Abstract
The histology of the primary tumor in metastatic spine disease plays an important role in its treatment and prognosis. However, there is paucity in the literature of histology-specific analysis of spinal metastases. In this study, prognostic variables were reviewed for patients who underwent surgery for breast metastases to the spinal column. Respective chart review was done to first identify all patients with breast cancer over an 8-year period at a major cancer center and then to select all those with symptomatic metastatic disease to the spine who underwent spinal surgery. Univariate and multivariate analyses were used to assess several prognostic variables. Presence of visceral metastases, multiplicity of bony lesions, presence of estrogen receptors (ER), and segment of spine (cervical, thoracic, lumbar, sacral) in which metastases arose were compared with patient survival. Eighty-seven patients underwent 125 spinal surgeries. Those with estrogen receptor (ER) positivity had a longer median survival after surgery compared to those with estrogen receptor negativity. Patients with cervical location of metastasis had a shorter median survival compared with those having metastases in other areas of the spine. The presence of visceral metastases or a multiplicity of bony lesions did not have prognostic value. In patients with spinal metastases from breast cancer, aggressive surgical management may be an option for providing significant pain relief and preservation/improvement of neurological function. Interestingly, in patients undergoing such surgery, cervical location of metastasis is a negative prognostic variable, and ER-positivity is associated with better survival, while presence of visceral or multiple bony lesions does not significantly alter survival.
Keywords: Breast cancer, Estrogen receptor, Metastases, Prognosis, Spine surgery
Introduction
Carcinoma of the breast is the most common malignancy and the second most common cause of cancer-related death in North American and Western European women [8, 13, 20]. When breast cancer becomes metastatic, skeletal involvement is very frequent, with reported incidences between 47–85% in autopsy series and 69–80% when defined radiographically [8, 13, 16, 20, 30]. In addition, the majority of such skeletal metastases occur in the spine [37]. About one-third of these spinal metastases become symptomatic, causing intractable pain, neurological deficits, and/or biomechanical instability requiring surgical treatment [18, 32], all which severely affect the patient’s quality of life.
In patients with metastatic spinal tumors, the histopathology of the primary cancer has frequently been shown to have significant prognostic value [2, 3, 25, 27, 32, 39]. Patients with metastatic breast cancer often survive significantly longer than similar patients with metastatic spine disease from lung cancer [36]. In this way, aggressive surgical options used to improve quality of life may become increasingly important options for care. However, despite the paramount importance of primary tumor histopathology, few studies in the literature on spinal metastases are pathology specific. In this study, the outcomes of a large number of patients undergoing spinal surgery for metastatic breast cancer at a major cancer center were reviewed. Particular attention was given to hormone status, presence of visceral metastases, number of spinal metastases, and location of metastasis within the spine.
Methods
Patient population and selection criteria
A retrospective review was performed of all patients treated at The University of Texas M. D. Anderson Cancer Center from June 1, 1993 to June 30, 2001 for histologically confirmed breast cancer with metastases to the spine. During this period, a total of 16,977 patients were diagnosed with breast cancer at the institution, as identified through a search of the tumor registry. During the same time period, there were 2,641 patients diagnosed with spinal metastases from a variety of primary cancer types, 479 of which were from breast cancer. Eighty-seven of these patients (18%) underwent surgery for spinal metastases.
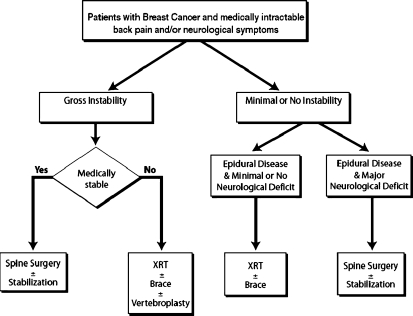
The selection criteria for undergoing surgical intervention for spinal metastases from breast cancer required all patients to be deemed medically stable enough to undergo the proposed surgery and to have at least one of the following conditions: (1) obvious spinal deformity with intractable pain, (2) retropulsed bone or disc fragment in the spinal canal causing significant spinal cord compression or (3) prior irradiation of the site of progressive spinal involvement with cord compression or (4) medically intractable mechanical, local, or radicular pain. Patients excluded from surgery were those with end-stage disease (e.g, estimated survival < 3 months), absence of biomechanical instability or significant spinal deformity, and those who refused surgery. Life expectancy was estimated by the medical oncology service using multiple characteristics of the individual patient, including but not limited to: histopathology, age, functional status, concomitant comorbidities, presence of untreatable visceral metastases, hormone receptor status, response to adjuvant therapy, quality of life issues, and presence of psychosocial problems. These patients, when treated, underwent radiation therapy and/or chemotherapy/hormonal therapy (Fig. 1). Eighty-seven patients met the above criteria and constituted the study group for this paper. These patients underwent a total of 125 spinal operations over the duration of follow up.
Fig. 1.
Proposed algorithm for treatment of patients with symptomatic spinal metastases from breast cancer
Data collection
Medical records were retrospectively reviewed for demographic, clinical, radiographic, and histological data. Data collected regarding the primary breast cancer included dates of initial diagnosis, surgery, radiation and/or chemotherapy/hormonal therapy and the presence of other metastases at the time of spinal surgery.
The anatomical location of the spinal lesions was assessed using magnetic resonance (MR) imaging and plain X-rays. The total number of vertebral segments involved with tumor was noted and grouped into categories (e.g., single lesion, two lesions, and three or more lesions) for analysis of survival and complications. Metastatic spinal lesions requiring surgery were classified as follows: cervical (C, C1-6), cervicothoracic (CT, C7-T1), thoracic (T, T2-11), thoracolumbar (TL, T12-L1), lumbar (L, L2-4), or lumbosacral (LS, L5-S1). Lesions encompassing two or more of these regions such as cervicothoracic, thoracolumbar, and lumbosacral regions were considered to be at a junctional level.
All patients had histologically verified breast cancer treated at M. D. Anderson. Although the original diagnosis was often made at a referring institution, all available pathology slides from outside hospitals were reviewed at M. D. Anderson to confirm the diagnosis. A review of the hospital charts and pathology reports for each patient was performed to determine the original histopathological tumor type, the degree of involvement of lymph nodes by the tumor, and whether the tumor expressed estrogen or progesterone receptors. The anatomical location and extent of disease dictated the surgical approach as has been previously published by Fourney and Gokaslan [14]. In patients with tumor-related spinal instability, posterior arthrodesis was performed using allograft bone, and posterior stabilization was achieved by implanting instrumentation.
Details of patient evaluation at the time of discharge and at around 1 month, 3 months, 6 months, and 1 year after surgery were reviewed. Follow-up spine MR images and plain X-rays were also evaluated. Clinical or radiographic evidence of local or distant metastatic tumor recurrence in the spine was noted. Tumor growth at the operation site was considered local recurrence, and tumor growth at another site in the spine was considered distant recurrence.
Statistical methods
Chi-square and Fisher exact tests were used for categorical variables, and Student’s t test and the Mann–Whitney test were used for continuous and ordinal variables, as appropriate. The Wilcoxon signed-rank test was used to compare the paired outcomes at various follow-up points. The Kaplan–Meier method was used to estimate postoperative survival and survival time after primary breast cancer diagnosis [26]. Univariate and multivariate predictors of overall survival were assessed using the Cox proportional hazards model. Variables significant at P < 0.25 in the univariate analysis were tested through a backward stepwise selection process for their independent effect on overall survival. Rate ratios and their 95% confidence intervals (CIs) were computed. Odds ratios and their 95% CIs were computed. A P value ≤0.05 was considered significant.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of these patients are illustrated in Table 1.
Table 1.
Demographics and clinical characteristics of 87 patients with breast cancer who underwent surgery for spinal metastases
| Age (years) | |
| Median | 53 years |
| Range | 35–84 years |
| Median time between primary breast cancer diagnosis and first metastatic spine surgery | 3.9 years |
| Characteristics | n (%) |
| Original breast cancer histopathology | |
| Invasive ductal carcinoma | 74 (85) |
| Adenocarcinoma | 13 (15) |
| Original hormone receptor statusa | |
| ER positive | 46 (72) |
| PR positive | 32 (56) |
| Original lymph node statusa | |
| Positive | 44 (68) |
| Other sites of metastases at time of spine surgery | |
| No other metastases (spine only) | 29 (33) |
| Skeletal (skull, ribs, pelvis, long bones) | 53 (61) |
| Liver | 17 (19) |
| Lungs | 12 (14) |
| Brain | 6 (7) |
| Pre-op visual analog pain score [Median (range)] | 6 (1–10) |
| Pre-op pain medication score [Median (range)] | 4 (1–5) |
| Radicular | 34 (39) |
| Axial | 31 (36) |
| Local | 22 (25) |
| Frankel grade at presentation | |
| E | 52 (60) |
| D | 24 (28) |
| C | 8 (9) |
| B | 1 (1) |
| A | 2 (2) |
| Pre-op adjuvant spine treatment | |
| Both chemotherapy and radiation | 40 (46) |
| Chemo/hormonal only | 39 (45) |
| None | 5 (6) |
| Spinal radiation alone | 3 (3) |
| Post-op adjuvant spine treatment | |
| Chemotherapy only | 43 (50) |
| Both radiation and chemotherapy | 26 (30) |
| None | 8 (9) |
| Spinal radiation only | 1 (1) |
| Unknown | 9 (10) |
aAmong patients with available information
Number and location of spinal metastases
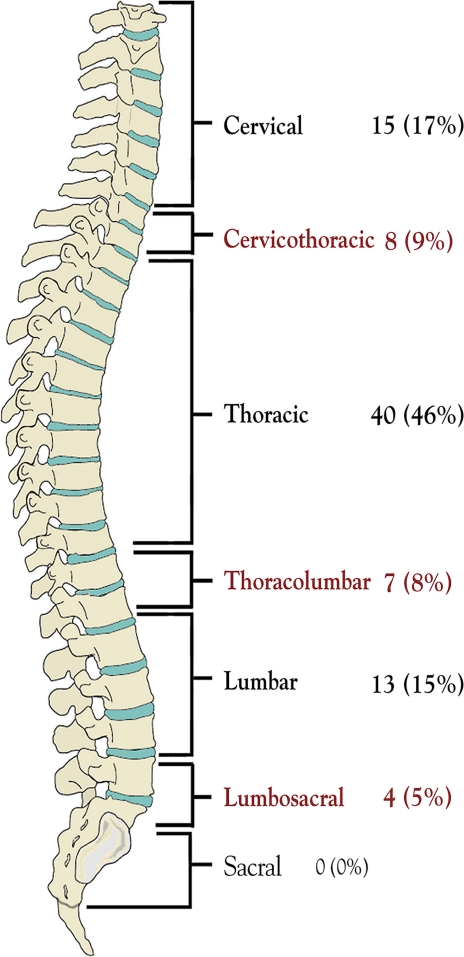
Twenty-six patients (30%) had tumor involvement of one vertebral body, as diagnosed by MR imaging criteria. The remaining 61 patients (70%) had multiple locations of metastases within the spinal column. Twenty-two (25%) had two, 23 (26%) had three, and 16(18%) had four or more vertebral bodies involved with tumor material. The anatomical distribution of spinal metastases requiring surgical treatment is illustrated in Fig. 2.
Fig. 2.
Anatomical distribution of spinal metastases requiring surgical treatment
Tumor recurrence
The median overall duration of follow up was 13 months (range <1 to 70 months). Patients remaining alive were followed for a median of 10 months (range <1 to 70 months). A total of 20 patients (23%) experienced tumor recurrences. Of these recurrences, seven were local (35%), ten were distant sites of new metastasis (50%), and three patients (15%) had both local recurrences and distant sites of new metastasis. Treatment for recurrence included surgery in 11 patients and radiation therapy in 9 patients.
Survival
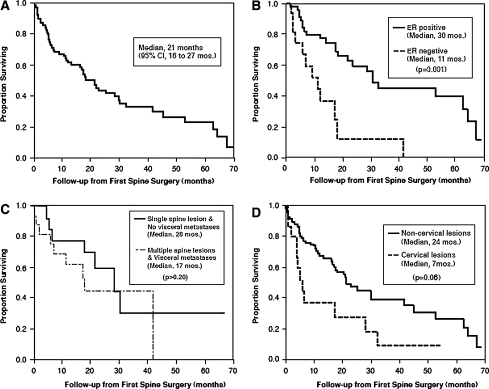
The median survival interval of patients after the original breast cancer diagnosis was 80 months (6.6 years; 95% CI 5.4–7.7 years). The patient survival rate after the date of primary breast cancer diagnosis was 96% at 1 year, 81% at 3 years, and 69% at 5 years. The patients’ median survival time after their first spinal surgery was 21 months (95% CI 16–27 months). The overall survival rate of patients after their first spinal surgery was 62% at 1 year, 44% at 2 years, 33% at 3 years, 27% at 4 years, and 24% at 5 years, Fig. 3a–d.
Fig. 3.
Kaplan–Meier survival curves. Survival from time of first surgery for spinal metastases (median survival, 21 months) (a). Improved survival for patients with estrogen receptor positivity (b). Unchanged survival for patients with visceral metastases with or without multiple spine metastases (c). Shortened survival for patients with cervical lesions (d)
Prognostic variables
Several variables were assessed for prognostic value in overall survival and are listed in Table 2. Estrogen receptor positivity of the tumor was significantly associated with better prognosis (multivariate rate ratio 3.7; 95% CI 1.6–7.1; P = 0.001). The multiplicity of spinal lesions and presence or absence of visceral metastases was assessed, and neither contributed significant survival value. A cervical tumor location showed a trend towards shorter survival (multivariate rate ratio 2.1, 95% CI, 1.0–4.5; P = 0.06).
Table 2.
Univariate and multivariate predictors of survival after surgery in 87 patients with breast cancer metastases to the spine
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Rate ratioa | 95% CI | P value | Median survival (mo) | Rate ratioa | 95% CI | P value | |
| Age | |||||||
| <65b | 1.0 | – | – | 17.9 | |||
| ≥65 | 0.6 | 0.2–1.6 | 0.30 | 42.7c | NI | ||
| Interval from breast cancer diagnosis to first spinal metastasis (months) | |||||||
| ≥60b | 1.0 | – | – | 28.0 | |||
| 12–60 | 1.2 | 0.6–2.5 | 0.68 | 21.5 | NI | ||
| <12 | 1.5 | 0.6–4.0 | 0.39 | 11.8 | |||
| Tumor histology | |||||||
| Ductal carcinomab | 1.0 | – | – | 21.1 | |||
| Adenocarcinoma | 1.4 | 0.7–2.8 | 0.32 | 21.3 | NI | ||
| Lymph node status | |||||||
| Negativeb | 1.0 | – | – | 28.0 | |||
| Positive | 1.4 | 0.7–2.9 | 0.33 | 17.2 | NI | ||
| Preop Frankel grade | |||||||
| Ambulatory Eb | 1.0 | – | – | 21.1 | |||
| Ambulatory D | 1.2 | 0.6–2.1 | 0.63 | 21.5 | NI | ||
| Non-ambulatory (A-C) | 1.3 | 0.5–3.5 | 0.56 | 3.1 | |||
| Extent of metastatic disease | |||||||
| Spine onlyb | 1.0 | – | – | 28.0 | |||
| Spine plus Visceral | 1.4 | 0.7–3.0 | 0.32 | 17.4 | NI | ||
| Spine plus Non-visceral | 1.5 | 0.8–2.9 | 0.26 | 17.2 | |||
| Preoperative radiation to operative site | |||||||
| Nob | 1.0 | – | – | 17.4 | NI | ||
| Yes | 0.7 | 0.4–1.3 | 0.25 | 21.5 | |||
| Surgical approach | |||||||
| Single (anterior or posterior)b | 1.0 | – | – | 17.9 | NI | ||
| Combined | 0.8 | 0.4–1.7 | 0.63 | 25.1 | |||
| Number of vertebral bodies removed | |||||||
| Noneb | 1.0 | – | – | 16.2 | |||
| One | 0.9 | 0.4–1.7 | 0.64 | 21.3 | NI | ||
| Two or more | 1.1 | 0.5–2.4 | 0.79 | 21.5 | |||
| Number of spine lesions by MRI | |||||||
| 1b | 1.0 | – | – | 22.9 | |||
| 2 | 1.7 | 0.8–3.6 | 0.14 | 17.0 | NS | ||
| 3 or more | 1.5 | 0.7–2.9 | 0.26 | 16.5 | |||
| Major postoperative complications | |||||||
| Nob | 1.0 | – | – | 22.9 | |||
| Yes | 1.7 | 0.8–3.5 | 0.13 | 11.1 | NS | ||
| Spine tumor location | |||||||
| Non-cervicalb | 1.0 | – | – | 25.1 | 1.0 | − | − |
| Cervical | 2.3 | 1.3–4.2 | 0.006 | 6.8 | 2.1 | 1.0–4.5 | 0.06 |
| Estrogen receptor | |||||||
| Positiveb | 1.0 | – | – | 30.0 | 1.0 | − | − |
| Negative | 3.4 | 1.6–7.1 | 0.001 | 11.0 | 3.7 | 1.7–8.1 | 0.001 |
| Progesterone receptor | |||||||
| Positiveb | 1.0 | – | – | 30.0 | |||
| Negative | 2.6 | 1.3–5.3 | 0.007 | 11.0 | NS | ||
CI confidence interval, NI not included, NS not significant
Bold values represent variables that showed statistical significance (or close to it) during the univariate and multivariate analysis
aRate ratio > 1.0 indicates a faster rate of death, <1.0 indicates a slower rate of death, rate ratio of 1.0 indicates a similar rate of death. Cox proportional Hazard analysis used
bReferent group (group that others are compared to)
cMean; median not reached
Discussion
Within the Western World, the incidence of breast cancer has continued to rise over the last few decades. In patients with metastatic breast cancer, skeletal involvement is very frequent [8, 13, 16, 20, 30, 44], and among reported clinical series of spinal epidural metastases, breast cancer is again common, accounting for 9–40% of all cases [12, 19, 21–23, 27, 46–49, 52, 53]. In this current series, the incidence of spinal metastases from breast cancer was only 18%, likely reflecting the large number of rarer spinal metastases that are referred to this tertiary oncology center. Despite the high incidence of breast cancer metastases to the spine however, few series in the literature on metastatic spinal disease deal specifically with metastases from breast cancer. The histopathology of the primary cancer has significant implications for treatment and defines the tumor’s radiosensitivity, chemosensitivity, vascularity, growth pattern as well as the prognosis [2, 3, 25, 27, 32, 39]. Because of the large number of cancer patients treated at M. D. Anderson, a pathology-specific study of a large number of patients is feasible. Although spinal metastases are most often clinically silent, they can often cause significant morbidity, pain, and neurological dysfunction that may adversely affect quality of life.
A multidisciplinary approach including medical treatment, radiotherapy, and/or surgery is the ideal treatment of breast metastasis [3, 32]. Medical therapies include systemic chemotherapy/hormonal therapy and medications specific for spinal metastases, such as steroids, analgesics, and bisphosphonates [7, 13, 30, 32, 43]. For patients with localized bone pain that has not responded to systemic therapy and analgesics, external beam irradiation (30 Gy in ten fractions) has traditionally been the treatment of choice and usually provides good pain relief [29–31, 42]. Spinal stereotactic radiosurgery (SRS) is a relatively new radiation treatment option for spinal metastases that not undergone rigorous, long-term investigation, but may have advantages over conventional XRT for the treatment of metastatic spine disease. Interestingly, a number of studies involving SRS for metastatic spine disease have provided encouraging results in relation to pain control and improvement in neurological function [11, 17]. In addition, percutaneous vertebroplasty and kyphoplasty have been shown to be safe and effective techniques for treating intractable pain secondary to pathological vertebral fractures of metastatic spine disease [15].
Indications for surgery
The exact indications for surgery in patients with metastatic spine disease are controversial [10], although it is generally agreed that the surgery is palliative, not curative. Despite the efficacy of spinal radiotherapy, there are clinical situations in which surgical intervention should take precedence (Fig. 1). Assuming the overall medical condition of the patient is suitable to tolerate the proposed operation and the patient does not have a limited life expectancy (<3 months), patients may benefit significantly from surgery, as has been shown in a prospective, randomized clinical trial by Patchell et al. [40]. Generally, surgical indications include: progressive neurological deficits due to compression from structural disintegration of the vertebral elements and disc, mechanical instability, deformity, radiation resistant tumors or tumors that progress despite undergoing maximal radiation dosages, and medically intractable pain [29].
Reduction in pain and preservation of motor and/or sphincter control in patients with spinal metastases may significantly improve the quality of remaining life. For this reason, aggressive surgical intervention is often considered in patients with metastatic spine disease who can tolerate surgery [5, 19, 24, 33, 38, 39, 47, 48, 53, 54]. Recently, Ogihara et al. [36] reviewed patients with lung cancer metastatic to the spine in attempt to identify prognostic factors that may aid in stratifying patients towards medical or surgical treatment. Not surprisingly, overall general condition of the patient (Karnofsky’s performance status) was shown to be one of the strongest prognostic indicators for survival. With improvements in adjuvant care for breast cancer, patients have benefited from relatively long life expectancy compared to patients with other types of cancer. For this reason, patients with metastatic breast cancer to the spine may form a subset in which aggressive surgical intervention should be commonly considered.
Several schemes, systems, and algorithms have been proposed in the past to determine which patients with spinal metastases in general, would benefit most from surgery [51, 52]. However, they fall short of determining factors that affect outcome in pathology-specific subgroups. Tokuhashi et al. [51] proposed a preoperative scoring system consisting of six parameters that typically affect prognosis. In this scheme, each parameter is given equal weight, and the scores are added together. However, the scoring system was based on only 64 patients who had tumors with a wide variety of histopathologies (at least 11 types) and included only 13 patients with breast cancer. In an effort to increase the reliability of predicting prognosis from such scoring systems, Tokuhashi et al. [50] modified the original scoring system to provide more prognostic weight to primary tumor histology. Tomita et al. [52] also proposed a prognostic scoring system and surgical strategy for patients with spinal metastases. However, it also is not pathology specific, including nine different cancer histologies in 61 patients, only 16 of which possessed breast cancer. Thus, although these scoring systems may provide a useful guide to managing spinal metastases in general, they are not entirely applicable for all histological types of tumors, in particular, spinal metastases from breast cancer. Another criticism of these two scoring systems is the fact that neither takes into account medically intractable pain as an indication for surgery.
Hormone receptors
Hormone receptor status of the tumor in patients with breast cancer has significant prognostic value with respect to spinal metastases and patterns of tumor spread [8, 28]. First, breast cancer bone metastases are more common in well-differentiated receptor positive tumors, while liver metastases are more frequent with receptor negative anaplastic tumors [8]. Koenders et al. [28] report that patients with ER-positive breast tumors had bone metastases at first relapse three times more often than patients with ER-negative tumors. In our series, patients having tumors with ER-positivity had a median survival time almost three times longer than patients whose tumors were estrogen receptor negative (P = 0.001). This finding is similar to that reported in a series of 367 patients with breast cancer whose first distant metastases was in the skeleton [9].
Anatomic location
The anatomical location of the metastatic lesion from breast cancer also demonstrated some prognostic value in our series. Previous authors have noted that cervical location may be associated with a negative influence on the survival rate [1], while others have considered cervical spine metastases a favorable prognostic factor [27]. In our series, patients with metastases involving the cervical spine or the cervicothoracic junction (C7-T1) showed a trend towards poorer median postoperative survival time compared to those with non-cervical lesions. The exact reason for this observation is unclear, although a number of theories can be hypothesized. Firstly, lesions in the cervical and cervicothoracic spine may present at a later stage during the breast cancer disease process. The median survival after initial breast cancer diagnosis is the same for patients with and without cervical lesions suggesting that there is a similar finite life expectancy in these patients.
Another reason for cervical lesions carrying a worse prognosis in this group may be due to possible delayed detection of metastatic tumors in the cervical region. In our population, there was a strong trend (P = 0.17) for patients with cervical lesions to have a longer interval between primary breast cancer diagnosis and first spinal surgery for metastases (median 50 months) relative to patients with noncervical lesions (median 31 months). Delayed detection, if present, could be due to the fact that the cervical spinal canal is relatively wide compared to the thoracic spine, and thus lesions may need to grow larger before coming to clinical attention. Consequently, such lesions may represent a later stage in the disease process. In addition, there is evidence that breast cancer metastases may be particularly difficult to detect in the cervical spine during routine screening skeletal scintigraphy [41]. Furthermore, bone scintigraphy has a relatively high false-negative rate in patients with ER-negative or highly proliferative tumors [35], which may be clinically more aggressive. A third, but less likely theory for why patients undergoing surgery for cervical breast cancer metastases exhibit a trend towards shorter survival may be related to the morbidity associated with surgery in this region. However in our series, the risk of major early complications for patients undergoing cervical surgery was not significantly different from that of patients undergoing surgery on non-cervical regions of the spine.
Visceral metastases
In distinct contrast to the scoring systems of Tokuhashi [50, 51] and Tomita [52] and other reports from the literature on spine metastases from varied histologies [2–4, 10, 19, 21], our current study reveals an important difference with respect to presence of visceral metastases on overall survival in patients with breast cancer metastases to the spine. Patients with both spinal and visceral metastases did not have a significantly shorter survival time than patients with metastatic disease confined to the spinal column. This is in contrast to reports in the literature, that suggest, that breast metastases confined to the skeletal system have a better overall prognosis [6, 8, 34, 43, 45]. In our series, which includes patients with and without visceral metastases, the overall median survival from time of surgery was 21 months, which is comparable to some reported series [52] and longer than others [39, 46, 47, 49, 53]. Furthermore, the extent of metastatic disease was not associated with the risk of major postoperative complications.
It is unclear why the presence of visceral metastases may not carry the same dismal fate in breast cancer as it does in other primary cancers such as carcinoma of the lung [36]. This discrepancy may be due to the relative effectiveness of adjunctive systemic treatments, such as chemotherapy, hormonal therapy, and irradiation in breast cancer. It is also possible that there was bias introduced into our selection of patients for surgery (Fig. 1), such that patients with poor performance scores or shorter expected survival were excluded from surgery. In this way, sicker patients, likely with a greater proportion of visceral metastases, were excluded, thus falsely showing good outcomes in the patients undergoing surgery who possessed visceral metastases. If this were the case however, the presence of visceral metastasis would still be a non-significant prognostic indicator, but the prognostic value of performance score would be further strengthened.
Number of spinal metastases
Another way in which the results of the study differ from the scoring systems of Tokuhashi [50, 51] and Tomita [52], is that the number of vertebral bodies involved with breast cancer metastases did not have prognostic value in our series. The majority of our patients (70%) had multiple spinal lesions. Furthermore, the number of vertebral bodies involved with tumor was not associated with the risk of major early postoperative complications. The lack of effect that the number of spinal lesions has on the overall survival time of patients in our study differs from many reported series of spinal metastases [51], a finding that may have become apparent only by restricting our series to breast cancer patients. Nonetheless, even though the number of lesions metastatic to the spine may not influence overall survival, the presence of multiple lesions may drastically affect surgical management in regard to both approach and subsequent reconstruction.
Conclusions
ER-positivity of the breast cancer was associated with better survival after surgery for spinal metastases, while cervical location of a spinal metastasis is a possible negative prognostic variable. Neither the number of vertebral bodies involved with tumor nor the presence of visceral metastases had prognostic significance in our series. Future reports on surgical outcomes for patients with metastatic spinal disease should be pathology-specific whenever possible, considering its paramount implications for optimal treatment and prognostication.
References
- 1.Atanasiu JP, Badatcheff F, Pidhorz L. Metastatic lesions of the cervical spine. A retrospective analysis of 20 cases. Spine. 1993;18:1279–1284. doi: 10.1097/00007632-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Barcena A, Lobato RD, Rivas JJ, Cordobes F, Castro S, Cabrera A, Lamas E. Spinal metastatic disease: analysis of factors determining functional prognosis and the choice of treatment. Neurosurgery. 1984;15:820–827. doi: 10.1097/00006123-198412000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky MH, Lis E, Raizer J, Lee H, Boland P. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459–469. [PubMed] [Google Scholar]
- 4.Chataigner H, Onimus M. Surgery in spinal metastasis without spinal cord compression: indications and strategy related to the risk of recurrence. Eur Spine J. 2000;9:523–527. doi: 10.1007/s005860000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LH, Chen WJ, Niu CC, Shih CH. Anterior reconstructive spinal surgery with Zielke instrumentation for metastatic malignancies of the spine. Arch Orthop Trauma Surg. 2000;120:27–31. doi: 10.1007/pl00021238. [DOI] [PubMed] [Google Scholar]
- 6.Chiedozi LC. Prognostic significance of exclusive skeletal metastases in stage IV primary carcinoma of the breast. Surg Gynecol Obstet. 1988;167:303–306. [PubMed] [Google Scholar]
- 7.Ciray I, Lindman H, Astrom KG, Bergh J, Ahlstrom KH. Early response of breast cancer bone metastases to chemotherapy evaluated with MR imaging. Acta Radiol. 2001;42:198–206. doi: 10.1034/j.1600-0455.2001.042002198.x. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336–340. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coraddu M, Nurchi GC, Floris F, Meleddu V. Surgical treatment of extradural spinal cord compression due to metastatic tumours. Acta Neurochir (Wien) 1991;111:18–21. doi: 10.1007/BF01402508. [DOI] [PubMed] [Google Scholar]
- 11.Degen JW, Gagnon GJ, Voyadzis JM, McRae DA, Lunsden M, Dieterich S, Molzahn I, Henderson FC. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2:540–549. doi: 10.3171/spi.2005.2.5.0540. [DOI] [PubMed] [Google Scholar]
- 12.Enkaoua EA, Doursounian L, Chatellier G, Mabesoone F, Aimard T, Saillant G. Vertebral metastases: a critical appreciation of the preoperative prognostic tokuhashi score in a series of 71 cases. Spine. 1997;22:2293–2298. doi: 10.1097/00007632-199710010-00020. [DOI] [PubMed] [Google Scholar]
- 13.Esteva FJ, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN. Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist. 2001;6:133–146. doi: 10.1634/theoncologist.6-2-133. [DOI] [PubMed] [Google Scholar]
- 14.Fourney DR, Gokaslan ZL. Use of “MAPs” for determining the optimal surgical approach to metastatic disease of the thoracolumbar spine: anterior, posterior, or combined. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2005;2:40–49. doi: 10.3171/spi.2005.2.1.0040. [DOI] [PubMed] [Google Scholar]
- 15.Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, Rhines LD, Gokaslan ZL. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 16.Galasko CS. Skeletal metastases and mammary cancer. Ann R Coll Surg Engl. 1972;50:3–28. [PMC free article] [PubMed] [Google Scholar]
- 17.Gerszten PC, Welch WC. Cyberknife radiosurgery for metastatic spine tumors. Neurosurg Clin N Am. 2004;15:491–501. doi: 10.1016/j.nec.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Gokaslan ZL. Spine surgery for cancer. Curr Opin Oncol. 1996;8:178–181. doi: 10.1097/00001622-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gokaslan ZL, York JE, Walsh GL, McCutcheon IE, Lang FF, Putnam JB, Jr, Wildrick DM, Swisher SG, Abi-Said D, Sawaya R. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599–609. doi: 10.3171/jns.1998.89.4.0599. [DOI] [PubMed] [Google Scholar]
- 20.Harrison KM, Muss HB, Ball MR, McWhorter M, Case D. Spinal cord compression in breast cancer. Cancer. 1985;55:2839–2844. doi: 10.1002/1097-0142(19850615)55:12<2839::AID-CNCR2820551222>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Hatrick NC, Lucas JD, Timothy AR, Smith MA. The surgical treatment of metastatic disease of the spine. Radiother Oncol. 2000;56:335–339. doi: 10.1016/S0167-8140(00)00199-7. [DOI] [PubMed] [Google Scholar]
- 22.Helweg-Larsen S, Sorensen PS. Symptoms and signs in metastatic spinal cord compression: a study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer. 1994;30A:396–398. doi: 10.1016/0959-8049(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 23.Hosono N, Yonenobu K, Fuji T, Ebara S, Yamashita K, Ono K. Vertebral body replacement with a ceramic prosthesis for metastatic spinal tumors. Spine. 1995;20:2454–2462. doi: 10.1097/00007632-199511001-00015. [DOI] [PubMed] [Google Scholar]
- 24.Jackson RJ, Gokaslan ZL. Spinal-pelvic fixation in patients with lumbosacral neoplasms. J Neurosurg. 2000;92:61–70. doi: 10.3171/spi.2000.92.1.0061. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson B, Petren-Mallmin M, Jonsson H, Jr, Andreasson I, Rauschning W. Pathoanatomical and radiographic findings in spinal breast cancer metastases. J Spinal Disord. 1995;8:26–38. [PubMed] [Google Scholar]
- 26.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 27.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien) 1998;140:957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 28.Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res Treat. 1991;18:27–32. doi: 10.1007/BF01975440. [DOI] [PubMed] [Google Scholar]
- 29.Landreneau FE, Landreneau RJ, Keenan RJ, Ferson PF. Diagnosis and management of spinal metastases from breast cancer. J Neurooncol. 1995;23:121–134. doi: 10.1007/BF01053417. [DOI] [PubMed] [Google Scholar]
- 30.LoRusso P. Analysis of skeletal-related events in breast cancer and response to therapy. Semin Oncol. 2001;28:22–27. doi: 10.1016/S0093-7754(01)90228-3. [DOI] [PubMed] [Google Scholar]
- 31.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-G. [DOI] [PubMed] [Google Scholar]
- 32.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/S0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 33.Miller DJ, Lang FF, Walsh GL, Abi-Said D, Wildrick DM, Gokaslan ZL. Coaxial double-lumen methylmethacrylate reconstruction in the anterior cervical and upper thoracic spine after tumor resection. J Neurosurg. 2000;92:181–190. doi: 10.3171/spi.2000.92.2.0181. [DOI] [PubMed] [Google Scholar]
- 34.Miller F, Whitehill R (1984) Carcinoma of the breast metastatic to the skeleton. Clin Orthop Relat Res:121–127 [PubMed]
- 35.Nishimura R, Nagao K, Miyayama H, Yasunaga T, Asao C, Matsuda M, Baba K, Matsuoka Y, Yamashita H, Fukuda M. Diagnostic problems of evaluating vertebral metastasis from breast carcinoma with a higher degree of malignancy. Cancer. 1999;85:1782–1788. doi: 10.1002/(SICI)1097-0142(19990415)85:8<1782::AID-CNCR19>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Ogihara S, Seichi A, Hozumi T, Oka H, Ieki R, Nakamura K, Kondoh T. Prognostic factors for patients with spinal metastases from lung cancer. Spine. 2006;31:1585–1590. doi: 10.1097/01.brs.0000222146.91398.c9. [DOI] [PubMed] [Google Scholar]
- 37.Oka H, Kondoh T, Seichi A, Hozumi T, Nakamura K. Incidence and prognostic factors of Japanese breast cancer patients with bone metastasis. J Orthop Sci. 2006;11:13–19. doi: 10.1007/s00776-005-0966-9. [DOI] [PubMed] [Google Scholar]
- 38.Okuyama T, Korenaga D, Tamura S, Maekawa S, Kurose S, Ikeda T, Sugimachi K. Quality of life following surgery for vertebral metastases from breast cancer. J Surg Oncol. 1999;70:60–63. doi: 10.1002/(SICI)1096-9098(199901)70:1<60::AID-JSO11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 39.Onimus M, Papin P, Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5:407–411. doi: 10.1007/BF00301969. [DOI] [PubMed] [Google Scholar]
- 40.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Mohiuddin M, Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 41.Petren-Mallmin M, Andreasson I, Nyman R, Hemmingsson A. Detection of breast cancer metastases in the cervical spine. Acta Radiol. 1993;34:543–548. doi: 10.3109/02841859309175404. [DOI] [PubMed] [Google Scholar]
- 42.Prie L, Lagarde P, Palussiere J, el Ayoubi S, Dilhuydy JM, Durand M, Vital JM, Kantor G. Radiotherapy of spinal metastases in breast cancer. Apropos of a series of 108 patients. Cancer Radiother. 1997;1:234–239. doi: 10.1016/s1278-3218(97)89770-3. [DOI] [PubMed] [Google Scholar]
- 43.Scheid V, Buzdar AU, Smith TL, Hortobagyi GN. Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer. 1986;58:2589–2593. doi: 10.1002/1097-0142(19861215)58:12<2589::AID-CNCR2820581206>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 44.Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favorable prognosis. Cancer. 1986;58:178–182. doi: 10.1002/1097-0142(19860701)58:1<178::AID-CNCR2820580130>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Metastatic breast cancer confined to the skeletal system. An indolent disease. Am J Med. 1986;81:381–386. doi: 10.1016/0002-9343(86)90286-X. [DOI] [PubMed] [Google Scholar]
- 46.Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: a prospective study. Neurosurgery. 1985;17:424–432. doi: 10.1097/00006123-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Sinardet D, Chabane A, Khalil T, Seigneuret E, Sankari F, Lemaire JJ, Chazal J, Irthum B. Neurological outcome of 152 surgical patients with spinal metastasis. Neurochirurgie. 2000;46:4–10. [PubMed] [Google Scholar]
- 48.Sundaresan N, Digiacinto GV, Hughes JE, Cafferty M, Vallejo A. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645–650. doi: 10.1097/00006123-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Sundaresan N, Galicich JH, Lane JM, Bains MS, McCormack P. Treatment of neoplastic epidural cord compression by vertebral body resection and stabilization. J Neurosurg. 1985;63:676–684. doi: 10.3171/jns.1985.63.5.0676. [DOI] [PubMed] [Google Scholar]
- 50.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 51.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 52.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 53.Weigel B, Maghsudi M, Neumann C, Kretschmer R, Muller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine. 1999;24:2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 54.York JE, Gokaslan ZL. Instrumentation of the spine in metastatic disease. Spine State Art Rev. 1999;13:335–350. [Google Scholar]