Abstract
Little is known about the natural history of scoliosis found in patients with syringomyelia, including the factors affecting scoliosis curve progression and the effect of syrinx drainage treatment. Twenty patients having scoliosis with syringomyelia diagnosed by MRI were followed up for 6.6 (range 2.0–12.6) years on an average. Various factors potentially influencing curve pattern or progression in these patients were then retrospectively reviewed. The convex side of major curve of scoliosis tended to be on the same side as the syrinx and as the unilateral neurologic abnormality. No correlation was found between the location and the size of the syrinx and the location and size of the major curve of the scoliosis, or between the severity of neurologic deficit and the size of the major curve of the scoliosis. In patients under the age of ten at the time of diagnosis of scoliosis and with a flexible curve, decompression of the syrinx improved or stabilized scoliosis. In most patients over the age of ten, surgical treatment of the scoliosis was necessary because of the large initial size of the curve or progression of the curve even after syrinx drainage. Other factors including gender, location of the syrinx, type of the curve, and severity of neurologic deficits did not correlate with the progression of the curve. The results of this retrospective study suggest that early diagnosis and decompression of a syrinx in scoliosis patients especially under the age of ten is crucial and may decrease the curve size and limit scoliosis curve progression.
Keywords: Scoliosis, Syringomyelia, MRI, Syrinx drainage
Introduction
Scoliosis is reported in 25–85% of syringomyelia cases [5, 13–15, 23]. Neurologic signs of syringomyelia include dissociated sensory loss of pain and temperature with sparing touch and proprioception, asymmetric deep tendon or superficial abdominal reflexes, and unilateral or bilateral muscle atrophy and weakness. However, these neurological signs have been reported to be frequently minimal or absent in patients with syringomyelia, even with a very large syrinx. Scoliosis with syringomyelia has been characterized by 44–50% incidence of left thoracic curves [7, 9, 15, 22], juvenile or skeletally immature patients [16], rapid curve progression [3, 18], less rotation than would otherwise be expected, and frequent association with headaches due to Chiari malformation.
Recently, with the aid of MRI, syringomyelia with minimal neurologic deficits can be easily diagnosed in patients with scoliosis. To date, little is known about relationship between syringomyelia and the curve pattern of the scoliosis. The natural history of the scoliosis, the factors affecting the curve progression and the effect of syrinx drainage upon the curve changes are still unclear. Therefore appropriate management of the scoliosis in syringomyelia patients remains controversial.
The purpose of this paper is to analyze the relationship between syringomyelia and the curve pattern of the scoliosis and to determine the factors affecting the curve changes after syrinx drainage.
Materials and methods
Among 27 patients diagnosed as scoliosis with syringomyelia at the first authors’ institution between 1988 and 2003, 20 with more than 2 years of follow-up were selected for analysis. Twelve patients among these 20 patients had a concomitant Chiari I malformation. Patients with post-traumatic syringomyelia, tumor associated syringomyelia, tethered cord syndrome, or congenital spinal deformities were excluded from analysis.
Twelve patients were boys and eight were girls. Scoliosis was the initial presenting symptom in 19 patients (95%) and was present in all cases at the initial examination. The age at diagnosis of scoliosis averaged 11.9 (range 2.8–23.3) years, and the age at diagnosis of syringomyelia averaged 12.3 (range 2.9–23.3) years. The mean duration of follow-up was 6.6 (range 2.0–12.6) years. Relationship between pattern of syrinx, neurologic deficits and pattern of curve was evaluated. Progression of scoliosis was analyzed on the basis of factors including age, gender, location of the syrinx, severity of neurologic deficit, type of the curve and syrinx decompression. Statistical analyses were done with SPSS 12.0 software (SPSS, Chicago, IL, USA).
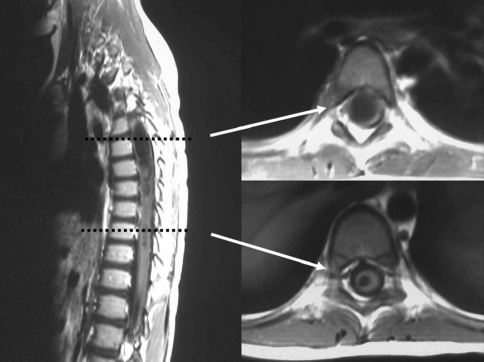
The location of syrinx was determined in sagittal view of MRI and the size and the side of syrinx in T1-weighted axial view of MRI. The sidedness of a syrinx was determined by lateralizing the syrinx at the upper and the lower end of syrinx on the assumption that syrinx is consisted of a single straight tube (Fig. 1). The sizes of the syrinxes at their widest level were classified as small if the syrinx was thin and slit-like, large when the diameter of syrinx was larger than the half of the AP diameter of the spinal cord, and medium for the others. There were two cases of small central syrinx which was not clearly distinguishable from hydromyelia.
Fig. 1.
Lateralizing a syrinx; at the both ends of a syrinx, the right side deviation of the large syrinx can be detected in T1-weighted axial view of a MRI
Results
The clinical–radiological features and outcomes of 20 patients are summarized in Tables 1 and 2.
Table 1.
Summary of clinical and radiological features
| Sex | Age at scoliosis Dx. | F/U (year) | Scoliosis type | Scoliosis level | Initial curve (°) | Chiari I | Syrinx size | Syrinx level | Syrinx side | Neurologic deficit | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 4.1 | 7.1 | Rt T | T7–L1 | 26 | None | Small | C3–C6 | Rt | Minor |
| 2 | M | 5.4 | 7.0 | Lt T | T7–L2 | 28 | None | Small | T1–T11 | Lt | Minor |
| 3 | M | 2.8 | 10.7 | Lt TL | T7–L3 | 31 | Yes | Large | C3–T8 | Lt | None |
| 4 | M | 3.8 | 8.5 | Lt T | T6–T12 | 29 | Yes | Large | C4–T10 | Lt | Minor |
| 5 | F | 4.8 | 8.0 | Rt T | T4–L1 | 49 | Yes | Large | C2–T6 | Rt | None |
| 6 | M | 9.8 | 7.0 | double T (Rt = Lt) | T2–T8 T8–L2 | 33/34 | Yes | Medium | C5–C7 | Rt | Rt sensory |
| 7 | F | 13.8 | 12.6 | Lt T | T5–T11 | 19 | Yes | Large | C2–T6 | Lt | Lt sensory motor |
| 8 | F | 12.2 | 9.8 | Lt T | T7–L1 | 28 | Yes | Large | Holocord | Rt | Rt motor |
| 9 | F | 13.8 | 5.9 | Lt T | T2–T8 | 43 | Yes | Medium | C2–T8 | Lt | Rt sensory |
| 10 | M | 15.3 | 3.8 | Lt T | T7–T12 | 50 | Yes | Large | Holocord | Lt | Lt sensory |
| 11 | F | 12.6 | 6.9 | Lt T | T3–T10 | 57 | Yes | Large | C4–T5 | Lt | None |
| 12 | F | 11.9 | 8.7 | Rt T | T8–L1 | 30 | Yes | Large | C2–T9 | Rt | Rt sensory motor |
| 13 | F | 11.9 | 6.1 | Rt T | T6–L1 | 28 | Yes | Small | C5–C7 | Central | Rt motor |
| 14 | M | 12.8 | 2.5 | Lt TL | T9–L2 | 44 | None | Medium | T3–L1 | Lt | Minor |
| 15 | M | 13.0 | 2.0 | Lt T | T6–L1 | 86 | Yes | Small | C6–T6 | Lt | Minor |
| 16 | M | 13.8 | 2.0 | Rt T | T7–L2 | 83 | None | Medium | Lower T | Lt | Rt sensory |
| 17 | M | 23.3 | 5.8 | double T (Rt > Lt) | T1–T7 T7–T12 | 63/71 | None | Small | C4–C7 | Rt | Rt motor |
| 18 | M | 15.7 | 2.0 | double T (Rt > Lt) | T5–T10 T10–L2 | 42/35 | None | Small | C6–T1 | Rt | Lt motor |
| 19 | M | 17.6 | 7.8 | double T (Rt < Lt) | T1–T5 T5–T12 | 72/59 | None | Small | Upper and lower T | Central | None |
| 20 | F | 20.4 | 7.0 | Rt T | T5–T11 | 75 | None | Small | C2–C6 | Rt | Rt sensory |
Table 2.
Outline of treatments and clinical outcomes
| Initial curve (°) | Age at syrinx drainage | Age at scoliosis surgery | Curve size at the time of syrinx drainage (°) | Curve size at the time of scoliosis surgery(°) | Curve size at immediate post. correction (°) | Final curve (°) | Treatment of syringomyelia | Treatment of scoliosis | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | NP | NP | NP | NP | NP | 29 | observation | TLSO |
| 2 | 28 | NP | NP | NP | NP | NP | 21 | observation | TLSO |
| 3 | 31 | 3.1 | NP | 31 | NP | NP | 33 | FMD | TLSO |
| 4 | 29 | 4.0/5.2 | NP | 30 | NP | NP | 5 | SPS + SSS | TLSO |
| 5 | 49 | 4.9 | NP | 49 | NP | NP | 7 | FMD | TLSO |
| 6 | 33/34 | 10.3 | NP | 33/33 | NP | NP | 42/34 | FMD | Observation |
| 7 | 19 | 13.9 | NP | 19 | NP | NP | 6 | FMD | Milwaukee brace |
| 8 | 28 | 12.4 | NP | 31 | NP | NP | 37 | VPS | Observation |
| 9 | 43 | 14.3 | 14.7 | 43 | 45 | 17 | 17 | FMD | Post. ICF |
| 10 | 50 | 15.3 | 16.3 | 50 | 52 | 18 | 18 | FMD | Post. ICF |
| 11 | 57 | 12.7/12.9 | 13.8 | 57 | 70 | 37 | 47 | FMD + SPS | Post. ICF |
| 12 | 30 | 12.2 | 14.4 | 30 | 66 | 18 | 31 | SSS + SOC | Post. ICF |
| 13 | 28 | NP | 16.7 | NP | 40 | 9 | 16 | Observation | Post. ICF |
| 14 | 44 | NP | 14.6 | NP | 45 | 17 | 18 | Observation | Ant. ICF |
| 15 | 86 | NP | 13.1 | NP | 88 | 22 | 27 | Observation | Post. ICF |
| 16 | 83 | NP | 13.9 | NP | 83 | 28 | 33 | Observation | Post. ICF |
| 17 | 63/71 | NP | 23.3 | NP | 62/72 | 50/50 | 45/50 | Observation | Post. ICF |
| 18 | 42/35 | NP | 15.8 | NP | 45/39 | 14/14 | 16/20 | Observation | Post. ICF |
| 19 | 72/59 | NP | 17.7 | NP | 72/59 | 53/34 | 47/36 | Observation | Post. ICF |
| 20 | 75 | NP | 20.8 | NP | 75 | 32 | 36 | Observation | AP ICF |
NP Operation not performed, FMD foramen magnum decompression, VPS ventriculoperitoneal shunt, SPS syringoperitoneal shunt, SSS syringosubarachnoidal shunt SOC; suboccipital craniectomy, ICF instrumented correction and fusion
Relationship between neurologic deficits and the convex side or size of the major curve of scoliosis
Sixteen patients (80%) had neurologic deficits; five had only minor deficits such as increased deep tendon reflexes or asymmetric superficial abdominal reflexes and the remainder (11) had rather significant unilateral neurologic deficit such as sensory and/or motor disturbances. In seven (64%) patients out of the 11 with unilateral sensory and/or motor disturbances, the convex side of major curve of scoliosis was on the same side as neurologic deficits. The presence or severity of the neurologic deficits (in terms of none, minor, unilateral neurologic deficit groups) did not correlate with the size of the major curve of scoliosis (Kruskal–Wallis test P > 0.05).
Relationship between the size of the syrinx and the size of the major curve of scoliosis
Eight patients (40%) had small syrinx on MRI according to our classification, four (20%) had medium one, and eight (40%) had large one. Syrinx with Chiari I malformation tended to be larger than that without malformation. There was no correlation between syrinx size and the size of the major curve of scoliosis (Spearman correlation test P > 0.05).
Relationship between the side of the syrinx and the convex side of the major curve of scoliosis
Syrinxes were located on the right side in eight patients, on the left side in ten, and centrally in two. The major curve of scoliosis was left thoracic in eight cases, left thoracolumbar in two, right thoracic in six, and double thoracic in four. The convex side of major curve was on the same side as the syrinx in 15 (83%) out of 18 patients in whom syrinx was located eccentrically (Fisher’s exact test P = 0.004).
Relationship between the location of the syrinx and the location of the major curve of scoliosis
Five lesions were located in the cervical spine, nine in the cervicothoracic spine, three in the thoracic spine, one in the thoracolumbar spine, and two in the whole spine. The major curve of scoliosis was thoracic in 18 cases, thoracolumbar in two cases. No correlation was found between the location of syrinx and that of the major curve of scoliosis (Fisher’s exact test P > 0.05).
Progression of scoliosis
Six patients were under age ten at the diagnosis of scoliosis. In four of the six patients, the syrinx was treated with neurosurgical decompression including decompression for Chiari I malformation or laminectomy with shunt, which resulted in neurological improvement and/or in decrease of syrinx size confirmed by MRI in all patients. The other two patients were observed without surgical intervention because of a small syrinx size (cases 1 and 2). Scoliosis was treated with TLSO in five of six cases and with simple observation without bracing in one case. The initial Cobb’s angles of these six patients averaged 33° (range 26°–49°). The average follow-up period was 8.0 (range 7.0–10.7) years, and the average age at the last follow-up was 13.2 (range 11.2–16.8) years. The last follow-up examination demonstrated that the Cobb’s angles improved in three cases and stabilized in two cases, averaging 20° (range 5°–33°), although these patients remains skeletally immature (Fig. 2). In one case in which the patient was skeletally matured at last follow-up (case 6), the curve improved for 4 years after syrinx decompression then progressed, but did not require surgical correction of the scoliosis—from 33° at 9.8 years of age, to 42° at 16.8 years.
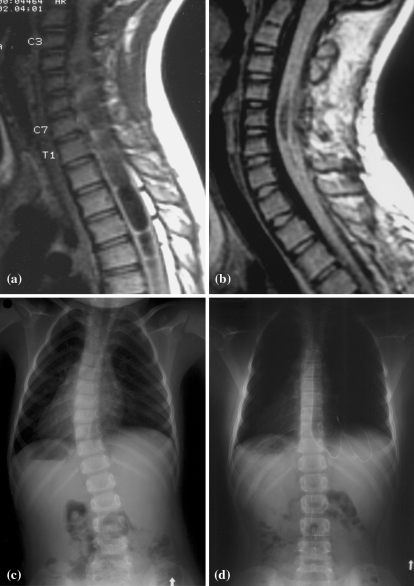
Fig. 2.
Case 4. MR images and radiographs of a 3.8-year-old boy with left thoracic scoliosis and minor neurological deficit. The size of the syrinx initially extending from C4 to T10 a was reduced significantly after syringoperitoneal and syringosubarachnoidal shunts b. The scoliosis (T6–T12), which measured 29° initially c, was gradually improved with TLSO to measure only 5° at the age of 12.3 years d
The other 14 patients were over the age of ten at the diagnosis of scoliosis. The initial Cobb’s angles of these patients averaged 52° (range 19°–86°). In six of the 14 patients, shunt for syrinx and/or decompression for Chiari I malformation was performed. Surgical intervention resulted in neurological improvement and/or in decrease of syrinx size confirmed by MRI in all six patients. In the other eight patients, syrinxes were observed without surgical intervention because of small syrinx size. Among the six patients over the age of ten who underwent surgical intervention, one (case 7) showed improvement of the curve—from 19° at 13.8 years of age to 6° at 26.2 years—after syrinx drainage, and one (case 8) showed acceptable progression—from 28° at 12.2 years of age to 37° at 22.0 years. The remaining four patients underwent surgical correction of scoliosis because of an initially large curve (over 45°) or continuing curve progression even after syrinx decompression. Among the latter eight patients in whom syrinx drainage was not carried out, seven underwent surgical correction of scoliosis due to an initially large curve (over 45°), and one because of curve progression. Therefore, a total of 12 patients (86%) out of 14 over the age of ten at initial diagnosis underwent surgical correction of scoliosis; three patients due to curve progression with or without syrinx decompression, and nine due to initially large curve. At the final follow up of the 12 patients who underwent surgical correction of scoliosis, six remained stable and six showed some loss of correction (range 5°–13°).
Other factors including gender (Fisher’s exact test P > 0.05), location of the syrinx (Kruskal–Wallis test P > 0.05), type of the curve (Fisher’s exact test or Kruskal–Wallis test P > 0.05), and severity of neurologic deficit (Kruskal–Wallis test P > 0.05) did not seem to be related to the progression of the curve.
Discussion
Most patients in our series had minimal neurologic signs regardless of the level or size of the syrinx. Therefore we agree with other authors [5, 9, 20, 22] that patients with atypical scoliosis such as left thoracic or scoliosis with an unusual appearance, a large curve at a young age, pain or neurologic deficits, less rotation than is expected, or headaches of unknown etiology should undergo a MRI to rule out syringomyelia.
The pathophysiology of the development of scoliosis with syringomyelia has not been yet established. Mass effect in the fetal life [11], damage to or influence on the anterior and/or posterior horn cells or roots [1, 6, 14, 15, 18, 24], and possible association with upper motor neuron signs [2] have been suggested. In the present series, most patients having motor disturbances showed essentially upper motor neuron signs as in the series of Farley et al. [9]. However, we are not certain whether the upper motor neuron signs had any relationship with the development of scoliosis in these patients, because their neurologic signs were minimal and some of our series had no neurologic findings at all. Interestingly, the side of syrinx and the side of unilateral sensory and/or motor disturbances was the same as the convex side of the major curve of scoliosis in many cases. As in other series [9, 13, 20, 22, 24], the presence/absence or severity of neurologic deficits and the size of the syrinx did not appear to be related to the magnitude and progression of the scoliotic curve. There was also no correlation between the location of the syrinx and the location of major curve.
Although many authors support syrinx drainage for neurologic improvement or stabilization [2, 5, 12, 17], the effect of syrinx drainage on the scoliosis curve progression is still controversial. Some authors have reported stabilization or improvement of scoliosis after syrinx drainage [16, 21, 22], yet other authors have reported questionable or no effect of drainage on curve progression [5, 9, 12, 20, 23]. Eule et al. [8] and Brockmeyer et al. [4] reported that suboccipital decompression of Chiari malformation with syringomyelia showed improvement or stabilization of the scoliosis in patients under age ten at the time of decompression. In contrast, patients older than 10 years old with a curve greater than 40° before suboccipital decompression have either been fused or are awaiting fusion. Özerdemoglu et al. [19] evaluated the effect of various treatment modalities for syringomyelia. They concluded that suboccipital craniectomy for Chiari I malformation gave the best chance for syrinx reduction and scoliosis improvement, particularly in children younger than 10 years and that syrinx shunting improved none of the scolioses. However, Phillips et al. [20] reported that drainage of the syrinx delayed but did not prevent curve progression. Farley et al. [10] also stated that in nine children with scoliosis and Chiari I, despite initial curve stabilization after suboccipital decompression, at final follow-up eight curves were of a magnitude that required spinal fusion and neither bracing nor secondary neurosurgical procedures could arrest the progression of the scoliosis curve. In the present series, syrinx drainage resulted in neurological improvement and/or in decrease of syrinx size in all patients, confirmed by MRI. But the effect of syrinx drainage on scoliosis curve progression depended mainly on the age of the patient and the flexibility of the curve. In patients under the age of ten at the diagnosis of scoliosis, decompression of the syrinx improved or stabilized scoliosis. Furthermore, two patients under the age of ten showed improvement or stabilization of scoliosis with simple bracing and even without decompression of the syrinx. However, in patients over the age of ten, decompression had no effect on curve progression except two patients having small and flexible scoliosis curves. Surgical correction of scoliosis by instrumentation and fusion was performed in 12 patients (86%) due to curve progression even after decompressing syrinx or because of initially large curve.
The greatest limitation of our study is that in patients under age ten, the follow-up length is relatively short. All patients except one still remain skeletally immature at last follow-up and will require close follow-up until they reach skeletal maturity in order to observe the improvement or progression of their curves.
We believe that if syrinx decompression is necessary due to large syrinx size or neurologic deficits, decompression must be achieved before surgical correction of scoliosis. Such decompression may decrease the risk of neurologic deterioration after scoliosis correction [20] and even stabilize or improve scoliosis, especially in patients under the age of ten or with flexible curves.
Conclusion
In patients of scoliosis with syringomyelia diagnosed under the age of ten and with a flexible curve, decompression of the syrinx improved or stabilized scoliosis. In most patients over the age of ten, surgical treatment of scoliosis was most likely necessary due to a large initial scoliosis curve or curve progression even after syrinx drainage. Other factors including gender, location of the syrinx, type of the curve, and severity of neurologic deficits did not correlate with the progression of the curve.
References
- 1.Alexander MA, Bunch WH, Ebbesson SO. Can experimental dorsal rhizotomy produce scoliosis? J Bone Joint Surg. 1972;54-A:1509–1513. [PubMed] [Google Scholar]
- 2.Alvisi C, Cerisoli M. Long term results of the surgical treatment of syringomyelia. Acta Neurochir. 1984;71:133–140. doi: 10.1007/BF01401158. [DOI] [PubMed] [Google Scholar]
- 3.Baker AS, Dove J. Progressive scoliosis as the first presenting sign of syringomyelia. J Bone Joint Surg. 1983;65-B:472–473. doi: 10.1302/0301-620X.65B4.6874721. [DOI] [PubMed] [Google Scholar]
- 4.Brockmeyer D, Gollogly S, Smith JT. Scoliosis associated with Chiari I malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine. 2003;28(22):2505–2509. doi: 10.1097/01.BRS.0000092381.05229.87. [DOI] [PubMed] [Google Scholar]
- 5.Charry O, Koop S, Winter R, Lonstein J, Denis F, Bailey W. Syringomyelia and scoliosis: a review of twenty-five pediatric patients. J Pediatr Orthop. 1994;14:309–317. doi: 10.1097/01241398-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Chuma A, Kitahara H, Minami S, Goto S, Takaso M, Moriya H. Structural scoliosis model in dogs with experimental induced syringomyelia. Spine. 1997;22:589–595. doi: 10.1097/00007632-199703150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Coonrad RW, Richardson WJ, Oakes WJ. Left thoracic curves can be different. Orthop Trans. 1985;9:126–127. [Google Scholar]
- 8.Eule JM, Erickson MA, O’Brien MF, Handler M. Chiari I malformation associated with syringomyelia and scoliosis: a twenty-year review of surgical and nonsurgical treatment in a pediatric population. Spine. 2002;27(13):1451–1455. doi: 10.1097/00007632-200207010-00015. [DOI] [PubMed] [Google Scholar]
- 9.Farley FA, Song KM, Birch JG, Browne R. Syringomyelia and scoliosis in children. J Pediatr Orthop. 1995;15:187–192. [PubMed] [Google Scholar]
- 10.Farley FA, Puryear A, Hall JM, Muraszko K. Curve progression in scoliosis associated with Chiari I malformation following suboccipital decompression. J Spinal Disord Tech. 2002;15(5):410–414. doi: 10.1097/00024720-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Gardner JW, Collis JS. Skeletal anomalies associated with syringomyelia, diastematomyelia, and myelomeningocele. J Bone Joint Surg. 1960;42-A:1265. [Google Scholar]
- 12.Ghanem IB, Londono C, Delalande O, Dubousset JF. Chiari I malformation associated with syringomyelia and scoliosis. Spine. 1997;22:1313–1318. doi: 10.1097/00007632-199706150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gurr KR, Taylor TK, Stobo P. Syringomyelia and scoliosis in childhood and adolescence. J Bone Joint Surg. 1988;70-B:159. [Google Scholar]
- 14.Huebert HT, Mackinnon WB. Syringomyelia and scoliosis. J Bone Joint Surg. 1969;51-B:338–343. [PubMed] [Google Scholar]
- 15.Isu T, Iwasaki Y, Akino M, Abe H. Hydrosyringomyelia associated with a Chiari I malformation in children and adolescents. Neurosurgery. 1990;26:591–597. doi: 10.1097/00006123-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lewonowski K, King JD, Nelson MD. Routine use of magnetic resonance imaging in idiopathic scoliosis patients less than eleven years of age. Spine. 1992;17:S109–S116. doi: 10.1097/00007632-199206001-00008. [DOI] [PubMed] [Google Scholar]
- 17.Logue V, Edwards MR. Syringomyelia and its surgical treatment—an analysis of 75 patients. J Neurol Neurosurg Psychiatr. 1981;44:273–284. doi: 10.1136/jnnp.44.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordwall A, Wikkelso C. A late neurologic complication of scoliosis surgery in connection with syringomyelia. Acta Orthop Scand. 1979;50:407–410. doi: 10.3109/17453677908989783. [DOI] [PubMed] [Google Scholar]
- 19.Özerdemoglu RA, Transfeldt EE, Denis F. Value of treating primary causes of syrinx in scoliosis associated syringomyelia. Spine. 2003;28(8):806–814. doi: 10.1097/00007632-200304150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Phillips WA, Hensinger RN, Kling TF. Management of scoliosis due to syringomyelia in childhood and adolescence. J Pediatr Orthop. 1990;10:351–354. doi: 10.1097/01241398-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta DK, Dorgan J, Findlay GF. Can hindbrain decompression for syringomyelia lead to regression of scoliosis? Eur Spine J. 2000;9:198–201. doi: 10.1007/s005860000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson RJ, Wolfe MW, Nadall JM, Bennett JT, MacEwen GD. Syringomyelia and developmental scoliosis. J Pediatr Orthop. 1994;14:580–585. doi: 10.1097/01241398-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Weber FA. The association of syringomyelia and scoliosis. J Bone Joint Surg. 1974;56-B:589. [Google Scholar]
- 24.Williams B. Orthopaedic features in the presentation of syringomyelia. J Bone Joint Surg. 1979;61-B:314–323. doi: 10.1302/0301-620X.61B3.158024. [DOI] [PubMed] [Google Scholar]