Abstract
The standard treatment for osteoid osteomas is CT-guided radiofrequency ablation (RFA). This minimally invasive procedure is effective in terms of pain reduction as well as the recurrence rate. Nevertheless, the use for spinal lesions is limited due to a possible thermal damage of neural structures. Although the literature is contradictory, RFA should only be used when a cortical shell between the lesion and the spinal canal is existent. We present seven cases (five males, two females, mean age 23 years) with spinal osteoid osteoma in which RFA was not applicable and open resection with the use of probe-guided surgery (PGS) was performed. The principle of PGS is that after preoperative bone scintigraphy, a handheld radiation probe is used intraoperatively for tumour localisation. Here, exposure and bone resection can be minimised and completeness of tumour excision may be estimated. At the initial measurement we found a hot-spot (maximum count-rate) in all patients and after tumour resection, the signal decreased by a mean of 68% in the operative field. After a mean follow-up of 17 months one patient had residual pain but no patient had signs of tumour recurrence. The authors recommend to use PGS for those spinal osteoid osteomas where RFA is not applicable and intraoperative localisation—and here complete resection—of the tumour is difficult.
Keywords: Osteoid osteoma, Spine, Scintigraphy, Probe-guided surgery
Introduction
Osteoid osteomas represent approximately 10% of the benign bone tumours. They mainly affect long bones of children and of young adults and occur in the spine with a frequency of about 10% [11]. Traditional therapy of spinal lesions consists of open intralesional resection but recent papers report the use of CT-guided radiofrequency ablation (RFA) [6, 7, 12, 14, 18, 21, 23]. Nevertheless, RFA in the spine is limited due to the possible thermal damage of neural structures while it is the treatment of choice for osteoid osteomas of the extremities. During open resection, localisation of a small nidus can be difficult and therefore surgeons opt for wide surgical resection to ensure complete excision of the nidus which is a prerequisite for curative treatment. Spinal lesions usually occur in the posterior elements of the vertebrae and due to the proximity to neural structures and the potential for a subsequent iatrogenic instability, bone resection needs to be limited. A precise intraoperative localisation of the nidus is therefore desirable. Osteoid osteomas avidly accumulate bone-seeking tracers such as technetium-labelled phosphonates and their gamma radiation can be detected by handheld probes. Intraoperative use of a radiation probe to guide detection and resection of osteoid osteomas has been primarily reported in 1980 [4, 5] and probe-guided surgery (PGS) has been used to excise a spinal lesion the same year [20]. Since 1983 our own group has been using PGS [2] with good success in all skeletal regions [24]. The aim of the intraoperative use of the radionuclide is to locate the tumour more precisely to reduce removal of bone and to completely resect the nidus in order to reduce the recurrence rate. In the literature, there are only a few papers dealing with PGS of spinal osteoid osteomas [15–17, 20, 25, 26]. The aim of this report is to reconsider the indication of radioguided procedures for osteoid osteomas after CT-guided ablative procedures have been favoured even for spinal tumours. We report the results of seven patients for whom RFA was not applicable due to the proximity of the lesion to the spinal canal. This study is the largest report on the use of PGS for osteoid osteomas of the spine.
Materials and methods
Seven patients (five males, two females) with a mean age of 23 years (range 18–31 years) underwent open resection of a spinal osteoid osteoma. The tumour was found in the cervical spine in one, in the thoracic spine in three, in the lumbar spine in two and in the sacrum in one patient. All patients had intense preoperative diagnostic imaging including standard radiographs, CT and MRI as well as bone scintigraphy. All tumours were localised in the pedicle or the lamina. One patient (no. 7) had had two previous operations, all other patients had only the index operation. A bone scintigraphy was performed on the day of the operation. Patients received an i.v. application of approximately 600 MBq 99mTc-medronate (DuPont Pharma, Brussels, Belgium) and a whole body scan 2 h later. Surgery was performed about 2 h after completion of the bone scintigraphy. After standard posterior approach to the spine the posterior bony elements were surgically exposed and the vertebral levels were identified with an image intensifier. The nidus was further localised by a commercially available handheld radiation probe (CRYSTAL CXS-Sgo1, Crystal GmbH, Berlin, Germany) which was connected to a portable computer. The output was both an acoustic signal and displayed as an online bar graph on the computer screen. In addition, the count rates were indicated numerically on the screen, they were indexed and stored digitally for later review. The probe was placed on the exposed bone and displaced at a constant angle relative to the bone surface to maintain a constant recording geometry. Initially, normal bone uptake adjacent to the suspected lesion and maximum radioactivity over the tumour was determined (Figs. 1B, 2B). The highest point of activity (hot spot) could be located with a precision of approximately 2 mm [26] and lesion to background ratios are usually high. Depending on the anatomical situation, the sclerotic bone as well as the nidus were excised using a chisel, a curette or a punch. All specimens were used for histopathology. Further measurements were made of the excised bone as well as the nidus cavity until its counts approximated the normal background activity. If a lesion was resected in numerous small fragments rather than en bloc, an integral count of the resected tumour was methodologically impossible.
Fig. 1.
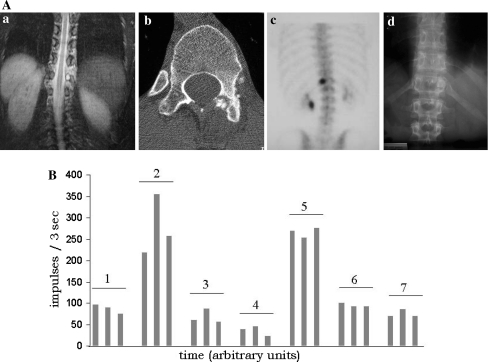
A Osteoid osteoma in the lamina of Th 12 in a 31-year-old female (no. 3). MRI shows the T2-weighed images with the highly intense lesion and the pain-induced scoliosis (a), CT shows that there is no continuous cortical bone between the lesion and the spinal canal (b) and posterior view bone scintigram (c) shows a hot spot on the left side; (d) shows the postoperative result. B Intraoperative measurements of the radioactive counts of the patient in A (three measurements shown for each time point). Normal bone (1), nidus (2), first excised fragments (3), second excised fragments (4), excised nidus (5), operative field after excision (6), healthy bone after resection (7)
Fig. 2.
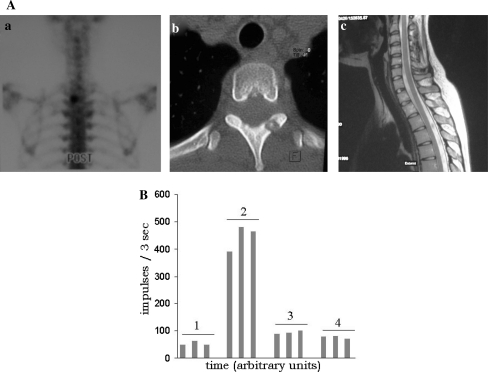
A 22-year-old female patient (no. 1) with an osteoid osteoma in the lamina of th2. Posterior view bone scintigram showing a hot spot in the left side of th2 (a) and CT (b) as well as MRI (c) showing the nidus in the lamina and absence of an intact bony lamella between the tumour and the epidural space. B Intraoperative measurements of the radioactive counts of the patient in a (three measurements shown for each time point). Healthy bone (1), nidus (2), operative field after excision (3), healthy bone after resection (4)
Results
The preoperative scintigram revealed a hot spot in every patient in a location correlating well with the nidus on MRI and CT. Illustrative examples are given in Figs. 1A and 2A. Intraoperatively the hot spot, i.e. the nidus, could be localised by the gamma probe in every patient (Table 1). The background radioactivity from normal bone was 74 while the nidus averaged 315 counts thus yielding a target to background ratio of >4. After resection the signal at the tumour site decreased to a mean of 98 representing an average percental decrease of 63%. The individual count rates are shown in Table 1, while the salient intraoperative counts of the two sample patients are given in Figs. 1B and 2B. The mean follow-up was 17 months (range 12–48). At the latest follow-up, no patient had signs of tumour recurrence and all but one patient (no. 2) were pain free. This patient was reoperated 3 years after the radioguided excision in an outside institution for recurrence of pain. At this time histopathology did not show tumour recurrence and even 5 years after the index operation CT and MRI were still negative. With PGS we have seen no intraoperative complications but one superficial wound infection (no. 2) requiring revision surgery. The histopathology of all patients confirmed the diagnosis of an osteoid osteoma. In one patient (no. 7) a spinal fusion from Th12–L2 was performed due to subsequent instability after tumour resection in the pedicle of Th11.
Table 1.
Time-course of intraoperatively recorded countrates for individual patients: each data point represents the average of three single measurements
| Sex | Age | Localisation | Operation | Normal bone | Countrate PGS (AU) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tumour ("hot spot”) | Resected tumour | Tumour site after resection | Reduction after resection (%) | ||||||
| 1 | F | 22 | th 2 | Intralesional resection | 50 | 480 | a | 100 | 79 |
| 2 | M | 16 | c 5 | Intralesional resection | 60 | 263 | a | 73 | 72 |
| 3 | F | 31 | th 12 | Excision pedicle | 85 | 350 | 270 | 100 | 71 |
| 4 | M | 22 | l 2 | Excision pedicle | 100 | 180 | 40 | 110 | 38 |
| 5 | M | 18 | th 10 | Part. laminectomy | 70 | 460 | 200 | 70 | 84 |
| 6 | M | 30 | s 4 | Intralesional resection | 80 | 290 | 190 | 90 | 69 |
| 7 | m | 23 | l 1 | Intralesional resection, post. instr. Th12 - L2 | 70 | 180 | 280 | 140 | 22 |
aMultiple bone chips resected, for methodological reasons integral countrates cannot be assessed reliably
Discussion
The diagnosis of an osteoid osteoma is based on CT scanning with thin sectioning [13] and CT is also used for the current standard treatment with minimally invasive techniques. RFA is the best described and most common thermoablative technique and has become established as the most effective method due to its high safety along with efficacy proven in numerous clinical studies [14]. As compared to conventional surgical methods, success and recurrence rates of CT-guided RFA is at least equivalent but with a lower complication rate, a shorter time of hospitalisation and faster reconvalescence [21, 22]. Nevertheless, the usage of RFA in lesions close to neural structures—like in the spine—is limited due to the risk of adjacent neural tissue heating. Accordingly, several authors are sceptical regarding RFA for spinal osteoid osteomas because of the potential neurologic risks [9, 12, 14, 19, 21] and therefore open resection is still the treatment of choice for lesions of the occipitocervical junction [3]. Nevertheless, radiofrequency ablation of a spinal osteoid osteoma has been primarily reported in 1998 [18] and in recent years several authors reported their experience [6, 7, 12, 14, 21, 23]. Animal models of RFA have shown that it may be performed safely in the vertebral body but the temperature of the surrounding soft tissue depends on the thickness of the cortical bone lamella [1, 7]. There seems to be agreement both from animal studies and from clinical experience that the lesion being treated with RFA should not be adjacent to neural structures and should be separated from them by a complete shelf of protective bone.
We present seven cases with an osteoid osteoma of the spine in which RFA was not applicable due to the proximity to the spinal canal. Images showed that no clear cancellous or cortical bone was left between the lesion and the spinal canal (Figs. 1A, 2A). We have performed open resection and in addition to that we have used PGS to localise the tumour and to control the extent of excision. Radioguided surgery for excision of osteoid osteomas has been used since 1980 [10, 20] and large studies using PGS at the extremities exist [26]. As compared to a standard open resection, nidus resection with the intraoperative use of a gamma probe has proven to be significantly more successful [8]. For spinal lesions, with the knowledge of the exact localisation of the tumour, extensive resection of normal bone and thus iatrogenic instability and the need for additional instrumentation can be avoided in some cases. But to date PGS for spinal osteoid osteomas has been described in only a few cases [15–17, 20, 26]. Results of a new technique with the combination of computer-assisted surgery with a gamma probe-guided drill excision are promising, but efficacy in a larger cohort of patients would be of interest [25].
The aim of this study is to assess the efficacy of PGS in our patients with spinal osteoid osteomas and to discuss today’s relevance of this technique in the light of a more frequent use of RFA. In all patients a hot spot correlating with the nidus was found. After resection, we found a mean decrease of the count rate at the tumour site of 63%, other studies reported a decline of 38% [25] and 20–40% [26], respectively. For the interpretation of the count rates a close cooperation with the radiologist in the operating room is necessary. The results of the preoperative scintigraphy also need to be considered. In all patients it has been judged that complete excision of the tumour was achieved. For the surgeon these informations are extremely helpful since it is of utmost importance to avoid both incomplete resection and iatrogenic instability. During follow-up no patient had signs of tumour recurrence.
One should be aware of the disadvantages of the method: Narrowness and depth of the operative field renders the application of the method technically demanding. In patient 7, initial measurement of the affected bone showed a lesser count rate than the one of the resected bone. This can probably be explained by the localisation of the tumour in the pedicle. The count rates measured may vary considerably if changes in distance between probe and the radioactive focus occur or the angle between the probe and the bone is varied. Both distance and probe tilt could not be kept constant in patient 7. These problems have also been mentioned by other authors [26]. Of course, some of these problems can be overcome by development of smaller probes with a geometry adapted to the anatomy of the operating field. Another limitation of the method is that in spinal lesions the nidus is most often excised in small fragments and therefore integral measurement of the resected bone is not reliable (patient nos. 1 and 2). In spite of that, signal intensity of the tumour site after resection revealed a significant decrease or equaled the rate of the normal bone thereby confirming complete excision. A further disadvantage of radioguided surgery is the radiation caused by the additional bone-scintigraphy prior to the operation. The radiation exposure of bone scintigraphy compares however very favourably with that of CT. Additionally, by PGS, use of the intraoperative X-ray is reduced since localisation of the tumour is facilitated by the gamma probe.
In conclusion, PGS for spinal osteoid osteomas is a useful and easy to perform technique by which effective removal of the nidus may be assessed intraoperatively and extent of excision may be minimised. PGS is still a good option when the tumour is difficult to localise or when RFA is not possible due to the proximity to neural structures.
Acknowledgments
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The authors thank V. Hehmann for help in editing the manuscript.
References
- 1.Bitsch R, Rupp R, Bernd L, et al. Osteoid osteoma in an ex vivo animal model: temperature changes in surrounding soft tissue during CT-guided radio frequency ablation. Radiology. 2006;238:107–112. doi: 10.1148/radiol.2381041500. [DOI] [PubMed] [Google Scholar]
- 2.Braun A, Lange D, Nagel A, et al. Intraoperative Niduslokalisation mit dem Szintillationsdetektor beim Osteoidosteom. In: Brussatis F, Hahn K, et al., editors. Nuklearmedizin in der Orthopädie. Berlin: Springer; 1990. pp. 252–262. [Google Scholar]
- 3.Bruneau M, Cornelius J, George B. Osteoid osteomas and osteoblastomas of the occipitocervical junction. Spine. 2005;30:E567–E571. doi: 10.1097/01.brs.0000180489.50171.ee. [DOI] [PubMed] [Google Scholar]
- 4.Colton C, Hardy J. The use of a sterilizable radiation probe during bone surgery. J Nucl Med. 1980;21:56. [Google Scholar]
- 5.Colton C, Hardy J. Evaluation of a sterilizable radiation probe as an aid to the surgical treatment of osteoid-osteoma. Technical note. J Bone Joint Surg [Am] 1983;65:1019–1022. [PubMed] [Google Scholar]
- 6.Cove J, Taminiau A, Obermann W, et al. Osteoid osteoma of the spine treated with percutaneous computer tomography-guided thermocoagulation. Spine. 2000;15:1283–1286. doi: 10.1097/00007632-200005150-00014. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy D, Hong R, Oliver B, et al. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. Am J Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 8.Etchebehere M, Etchebehere E, Reganin L, et al. Intraoperative localization of an osteoid-osteoma using a gamma probe. Int Orthop. 2004;28:379–383. doi: 10.1007/s00264-004-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18:36–41. doi: 10.1097/01.mop.0000193277.47119.15. [DOI] [PubMed] [Google Scholar]
- 10.Ghelman B, Thompson F, Arnold W. Intraoperative radioactive localization of an osteoidosteoma. J Bone Joint Surg [Am] 1981;63:826–827. [PubMed] [Google Scholar]
- 11.Greenspan A. Benign boneforming lesions: osteoma, osteoid osteoma and osteoblastoma. Skeletal Radiol. 1993;22:485–500. doi: 10.1007/BF00209095. [DOI] [PubMed] [Google Scholar]
- 12.Hadjipavlou A, Lander P, Marchesi D, et al. Minimally invasive surgery for ablation of osteoid osteoma of the spine. Spine. 2003;28:472–477. doi: 10.1097/01.BRS.0000092386.96824.DB. [DOI] [PubMed] [Google Scholar]
- 13.Hosalkar H, Sumeet G, Moroz L, et al. The diagnostic accuracy of MRI versus CT imaging for osteoid osteoma in children. Clin Orthop Relat Res. 2005;433:171–177. doi: 10.1097/01.blo.0000151426.55933.be. [DOI] [PubMed] [Google Scholar]
- 14.Lindner N, Ozaki T, Roedl R, et al. Percutanous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg [BR] 2001;83-B:391–396. doi: 10.1302/0301-620X.83B3.11679. [DOI] [PubMed] [Google Scholar]
- 15.Marchal C, Daxhelet B, Gobert P, et al. Surgical treatment of osteoid osteoma of the spine using intraoperative scintigraphy. Acta Orthop Belg. 2002;68:301–305. [PubMed] [Google Scholar]
- 16.Matejka J, Zahlava J. Vertebral osteoid osteoma: peroperative detection of its nidus using a surgical gamma probe. Acta Chir Orthop Traumatol Cech. 2003;70:187–190. [PubMed] [Google Scholar]
- 17.Osebold W, Lester E, Hurley J, et al. Intraoperative use of the mobile gamma camera in localizing and excising osteoid osteomas of the spine. Spine. 1993;18:1816–1828. doi: 10.1097/00007632-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Osti O, Sebben R. High-frequency radio wave ablation of osteoid osteoma in the lumbar spine. Eur Spine J. 1998;7:422–425. doi: 10.1007/s005860050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto G, Taminiau A, Vanderschuren G, et al. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: tricks of the trade. Am J Roentgenol. 2002;179:1633–1642. doi: 10.2214/ajr.179.6.1791633. [DOI] [PubMed] [Google Scholar]
- 20.Rinsky L, Goris M, Bleck E, et al. Intraoperative skeletal scintigraphy for localization of osteoid osteoma in the spine. J Bone Joint Surg [Am] 1980;62-A:143–144. [PubMed] [Google Scholar]
- 21.Rosenthal D, Hornicek F, Toriani M, et al. Osteoid osteoma: percutanous treatment with radiofrequency energy. Radiology. 2003;229:171–175. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal D, Hornicek F, Wolfe M, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg [Am] 1998;80:815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Samaha E, Ghanem I, Moussa R, et al. Percutanous radiofrequency coagulation of osteoid osteoma of the “neural spinal ring”. Eur Spine J. 2005;14:702–705. doi: 10.1007/s00586-004-0865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuknecht B, Daecke W, Bernd L, et al. Erfahrungen mit der intraoperativen Lokalisation von Osteoid Osteomen mit einem Szintillationsdetektor. Akt Traumatol. 1998;28:105–109. [Google Scholar]
- 25.Royen B, Baayen J, Pijpers R, et al. Osteoid osteoma of the spine: a novel technique using combined computer-assisted and gamma probe-guided high-speed intralesional drill excision. Spine. 2005;30:369–373. doi: 10.1097/01.brs.0000152531.49095.34. [DOI] [PubMed] [Google Scholar]
- 26.Wioland M, Sergent-Alaoui A. Didactic review of 175 radionuclide-guided excisions of osteoid osteomas. Eur J Nucl Med. 1996;23:1003–1011. doi: 10.1007/BF01084380. [DOI] [PubMed] [Google Scholar]