. During the stance of walking, an asymmetry of TV between left and right side was observed whereas the other force parameters (vertical, medio-lateral, antero-posterior) did not show a significant side asymmetry. The free moment changes have not been addressed in quiet standing balance.
We postulate that the free moment could be an indicator of body oscillations around the vertical axis and contributes to the horizontal plane postural control during standing balance. The objectives of this study were to test if the free moment variability can be utilized to characterize quiet standing balance in a group of able-bodied and non-treated scoliotic girls and to determine if it was associated with that of the COP among each group of subjects tested.
Methods
This study involved 46 adolescent girls. Half of them were diagnosed as adolescent idiopathic scoliosis (SCO) by an orthopaedic surgeon according to the criteria defined by Bunnel [11]. All SCO subjects presented a right thoracic curve with an average Cobb angle of 29.4 ± 9.4. No subject was under active treatment at the time of the experimentation. Their average age, height and mass were 12.2 ± 1.5 years, 154.0 ± 10.5 cm, 44.4 ± 9.8 kg, respectively.
The control group consisted of 23 able-bodied girls (AB). The same orthopaedic surgeon verified the absence of spinal deformity and the general good health of these subjects. The mean age, height and mass of this group were 13.4 ± 1.0 years, 161.5 ± 5.9 cm, 50.0 ± 11.1 kg. Since statistical differences were found between the scoliotic and able-bodied girls in terms of age (P = 0.005) and height (P = 0.005) but not for weight (0.089), these three factors were used as co-variables in the subsequent analyses. All girls and their parents gave their consent after being fully informed of the test procedure approved by the hospital ethics committee.
Standing balance was assessed with the subjects standing upright and bare feet on an AMTI (AMTI, Newton, MA, USA) force-plate with their feet oriented in a standardized position according to McIlroy and Maki [30] and Allard et al. [3]. The heels were spaced apart by about 23 cm and the feet were out-toeing by 15°.
Though standardizing the position of the feet could affect balance measurements, it was performed not only because it is the accepted procedure [28, 32, 35] but also to avoid confounding variables in the collected data due to different stances adopted by the subjects. Subjects were asked to fix a target placed at eye level at located a distance of 1.2 m while keeping their arms along their body. All subjects were tested three times in the eyes-opened condition. For each trial, the duration of the acquisition was set at 64 s, at a sampling frequency of 64 Hz [12, 32]. The participants had a 1 min rest period between each trial.
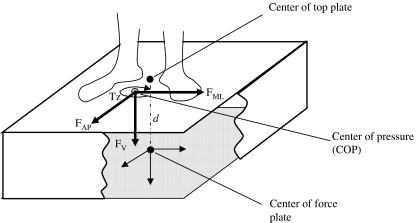
The force-plate provided three forces and moments calculated about an origin located near but not exactly at its geometric center. The antero-posterior forces (FAP) were positive along the forward direction; the medio-lateral forces (FML) towards the left while the vertical forces (Fv) were positive downwards (see Fig. 1). The corresponding moments (MAP, MML and Mv) were positive in the clock-wise direction along their respective axis.
Fig. 1.
Schematic view of the biomechanical parameters FAP the antero-posterior force; FML the medio-lateral force; FV the vertical force; TZ the free moment; d the distance between the center of force plate and its surface
The center of pressure (COP) excursion corresponds to the point of application of the resultant force acting on the force plate. It was calculated along the AP and ML axes from the ground reaction forces and moments according to
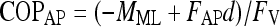 |
1 |
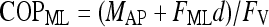 |
2 |
where d = 0.0394 m indicates the distance between the center of force-plate and its surface. The COP values of equations 1 and 2 are given with respect to the geometric center of the force-plate.
The free moment, Tv, is calculated from MV taken at the origin of the force-plate and two moments resulting from the antero-posterior and medio-lateral forces and COP positions.
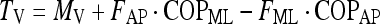 |
3 |
TV corresponds to MV in two conditions, namely, when the COP lies over the origin of the force-plate but this seldom occurs during quiet standing balance and when there is no horizontal force but in this condition, MV and TV would be zero.
For each direction (AP and ML), the mean, range and root mean square (RMS) values of the COP displacements were calculated according to Cornilleau-Pérès et al. [14]. In standing balance, forces acting at the centre of pressure control the centre of mass excursion at any given moment. Any changes in the COP position reflect the postural adjustments to control the body COM displacements. Since the centre of mass oscillates back and forth about the centre of pressure they have the same mean position [31]. The mean COP values correspond to the mean position of the vertical projection of the center of mass [31]. The mean COPAP was measured from the back of the heels and indicated how the subjects were leaning along the sagittal plane while the mean medio-lateral position COPML indicated if a subject was leaning laterally or not. The COP range corresponds to the body sway amplitude and was obtained by taking the differences between the maximal and minimal values of the COP position along the antero-posterior and medio-lateral axes. At least, the COP RMS represents the variability of the COP in maintaining a mean quiet standing position. These calculations provided six variables as concerns the COP excursions.
The free moment about the vertical axis varies during quiet standing though its mean value was close to zero since there is no self-imposed body rotation in a particular direction. But there exists a rotational oscillation around the vertical axis just as there is a migration of the COP in quiet standing (see Eq. 3). This oscillation around the vertical axis could be associated with standing imbalance to maintain quiet stance. The root mean square, TV RMS, and the range, TV RANGE, of the free moment, were calculated to quantify the rotational oscillation associated to the postural imbalance. These calculations provided two variables as concerns the free moment.
Though the force-plate zero-offset was corrected prior to the experimentation, the RMS and range of the free moment measured during quiet standing are relatively small. The RMS and range of the free moment calculated from an unloaded force-plate were compared to those obtained during balance to ensure that the latter were well above those registered during an unloaded force-plate condition. For the able-bodied subjects, they were about eight times higher than those recorded when the force-plate was unloaded and ten times higher for the scoliotic girls.
At all, from the COP excursion and the free moment data, eight parameters were calculated for each trial and were averaged over the three trials before further statistical analysis. One-way ANCOVAs with age, height and weight as co-variables were performed to compare the scoliotic and able-bodied girls. These were followed by Bonferronni post_hoc tests if a statistical difference was reported. Coefficients of correlation were calculated to determine if the variability in the free moment was associated with the variability in the COP displacements. For all tests, statistical significance was accepted for P < 0.05.
Results
Scoliotic subjects positioned themselves about 9 mm closer to their heels (62.3 ± 10.7 mm) than able-bodied girls (71.3 ± 14.3 mm, P = 0.043). According to Allard et al. [2] a mean sagittal COP position located closer to the heel is indicative of a hypokyphotic stance. In scoliotic subjects, a backward tilted trunk was previously reported by Nault et al. [32] and a sagittal C7–S1 angle by Bernard and Valero [7]. The mean medio-lateral COP (3.3 ± 12.0 mm to the right) was not statistically different from the non-scoliotic group (3.4 ± 6.8 mm to the left). Though the mean medio-lateral COP is close to zero the standard deviation is very large. Subjects have a tendency to stand either on their right or left but as a group this effect is masked by the mean. Similar results were reported by Silferi et al. [41], Allard et al. [2] and Nault et al. [32] in scoliotic subjects.
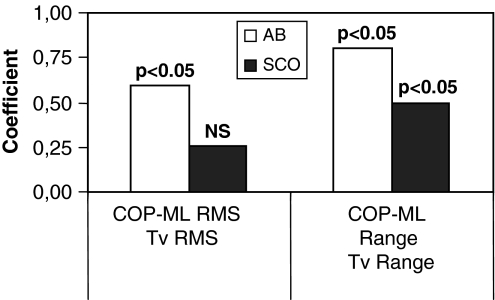
Figures 2 and 3 illustrate the antero-posterior and medio-lateral COP RMS and range for the scoliotic and non-scoliotic girls. Generally, the scoliotic group displayed higher variability by about 24% than the able-bodied girls. Statistical differences were noted for the medio-lateral COP RMS (P = 0.01046) and range (P = 0.00137) and for the COP antero-posterior range (P = 0.01626). Similarly, the free moment RMS (P = 0.00136) and range (P = 0.00197), shown in Fig. 4 were statistically higher by about 42% in the scoliotic group.
Fig. 2.
RMS values of center of pressure. Mean and standard deviation antero-posterior and medio-lateral center of pressure RMS values for the able-bodied and scoliotic girls where P < 0.05 denote a statistical difference between groups. AB able-bodied; SCO scoliotic
Fig. 3.
Range values of center of pressure. Mean antero-posterior and medio-lateral center of pressure range values for the able-bodied and scoliotic girls where P < 0.05 denote a statistical difference between groups. AB able-bodied; SCO scoliotic
Fig. 4.
RMS and range values of free moment. Mean free moment RMS and range values for the able-bodied and scoliotic girls where P < 0.05 denote a statistical difference between groups. AB able-bodied; SCO scoliotic
The coefficients of correlation between the free moment and the COP parameters are shown in Figs. 5 and 6 for the antero-posterior and medio-lateral directions respectively. Along the antero-posterior axis, the only statistically significant correlations were observed for the scoliotic group. In the medio-lateral direction, the statistically significant correlations between the RMS and range of the free moment and those of the COP were about 0.7 for the able-bodied group and 0.5 for the medio-lateral COP range for the scoliotic group girls. All the other coefficients of correlation were not statistically significant.
Fig. 5.
Correlation between free moment and antero-posterior COP. Pearson coefficients of correlation between the free moment and the antero-posterior COP values where P < 0.05 denote a significant correlation. AB able-bodied; SCO scoliotic
Fig. 6.
Correlation between free moment and medio-lateral COP. Pearson coefficients of correlation between the free moment and the medio-lateral COP values where P < 0.05 denote a significant correlation. AB able-bodied; SCO scoliotic
Discussion
The first objective of this study was to test if the free moment about COP can be utilized to characterize quiet standing balance in a group of able-bodied girls and in AIS girls who were not treated with a body brace nor had spinal surgery. To our knowledge, few authors reported the use of the free moment [8, 9, 20, 23, 40]. Though the free moment measurement is useful in dynamic movement as running or walking, little attention has been paid to its measurement in standing balance. This parameter is mainly used to indicate for examples, the rearfoot pronation [20] or the initiation of the arm movement [9]. Shiratoni and Aruin [40] measured the reaction vertical moment (MV) calculated at the force-plate origin rather than the free moment, they were able to associate it with arm movement in the sagittal plane involving axial rotation of the trunk. Holden and Cavanagh [20] found that increased rearfoot pronation in during running causes significant increase in the free moment while Bleuse et al. [8] found that it was related to anticipatory arm raising movements. Only, Kramers-de Quervain et al. [23] reported data in adolescent idiopathic scoliosis girls. They underlined that free moment asymmetry was the single dynamic parameter related to the abnormal transverse plane acromion-pelvis alignment. This study addressed the use of free moment measurement in gait analysis in idiopathic scoliosis patients. All these studies considered the contribution of the moment about the vertical axis to resist rotational perturbations but did not recognize the free moment as a possible mechanism involved in stability maintenance.
The relations between free moment and segmental movements stress possible changes of the free moment during standing balance. Although it is argued that the whole body moves in a block to control the body sway [17] or that movement from body segments act individually to locally stabilize joints [1, 40], these actions should affect the forces and moments acting at the COP. In standing balance, the mean free moment value was close to zero since there is no self-imposed body rotation but horizontal body or segment oscillations could be responsible for its variation. This variability in the free moment about COP was observed in the group of able-bodied-girls during quiet standing. Several factors such as venous return [21], breathing [10], etc., are responsible for the COP displacements in able-bodied subjects during quiet standing. It was shown recently that other factors such as morphological body somatotypes in adolescent girls [3] and gender [15] affect significantly normal standing balance. It is reasonable to assume that standing stability can be perturbed in able-bodied subjects while remaining within a normal range. These factors could explain in part the presence of a variable torque suggesting an asymmetric control of the body segments around the vertical axis during standing balance.
The free moment variability was statistically more important in adolescent idiopathic scoliotic girls than in non-scoliotic subjects. This could be the result of a mean COP position closer to their ankles, thus closer to the rearward imbalance limit generating higher COP RMS and range values. Adolescent idiopathic scoliosis patients have an important horizontal trunk torsion as well as head and pelvis postural compensations [26] that not only modify their COP position but also can interfere with standing balance. Nault et al. [32] were the first to associate standing imbalance with body posture attitude in SCO girls under observation though Sawatzky et al. [39] and Masso and Gorton [29] reported changes in standing body segment alignment following spinal instrumentation. The type of curves was also shown to influence static and dynamic postural control in SCO [18] as well as body morphology was associated with standing imbalance in SCO [2].
Balance impairments in SCO girls was reported by Sahlstrand et al. [36] and later associated with a deficit on a cerebral level [19], vestibular imbalance [37, 38] and proprioceptive disorders [5, 22]. Asymmetries in electromyographic responses of paraspinal muscles to postural disturbance were reported by Perret and Robert [34] in scoliotic children. This fact emphasized the non-symmetrical control of the trunk by the scoliotic subjects in standing balance. These results suggest that both morphological changes due to the deformed spine and trunk as well as sensory and motor deficits [43, 44] could lead to a balance dysfunction. The latter reported a sensory integration problem AIS in subjects. This is reflected in part by a greater variability in the free moment about the vertical axis. This strengthens the use of postural control in the treatment of adolescent idiopathic scoliosis as Weiss [45] proposes.
The second objective was to test if the variability of the free moment about COP was associated with that of the COP. Non-scoliotic girls displayed a statistically significant coefficient of correlation between Tv RMS and the medio-lateral COP RMS (value 0.8). Our observations are in agreement with Ferdjallah et al. [16] who reported that horizontal body motions were found to contribute significantly to the right and left COP displacements registered during a quiet standing trial. The strong correlation with the medio-lateral direction could be attributed to hip, ankle control and foot intrinsic muscle activity [16]. The lack of correlation between the variability of the COP-AP and that of the free moment suggests that the COP sway in the antero-posterior direction is not under the free moment control but managed by the plantarflexor activity to maintain the mean COP position anterior to the ankle [46].
The free moment variability of the scoliotic girls was associated with that of the COP in the antero-posterior direction. It could be argued that sagittal profiles, kyphosis and lordosis may contribute in the correlations between free moment and COP along the AP axis. But single correlation between body posture and balance parameters explained on the average less than 10% of the variation [39] and with backward stepwise multiple regression analyses, the explained variation increased to 45% indicating that factors other that body posture parameters are responsible for standing imbalance in AIS. The increase was attributed to head, trunk and pelvis horizontal rotations [25]. Sawatzky et al. [39] underlined that trunk imbalance is related to the control sway in scoliotic patients. Ferdjallah et al. [16] also observed that body transverse rotation is critical in maintaining balance control in children with cerebral palsy. For the scoliotic girls, the ankle mechanism described by Winter et al. [47] could be dependent on the head trunk pelvis adjustments around the vertical axis.
These findings are consistent with Simoneau et al. [42] who reported that scoliotic subjects rely considerably on ankle proprioception to regulate body sway oscillations and to scale the amplitude of their balance control commands. The asymmetry in the response of paraspinal muscles to the standing disturbance [34] and their sensory disorder [6] could contribute to the free moment variability about the vertical axis. Results reveal that the horizontal oscillations in SCO girls reflect a combine biomechanical and motor control dysfunction.
Conclusions
The free moment variability about the COP measured during quiet standing can be suggestive of an asymmetry control of the trunk around the vertical axis during standing balance. Its variability was more pronounced in girls who have both a spinal deformity and balance impairments in quiet standing than in a group of able-bodied girls. The free moment variability of the scoliotic girls was associated with the antero-posterior COP variability reflecting both biomechanical and motor control deficits.
The results suggest that both morphological changes due to the deformed spine and trunk as well as sensory and motor deficits could lead to a balance dysfunction resulting in a greater variability in the free moment about the vertical axis. This strengthens the use of postural control in the treatment of adolescent idiopathic scoliosis.
Acknowledgments
The authors wish to express their gratitude to Mrs. Lamoux Kate for her technical assistance and to NSERC Canada for funding in part this research.
Abbreviations
- AP
Antero-posterior direction
- COM
The center of mass is a point equivalent of the total body mass resulting from the location of each body segment
- COP
The center of pressure is the location of the net ground reaction force.
- ML
Medio-lateral direction
- RMS
The root mean square is calculated by
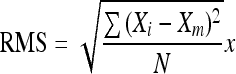 where Xm is the arithmetic mean of the variable X. Xi is the ith value of the variable X measured at the instant i, and N is the number of Xi values
where Xm is the arithmetic mean of the variable X. Xi is the ith value of the variable X measured at the instant i, and N is the number of Xi values
References
- 1.Accornero N, Capozza M, Rinalduzzi S, Manfredi GW. Clinical multisegmental posturography: age-related changes in stance control. Electroencephalogr Clin Neurophysiol. 1997;105:213–219. doi: 10.1016/S0924-980X(97)96567-X. [DOI] [PubMed] [Google Scholar]
- 2.Allard P, Chavet P, Barbier F, Gatto L, Labelle H, Sadeghi H. Effect of body morphology on standing balance in adolescent idiopathic scoliosis. Am J Phys Med Rehabil. 2004;83:689–697. doi: 10.1097/01.PHM.0000137344.95784.15. [DOI] [PubMed] [Google Scholar]
- 3.Allard P, Nault ML, Hinse S. Relationship between morphologic somatotypes and standing posture equilibrium. Ann Hum Biol. 2001;28:624–633. doi: 10.1080/03014460110047946. [DOI] [PubMed] [Google Scholar]
- 4.Baratto L, Morasso PG, Re C, Spada G. A new look at posturographic analysis in the clinical context: sway density versus other parametrization techniques. Motor Control. 2002;6:246–270. doi: 10.1123/mcj.6.3.246. [DOI] [PubMed] [Google Scholar]
- 5.Barrack RL, Whitecloud TS, 3rd, Burke SW, Cook SD, Harding AF. Proprioception in idiopathic scoliosis. Spine. 1984;9(7):681–685. doi: 10.1097/00007632-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Barrack RL, Whitecloud TS, Burke SW, Cook SD, Harding AF. Proprioception in idopathic scoliosis. Spine. 1984;9:681–685. doi: 10.1097/00007632-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bernard J-C, Valero J-P. Mesures stabilométriques des paramètres de l’équilibre statique de 26 patients scoliotiques avant et après traitement. Annales de kinésithérapie. 1999;26:145–153. [Google Scholar]
- 8.Bleuse S, Cassim F, Blatt J-L, Defebvre L, Derambure P, Guieu J-D. Vertical torque allows recording of anticipatory postural adjustments associated with slow, arm-raising movements. Clin Biomech. 2005;20(7):693–699. doi: 10.1016/j.clinbiomech.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Bleuse S, Cassim F, Blatt J-L, Defebvre L, Guieu J-D. Anticipatory postural adjustments associated with arm flexion: interest of vertical torque. Clin Neurophysiol. 2002;32:352–360. doi: 10.1016/S0987-7053(02)00335-0. [DOI] [PubMed] [Google Scholar]
- 10.Bouisset S, Duchene JL. Is body balance more perturbed by respiration in seating than in standing posture? Neuroreport. 1994;5:957–960. doi: 10.1097/00001756-199404000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Bunnel WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11:773–776. doi: 10.1097/00007632-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MG, Franck JS, Winter DA. Sampling duration effects on centre pressure summary measures. Gait Posture. 2001;13:35–40. doi: 10.1016/S0966-6362(00)00093-X. [DOI] [PubMed] [Google Scholar]
- 13.Chen PQ, Wang JL, Tsuang YH, Liao TL, Huang PI, Hang YS. The postural stability control and gait pattern of idiopathic scoliosis adolescent. Clin Biomech. 1998;13(Suppl 1):S52–S58. doi: 10.1016/S0268-0033(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 14.Cornilleau-Pérès V, Shabana N, Droulez J, Goh JCH, Lee GSM, Chew PTK. Measurement of the visual contribution to postural steadiness from the COP movement: methodology and reliability. Gait Posture. 2005;22:96–106. doi: 10.1016/j.gaitpost.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Farenc I, Rougier P, Berger L. The influence of gender and body characteristics on upright stance. Ann Hum Biol. 2003;30:279–294. doi: 10.1080/0301446031000068842. [DOI] [PubMed] [Google Scholar]
- 16.Ferdjallah M, Harris GF, Smith P, Wertsch JJ. Analysis of postural control synergies during quiet standing in healthy children and children with cerebral palsy. Clin Biomech. 2002;17:203–210. doi: 10.1016/S0268-0033(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 17.Gage WH, Winter DA, Franck JS, Adkin AL. Kinematic and kinetic validity of the inverted pendulum in quiet standing. Gait Posture. 2004;19:124–132. doi: 10.1016/S0966-6362(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 18.Gauchard GC, Lascombes P, Kuhnast M, Perrin PP. Influence of different types of progressive idiopathic scoliosis on static and dynamic postural control. Spine. 2001;26(9):1052–1058. doi: 10.1097/00007632-200105010-00014. [DOI] [PubMed] [Google Scholar]
- 19.Herman R, Mixon J, Fisher A, Maulucci R, Stuyck J. Idiopathic scoliosis and the central nervous system: a motor control problem. The Harrington lecture, 1983. Scoliosis Research Society. Spine. 1985;10(1):1–14. doi: 10.1097/00007632-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Holden JP, Cavanagh PR. The free moment of ground reaction in distance running and its changes with pronation. J Biomech. 1991;24(10):887–897. doi: 10.1016/0021-9290(91)90167-L. [DOI] [PubMed] [Google Scholar]
- 21.Inamura K, Mano T, Iwase S, Amagishi Y. One-minute wave in body fluid volume change enhanced by postural sway during upright standing. J Appl Physiol. 1996;81:459–469. doi: 10.1152/jappl.1996.81.1.459. [DOI] [PubMed] [Google Scholar]
- 22.Keessen W, Crowe A, Hearn M. Proprioceptive accuracy in idiopathic scoliosis. Spine. 1992;17(2):149–155. doi: 10.1097/00007632-199202000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kramers-de Quervain I, Muller R, Stacoff A, Grob D, Stussi E. Gait analysis in patients with idiopathic scoliosis. Eur Spine J. 2004;13:449–456. doi: 10.1007/s00586-003-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebiedowska MK, Syczewska M. Invariant sway properties in children. Gait Posture. 2000;12:200–204. doi: 10.1016/S0966-6362(00)00080-1. [DOI] [PubMed] [Google Scholar]
- 25.LeBlanc R, Labelle H, Forest F, Poitras B. Morphologic discrimination among healthy subjects and patients with progressive and nonprogressive adolescent idiopathic scoliosis. Spine. 1998;23(10):1109–1116. doi: 10.1097/00007632-199805150-00007. [DOI] [PubMed] [Google Scholar]
- 26.LeBlanc R, Labelle H, Rivard CH, Poitras B. Relation between adolescent idiopathic scoliosis and morphologic somatotype. Spine. 1997;22:2532–2536. doi: 10.1097/00007632-199711010-00013. [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc R, Labelle H, Rivard CH, Poitras B, Kratzenberg J. Three-dimensionnal (3D) postural evaluation of normal human subjects. Res Spinal Deform. 1997;1:293–296. [Google Scholar]
- 28.Maki B. selection of perturbations parameters for identification of the posture control system. Med Biol Eng Comput. 1986;24:561–568. doi: 10.1007/BF02446257. [DOI] [PubMed] [Google Scholar]
- 29.Masso PD, Gorton GE. Quantifying changes in standing body segment alignement following spinal instrumentation and fusion in idiopathic scoliosis using an optoelectronic measurement system. Spine. 2000;25(4):457–462. doi: 10.1097/00007632-200002150-00011. [DOI] [PubMed] [Google Scholar]
- 30.McIlroy WE, Maki BE. Prefered placement of the feet during quiet stance: development of a standardized foot placement for the balancing test. Clin Biomech. 1997;12:66–70. doi: 10.1016/S0268-0033(96)00040-X. [DOI] [PubMed] [Google Scholar]
- 31.Murray MP, Seireg A, Scholz RC. Center of gravity, center of pressure and supportive forces during human activities. J Appl Physiol. 1967;23:831–838. doi: 10.1152/jappl.1967.23.6.831. [DOI] [PubMed] [Google Scholar]
- 32.Nault ML, Allard P, Hinse S, Blanc RL, Caron O, Labelle H, Sadeghi H. Relationships between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine. 2002;27:1911–1917. doi: 10.1097/00007632-200209010-00018. [DOI] [PubMed] [Google Scholar]
- 33.Nigg BM. Biomechanics of running shoes. Urbana-Champaign: Human Kinetics; 1986. [Google Scholar]
- 34.Perret C, Robert J (2004) Electromyographic responses of paraspinal muscles to postural disturbance with special reference to scoliotic children. J Manipul Physiol Ther juil/août: 375–380 [DOI] [PubMed]
- 35.Rougier P. Automatic determination of the transition between succesive control mechanisms in upright stance by modelling of the centre of pressure. Arch Physiol Biochem. 1999;107:35–42. doi: 10.1076/apab.107.1.35.4350. [DOI] [PubMed] [Google Scholar]
- 36.Sahlstrand T, Ortengen R, Nachemson A. Postural equilibrium in adolescent idiopathic scoliosis. Acta Orthop Scand. 1978;49:354–365. doi: 10.3109/17453677809050088. [DOI] [PubMed] [Google Scholar]
- 37.Sahlstrand T, Petruson B. A study of labyrinthine function in patients with adolescent idiopathic scoliosis. I. An electro-nystagmographic study. Acta Orthop Scand. 1979;50(6 Pt 2):759–769. doi: 10.3109/17453677908991307. [DOI] [PubMed] [Google Scholar]
- 38.Sahlstrand T, Petruson B, Ortengren R. Vestibulospinal reflex activity in patients with adolescent idiopathic scoliosis. Postural effects during caloric labyrinthine stimulation recorded by stabilometry. Acta Orthop Scand. 1979;50(3):275–281. doi: 10.3109/17453677908989768. [DOI] [PubMed] [Google Scholar]
- 39.Sawatzky B, Tredwell S, Sanderson D. Postural control and trunk imbalance following Corel-Dubouisset instrumentation for adolescent idiopathic scoliosis. Gait Posture. 1997;5:116–119. doi: 10.1016/S0966-6362(96)01081-8. [DOI] [Google Scholar]
- 40.Shiratoni T, Aruin AS. Anticipatory postural adjustments associated with rotational perturbations while standing on fixed and free-rotating supports. Clin Neurophysiol. 2004;115:797–806. doi: 10.1016/j.clinph.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Silferi V, Rougier P, Labelle H, Allard P. Postural control and idiopathic scoliosis: comparison between healthy and scoliotic subjects. Revue de Chirurgie Orthopedique. 2004;90:215–225. doi: 10.1016/s0035-1040(04)70097-5. [DOI] [PubMed] [Google Scholar]
- 42.Simoneau M, Richer N, Mercier P, Allard P, Teasdale N (2005) Sensory deprivation and balance control in adolescent idiopathic scoliosis. Exp Brain Res accepted [DOI] [PubMed]
- 43.Simoneau M, Richer N, Mercier P, Allard P, Teasdale N. Does adolescent idiopathic scoliosis alter the central sensory integration mechnisms that control balance? BMC Neuroscience. 2006;7:68–77. doi: 10.1186/1471-2202-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simoneau M, Richer N, Mercier P, Allard P, Teasdale N. Sensory deprivation and balance control in adolescent idiopathic scoliosis. Exp Brain Res. 2006;170:576–582. doi: 10.1007/s00221-005-0246-0. [DOI] [PubMed] [Google Scholar]
- 45.Weiss HR. The effect of an exercise program on vital capacity and rib mobility in patients with idiopathic scoliosis. Spine. 1991;16:88–93. doi: 10.1097/00007632-199101000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Winter DA, (1995) A.B.C. (Anatomy, Biomechanics and Control) of balance during standing and walking, Waterloo Biomechanics, Waterloo
- 47.Winter DA, Prince F, Frank JS, Powell C, Zabjek K. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]