Abstract
Recombinant adeno-associated virus (rAAV) vectors were used in human trials as carriers of vaccines for HIV-1 after encouraging preclinical results. However, the clinical trials yielded disappointing results. Here we demonstrated that in mice, rAAV vectors expressing the gene encoding HIV-1 gag stimulated gag-specific CD8+ T cells, but these T cells failed to expand after a booster immunization with a replication-defective adenoviral (Ad) vector also expressing gag. We tested rAAV vectors of different serotypes expressing HIV-1 gag for induction of transgene product–specific CD8+ T cells and found that the immunoinhibitory effect of rAAV priming observed with different AAV serotypes was transgene product specific, was independent of the interval between prime and boost, and extended to boosts with vaccine modalities other than Ad vectors. rAAV vector–induced CD8+ T cells proliferated poorly, produced low levels of IFN-γ in response to gag stimulation, and upregulated immunoinhibitory molecules. These T cells did not protect efficiently against challenge with a surrogate pathogen. Finally, we showed that the impaired proliferative capacity of the T cells was caused by persistence of the antigen-encoding rAAV vectors and could be reversed by placing the CD8+ T cells in an antigen-free environment. Our data suggest that rAAV vectors induce functionally impaired T cells and could dampen the immune response to a natural infection.
Introduction
Vectors derived from adeno-associated viruses (AAVs) were initially developed for gene replacement therapy (1–3). They were shown to achieve sustained expression of the therapeutic protein in target tissues of experimental animals (4). This may be in part linked to the inability of recombinant AAV (rAAV) vectors to induce strong inflammatory responses or adaptive immune responses to the viral capsid antigens or the transgene product (5–7). Paradoxically, although rAAV vectors performed well in experimental animals as gene replacement vehicles, they were also shown to have merit as vaccine carriers (8–10).
AAVs are single-stranded DNA parvoviruses that infect both dividing and nondividing cells (11). AAVs do not cause disease. They are dependoviruses and rely on other viruses such as adenoviruses (Ads) or herpesviruses to complete their life cycle. AAV’s single-strand DNA genome of 4,700 nucleotides is flanked by 145 base palindromic inverted terminal repeat (ITR) elements (12, 13). The ITRs are minimally required in cis to generate rAAV vectors in which all other viral sequences are supplied in trans (14, 15). rAAV vectors thus do not introduce any of their viral genes into host cells.
Several AAV serotypes have been isolated from primates (16, 17), as well as other animals, and have been vectored and tested as gene delivery vehicles (18). The various AAV serotypes display different tissue and cell tropism in vivo and in vitro, and vectors based on AAV1 and AAV7, which efficiently transduce skeletal muscle (19), may have advantages over others that favor different target tissues (20–22).
Aside from efficient gene delivery for permanent gene replacement therapy, some of their characteristics make AAVs potentially attractive candidates as vaccine carriers. rAAV vectors used in clinical gene therapy trials were well tolerated (2, 23). There are several serotypes that could allow for heterologous prime/boosting. Also, because rAAV vectors fail to encode viral antigens, induction of CD8+ T cell–mediated responses against the vaccine carrier should be minimal.
In this study, we tested rAAV vectors expressing a codon-optimized truncated gag (gag37; ref. 24) of HIV-1 in prime/boost regimens with other vaccine vectors, such as replication-defective Ad vectors, carrying the same transgene. The gag-specific CD8+ T cells primed with the rAAV vaccines failed to efficiently expand upon subsequent restimulation with their cognate antigen supplied by different types of vaccines. Our data indicate that the CD8+ T cells induced in response to the transgene products of rAAV vectors are impaired and may thus potentially harm rather than benefit the recipients of rAAV vaccines.
Results
Vectors based on different serotypes of AAV elicit transgene product–specific CD8+ T cells.
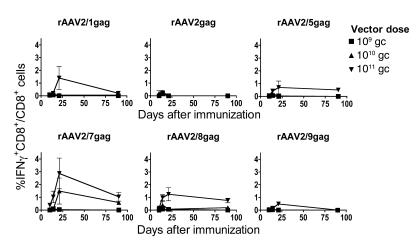
rAAV2 vectors pseudotyped with viral capsids from AAV serotypes 1, 2, 5, 7, 8, and 9 and carrying a truncated gag gene of HIV-1 clade B were tested for induction of transgene product–specific CD8+ T cells in mice. Groups of BALB/c mice were intramuscularly (i.m.) immunized in the lower leg with 109, 1010, or 1011 genome copies (gc) of each vector. Splenocytes were then harvested at different time points and analyzed by intracellular cytokine staining (ICS) to determine the frequency of gag-specific IFN-γ–producing CD8+ T cells (Figure 1). T cell frequencies were highest on days 20–21 for most of the vectors at the highest dose (rAAV2/1, rAAV2/5, rAAV2/7, rAAV2/8, and rAAV2/9), while the responses were better at around day 14 for the poorly immunogenic rAAV2 vectors or the other vectors given at lower doses. For most vectors, responses were sustained and still detectable 3 months after immunization. The highest frequencies of gag-specific CD8+ T cells were obtained with rAAV2/7 vectors, and we therefore focused on this construct for most of the experiments, although key experiments were confirmed with other serotypes.
Figure 1. Vectors based on different AAV serotypes induce gag-specific CD8+ T cell responses.
Groups of BALB/c mice were immunized with rAAV2 vectors carrying HIV-1 gag and pseudotyped with viral capsids from AAV1, AAV2, AAV5, AAV7, AAV8, and AAV9 at 3 different doses: 109, 1010, and 1011 gc. At various time points (days 10, 14, 21, and 80 shown here), splenocytes were stimulated with the AMQMLKETI peptide and analyzed for gag-specific IFN-γ–producing CD8+ T cells by ICS. Frequencies shown are representative of 3 experiments. Background frequencies (less than 0.1%) were subtracted prior to plotting. Error bars represent SD for 3 mice per group.
CD8+ T cells induced by rAAV vectors fail to expand upon reencounter of antigen.
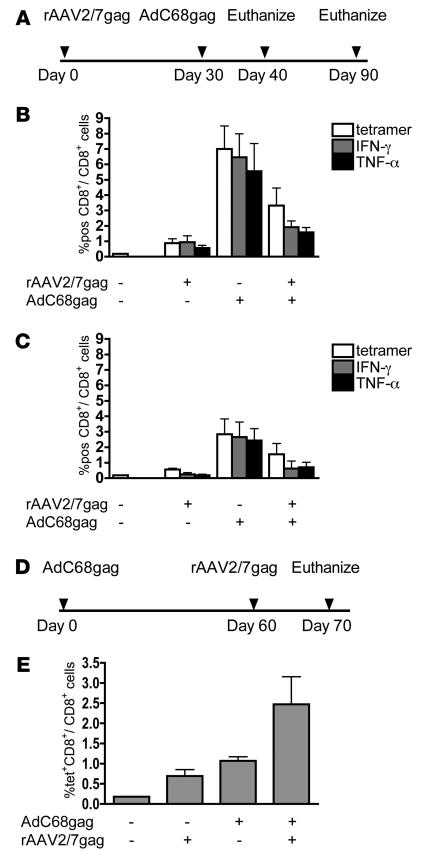
Because rAAV vectors induced only modest frequencies of gag-specific CD8+ T cells, we attempted to increase responses by a second immunization with a different vaccine carrier expressing the same antigen (Figure 2). rAAV2/7gag-primed mice were boosted with a chimpanzee-derived Ad vector, Ad chimpanzee serotype 68 (AdC68) (25, 26), that also expressed a codon-optimized truncated gag (AdC68gag). The first group of mice was primed with rAAV2/7gag at 1011 gc and not boosted. The second group received no priming with rAAV but a single immunization with 1010 viral particles (vp) AdC68gag vector. The third group was primed with rAAV2/7gag at 1011 gc and 1 month later boosted with AdC68gag at 1010 vp (Figure 2A). At 10 days (Figure 2B) and 2 months (Figure 2C) after AdC68 boost, lymphocytes from spleens were analyzed by ICS for gag-specific IFN-γ– and TNF-α–producing CD8+ T cells as well as gag-tetramer–binding (gag-tet–binding) CD8+ T cells. The mice receiving a single rAAV2/7gag immunization had only low levels of gag-specific IFN-γ–producing CD8+ T cells (0.9% at day 10 and 0.2% at month 2). The percentages of gag-specific TNF-α–producing CD8+ T cells and gag-tet CD8+ T cells in spleens from this group of mice were similar. Frequencies of gag-specific CD8+ T cells in mice immunized with AdC68gag were 6.5% at day 10 and 2.7% at month 2. Whereas a prime/boost regimen is supposed to elicit increased frequencies of antigen-specific CD8+ T cells that exceed those from a single immunization, mice primed with rAAV2/7gag and then boosted with AdC68gag had lower responses than did mice immunized with the AdC68gag vaccine alone. Mice receiving the 2 vaccines in a prime/boost regimen analyzed 2 months after the AdC68 immunization exhibited a similar pattern of lower responses. This lack of expansion of gag-specific CD8+ T cells upon booster immunization was also observed upon priming with vectors based on other AAV serotypes, i.e., AAV2, AAV2/5, and AAV2/8 (data not shown). Furthermore, rAAV2/7gag-induced CD8+ T cells produced significantly less IFN-γ per cell upon in vitro stimulation with their cognate antigen than did AdC68gag-induced CD8+ T cells (P < 0.01; data not shown). When we reversed the sequence of the vectors (i.e., immunized with 109 vp AdC68gag first, then with 1011 gc rAAV2/7gag; Figure 2D), frequencies of IFN-γ–producing gag-specific CD8+ T cells increased following the boost (Figure 2E), although any Ad boost given after the rAAV vaccine failed to further increase frequencies (data not shown). The latter results suggested that antigen presented by rAAV vectors not only elicited an impaired primary CD8+ T cell response, but also impaired a recall response of memory CD8+ T cells. It should be noted that in all experiments, overall numbers of gag-specific CD8+ T cells correlated to frequencies because neither rAAVs nor Ad vectors caused splenomegaly or a significant shift in the ratio of different lymphocyte populations.
Figure 2. Transgene product–specific CD8+ T cells fail to expand in response to a booster immunization.
(A) BALB/c mice were primed i.m. with 1011 gc AAV2/7gag, and then 1 month later boosted i.m. with 1010 vp AdC68gag. Control mice were immunized with only the rAAV vaccine or only the AdC68 vaccine. (B and C) At 10 days (B) and 2 months (C) after the AdC68 immunization, splenocytes were stained with an antibody to CD8 and a gag-specific tet. Splenocytes were also stimulated with the AMQMLKETI peptide and analyzed by ICS for frequencies of gag-specific IFN-γ– and TNF-α–producing CD8+ T cells. (D and E) The order of immunizations was switched (D), and gag-tet–specific CD8+ T cell frequencies were determined by flow cytometry (E). Background frequencies (less than 0.1%) were subtracted prior to plotting. Frequencies shown are representative of at least 3 independent experiments. Error bars represent SD for 5 mice per group.
Lack of expansion of rAAV vector–induced CD8+ T cells is transgene product specific.
To determine whether the lack of expansion of CD8+ T cells in rAAV-immunized mice is restricted to those responding to the transgene product of the vector, mice were immunized with 1011 gc rAAV2/7gag or rAAV2/7 expressing GFP (rAAV2/7GFP). Two months later, a subset of mice was boosted with 1011 vp AdC68gag. Control mice were only immunized with AdC68gag. At 10 days after the AdC68gag boost, splenocytes were analyzed by ICS for gag-specific CD8+ T cells producing IFN-γ (Figure 3A). The mice that were immunized with both rAAV2/7gag and AdC68gag had lower frequencies of gag-specific CD8+ T cells than did those immunized with AdC68gag only. In contrast, mice that were first injected with AAV2/7GFP and then with AdC68gag showed CD8+ T cell frequencies that were comparable to those of mice that only received AdC68gag.
Figure 3. Proliferative impairment of rAAV-induced transgene product–specific CD8+ T cells.
(A) Splenocytes of BALB/c mice immunized with 1011 gc AAV2/7gag or AAV2/7GFP and boosted 2 months later with 1011 vp AdC68gag were analyzed by ICS at 10 days after AdC68gag boost. (B) Thy1.2+ BALB/c mice were immunized with 1011 gc AAV2/7gag or 1010 vp AdC68gag; 1 month later splenic CD8+ cells were stained with 5 μM CFSE and transferred to Thy1.1+ mice that had been immunized i.p. with Vacgag (open histogram) or VacGFP (filled histogram). Three days later, splenocytes were stained with antibodies to CD8 and Thy1.2 and the gag-specific tet and analyzed for levels of CFSE in gag-tet+CD8+Thy1.2+ T cells. (C) Mice were primed with 1011 gc AAV2/7gag, followed 2 months later by 1010 vp AdC68gag or 200 μg gag DNA vaccine and additionally boosted 2 months later with 1010 vp AdHu5gag or 106 PFU MVAgag; controls received no rAAV. Ten days after the second boost, splenocytes were analyzed by ICS. (D) Mice were primed with 1011 gc rAAV2/7gag and boosted 2, 4, 7, 24, and 28 weeks later with 1010 vp AdC68gag; controls received no rAAV. At 10 days after the boost, splenocytes were analyzed by ICS. (E) Mice were immunized with different doses of AAV2/7gag and 1 month later boosted with 1010 vp AdC68gag; controls received no rAAV. At 10 days after the boost, splenocytes were analyzed by ICS. In A and C–E, the frequency of gag-specific CD8+ T cells that produced IFN-γ in response to the gag peptide is shown. Background frequencies (less than 0.1%) were subtracted before plotting. Error bars represent SD for 5 mice.
To further demonstrate that rAAV-induced CD8+ T cells have impaired proliferative capacity, we conducted an in vivo CFSE proliferation assay (Figure 3B). CD8+ T cells were isolated from rAAV2/7gag- or AdC68gag-immunized BALB/c (Thy1.2) mice, labeled with CFSE, and transferred into naive Thy1 congenic recipient mice. Recipients (Thy1.1) had been immunized with a vaccinia virus vector expressing gag (Vacgag) 24 hours prior to the adoptive transfer. Control recipients were immunized with a vaccinia vector expressing GFP (VacGFP). Three days later, splenocytes from all recipients were harvested and stained for the donor Thy1.2 phenotype and CD8 and with the gag-tet and analyzed for decrease in CFSE intensity as a parameter for cell division. rAAV-induced gag-specific CD8+ T cells, unlike those induced by AdC68, proliferated poorly upon encountering gag antigen in Vacgag-injected recipient mice (Figure 3B).
rAAV-induced CD8+ T cells fail to expand upon repeated booster immunizations with different vaccine modalities.
To test whether the inability of rAAV-induced CD8+ T cells to expand in vivo to antigenic stimulation is restricted to antigens presented by Ad vectors, other vaccine modalities were tested. Mice primed with the rAAV2/7gag vector either were boosted with a DNA vaccine expressing gag (DNAgag) and then again with a modified vaccinia ankara vector expressing gag (MVAgag), or received sequentially in 2-month intervals AdC68gag and then an Ad vector of human serotype 5 expressing gag (AdHu5gag). Animals were primed with 1011 gc rAAV2/7gag, boosted with either 200 μg DNAgag followed by 106 PFU MVAgag or 1010 vp AdC68gag followed by 1010 vp AdHu5gag. Ten days after the last immunization, splenocytes were tested by ICS for gag-specific IFN-γ–producing CD8+ T cells (Figure 3C). While the groups of mice that did not receive the initial priming with the rAAV2/7gag vector exhibited high frequencies (20%–30%) of gag-specific IFN-γ–producing CD8+ T cells upon either of the booster immunization protocols, mice that were primed with rAAV2/7gag, independent of the type of the subsequent boost, had significantly lower CD8+ T cell responses (P < 0.05). These data show that the poor proliferative capacity of rAAV-induced CD8+ T cells was not rescued by different types of immunogens and raises the possibility that upon natural infection, rAAV-induced CD8+ T cells may expand very poorly.
Lengthening the time interval between priming with rAAV and boosting with an Ad vector does not rescue the proliferative capacity of rAAV-induced CD8+ T cells.
While the CD8+ T cell response to gag delivered by AdC68 peaked around days 10–14, the responses to the rAAV-delivered gag transgene product peaked later. This could be due to the relatively longer time required for the rAAV vectors’ single-stranded DNA to convert into double-stranded DNA before transcription commences. To test whether the kinetics of the response affected antigen-driven T cell expansion, we explored different time intervals between priming and boosting. Groups of mice were primed as described above with 1011 gc rAAV2/7gag and then boosted 2–28 weeks later with 1010 vp AdC68gag. Ten days after boosting, splenocytes were analyzed for gag-specific IFN-γ–producing CD8+ T cells by ICS (Figure 3D). Lengthening the interval between vaccinations had no effect on the CD8+ T cell response, which was significantly lower in rAAV2/7gag-primed mice than in recipients of a single immunization of AdC68gag at all time points.
High doses of the rAAV vector are required to impair the proliferative capacity of the rAAV-induced CD8+ T cells.
We tested whether the lack of proliferative capacity of rAAV-induced transgene product–specific CD8+ T cells depended on the dose of the rAAV vector. Mice were primed with 109 gc, 1010 gc, and 1011 gc rAAV2/7gag and then 1–2 months later boosted with 1010 vp AdC68gag. Ten days after the last immunization, splenocytes were analyzed to determine the frequency of gag-specific IFN-γ–producing CD8+ T cells by ICS (Figure 3E). The CD8+ T cell frequency in mice primed with the lowest rAAV dose (109 gc) was similar to that in mice receiving only AdC68gag immunization (P > 0.05). Frequencies in mice primed with 1010 gc and 1011 gc rAAV2/7gag and then boosted with AdC68gag were significantly lower than those in mice receiving the single AdC68gag immunization (P < 0.05). A second experiment using 106-, 107-, and 108-gc doses of rAAV in a similar prime/boost regimen also showed that lower doses of rAAV failed to reduce the CD8+ T cell response to the AdC68gag vector (data not shown). At none of the doses tested did priming with the rAAV2/7gag vector enhance the response to the AdC68gag vector.
rAAV-induced CD8+ T cells are lytic but only provide partial protection in a challenge model.
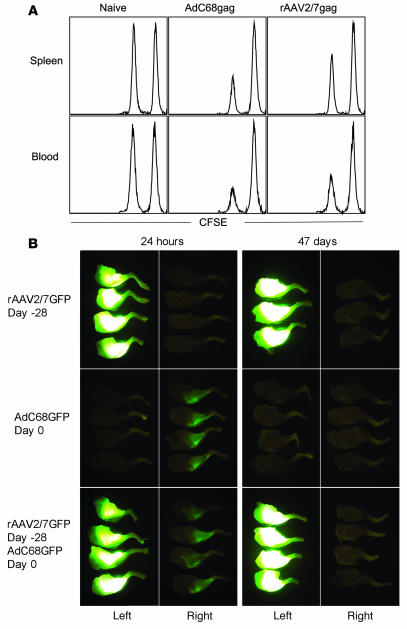
Further characterization of rAAV-induced CD8+ T cells revealed that they were partially functional: an in vivo killing assay showed that cells pulsed with the gag peptide were lysed when adoptively transferred into recipient mice that had been immunized prior with rAAV2/7gag (Figure 4A). The relative level of killing was lower in the rAAV2/7gag-immunized mice than in the AdC68gag-immunized mice. This may have been due to the higher frequencies of gag-specific CD8+ T cells in AdC68gag-immunized mice. Although rAAV-induced CD8+ T cells lysed gag peptide–pulsed splenocytes in vivo, they failed to eliminate rAAV-transduced muscle cells (Figure 4B). This was shown upon injection of mice with rAAV2/7GFP into the left leg, followed 4 weeks later by injection of AdC68GFP into the right leg. It should be noted that GFP carries an epitope that is recognized by CD8+ T cells of the H-2d haplotype (27). Muscle imaging 24 hours after the last immunization (i.e., 29 days after injection of the rAAV vector) revealed that both legs expressed GFP, although expression was markedly higher in the leg injected with the rAAV2/7GFP vector. When tested 47 days later, the leg that was immunized with AdC68GFP was no longer fluorescent, while the leg immunized with the rAAV2/7GFP vector expressed GFP at apparently undiminished levels. The experimental design does not allow for distinguishing whether the AdC68GFP-transduced cells had been eliminated by GFP-specific CD8+ T cells or by CD8+ T cells directed to antigens of Ad. Nevertheless, these results clearly show lack of clearance of the rAAV2/7GFP-transduced muscle cells.
Figure 4. rAAV-induced CD8+ T cells are lytic.
(A) In an in vivo killing assay, splenocytes harvested from naive BALB/c mice were either pulsed with the gag AMQMLKETI peptide and stained with 0.5 μM CFSE or pulsed with an irrelevant peptide and stained with 5 μM CFSE, then adoptively transferred via tail vein injection into BALB/c mice that were nonimmunized or immunized with 1011 gc rAAV2/7gag or 1010 vp AdC68gag 2 months prior to the transfer. At 24 hours after the transfer, splenocytes and peripheral blood lymphocytes were harvested from recipient mice and analyzed by flow cytometry. Levels of CFSE expression are shown. (B) In the muscle imaging study, mice were immunized with 1011 gc rAAV2/7GFP in the left leg and 27 days later immunized with 1011 vp AdC68GFP in the right leg; control mice received either rAAV2/7GFP only or AdC68GFP only (n = 3–4 per group). At 24 hours and 47 days after the AdC68GFP immunization, mice were killed and legs were removed for imaging.
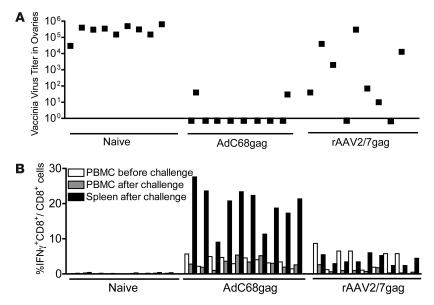
The functionality of rAAV-induced CD8+ T cells was further tested in challenge experiments in which rAAV2/7gag- or AdC68gag-immunized mice were injected i.p. with Vacgag to allow for its replication in the animals’ ovaries (28). A relatively low dose of AdC68gag was used for these experiments to ensure that frequencies of gag-specific CD8+ T cells induced by either vaccine were comparable. Naive mice were used as negative controls. Mice were bled before challenge to determine frequencies of gag-specific CD8+ T cells (Figure 5B). At 5 days after challenge the mice were sacrificed, and Vacgag titers in ovaries were determined (Figure 5A). In addition, at the same time, frequencies of gag-specific CD8+ T cells were measured from blood and spleens to determine whether the challenge caused their expansion (Figure 5B). Immunization with AdC68gag induced complete protection in 8 of 10 vaccinated mice, which had no detectable titers of Vacgag. The remaining 2 mice, which had the lowest frequencies of gag-specific CD8+ T cells in their blood before challenge, had low titers; protection compared with naive mice was statistically significant (P < 0.0001; Figure 5B). Upon challenge, frequencies of AdC68gag-induced CD8+ T cells did not increase in blood, while frequencies in the spleen were high. This probably reflects that T cell frequencies were measured fairly early, i.e., 5 days after the challenge, before the reactivated T cells circulated through the blood. In the rAAV2/7gag-primed group of 9 mice, 3 animals eliminated Vacgag to levels below detection, 4 animals were partially protected and had lower titers than did the control animals, and 2 animals had titers that were comparable to those in unvaccinated animals; protection compared with naive mice was statistically significant (P < 0.01). Overall, the level of clearance of vaccinia virus correlated with CD8+ T cell frequencies before challenge. Nevertheless, several of the rAAV2/7gag-vaccinated mice that had prechallenge frequencies of circulating gag-specific CD8+ T cells similar to those in AdC68gag-immunized mice cleared the infection less efficiently. The frequency of gag-specific CD8+ T cells in the blood of rAAV-immunized mice tested 5 days after challenge was below that seen before challenge. In the AdC68gag-vaccinated group, the frequency in spleen was higher than that in blood. In some animals in the rAAV-immunized group, the frequency in spleen exceeded that in blood before challenge, indicating some expansion, while in other animals frequencies were similar to or below those prior to challenge. None of the rAAV2/7gag-vaccinated mice developed the high frequencies of splenic gag-specific CD8+ T cells seen in all the AdC68gag-vaccinated mice.
Figure 5. rAAV-induced gag-specific CD8+ T cells provide partial protection against challenge with Vacgag.
(A) To test for protection, groups of nonimmunized mice and mice immunized with 1011 gc rAAV2/7gag or 109 vp AdC68gag were challenged 1 month after vaccination with Vacgag. After 5 days, ovaries were harvested and vaccinia virus titers were determined. (B) PBMCs prior to challenge and PBMCs and splenocytes following challenge were analyzed by ICS for frequencies of gag-specific IFN-γ–secreting CD8+ T cells. Background frequencies (less than 0.1%) were subtracted prior to plotting. Data are representative of 3 repeated experiments.
rAAV vectors efficiently prime transgene product–specific antibody responses.
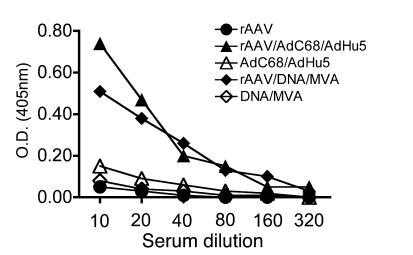
To determine whether priming with rAAV vectors also impaired transgene product–specific antibody responses, sera from animals as described above (mice that did or did not receive rAAV2/7gag followed by 2 sequential immunizations with DNAgag and MVAgag or AdC68gag and AdHu5gag; see Figure 3C) were tested for antibodies to gag by ELISA (Figure 6). Mice primed with rAAV2/7gag followed by either of the 2 booster modalities produced more antibodies to gag than did mice that were not primed with rAAV. Gag-specific antibodies were mainly of IgG2a and IgG1 isotypes, suggesting that the response was T helper cell dependent (data not shown).
Figure 6. rAAV priming increases the transgene product–specific antibody response to subsequent booster immunizations.
Sera collected from the same mice described in Figure 3C were tested for gag-specific antibodies by ELISA.
Regulatory CD4+ T cells are induced by rAAV but do not affect the proliferative capacity of rAAV-induced CD8+ T cells.
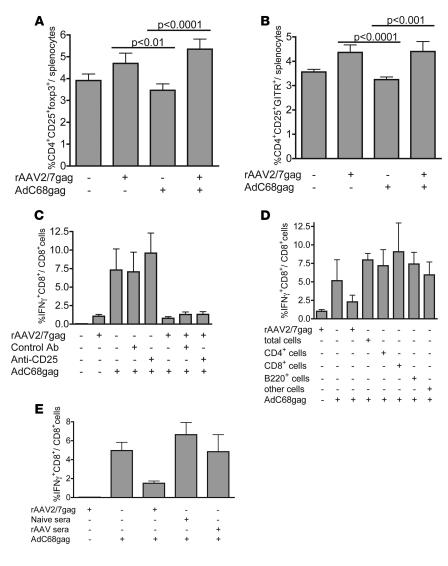
The impairment of the proliferative ability of the transgene product–specific CD8+ T cells induced by rAAV vectors may be attributed to an in vivo suppression mechanism. Previous gene therapy studies have shown that regulatory CD4+ T cells induce tolerance to the transgene product (29, 30). To test for activation of regulatory cells, splenocytes of BALB/c mice immunized with the rAAV2/7gag and AdC68gag vaccine regimens were analyzed for changes in the CD4+ population. Mice that were immunized with rAAV2/7gag with and without a booster vaccine showed a small increase in CD4+CD25+ cells expressing the transcription factor FoxP3, indicative of naturally occurring CD4+ regulatory T cells (Figure 7A). CD4+ regulatory T cells constitutively express CD25 and glucocorticoid-induced TNF receptor (GITR) on the cell surface, and we found a correlating increase of CD4+CD25+GITR+ splenocytes in rAAV-immunized mice (Figure 7B).
Figure 7. rAAV vectors increase regulatory cells, which do not cause impairment of the proliferative capacity of rAAV-induced CD8+ T cells.
(A and B) BALB/c mice were immunized with 1011 gc rAAV2/7gag and 1 month later boosted with 1010 vp AdC68gag; control mice received rAAV2/7gag or AdC68gag. At 10 days after the boost, splenocytes were analyzed for percentage of CD4+CD25+FoxP3+ cells (A) and CD4+CD25+GITR+ cells (B). (C) Mice immunized with 1011 gc rAAV2/7gag were treated 3 days before the 1010 vp AdC68gag boost with 200 μg antibody to CD25 i.p.; control mice were treated with 200 mg of control antibody. (D) For adoptive transfer, 2 × 106 CD4+, CD8+, B220+, or other cells were sorted from splenocytes of BALB/c mice immunized with 1011 gc rAAV2/7gag 1 month earlier and injected through the tail vein into naive mice. Recipient mice were immunized 1 day later with 1010 vp AdC68gag; control mice did not receive cells but did receive the 1011 gc rAAV2/7gag prime and 1010 vp AdC68gag boost. (E) Serum (200 μl) from naive mice or mice receiving an immunization of 1011 gc rAAV2/7gag was transferred i.v. into naive mice, and then 1 day later recipient mice were immunized with 1010 vp AdC68gag vector; control mice received no serum but were immunized with 1011 gc rAAV2/7gag followed by a 1010 vp AdC68gag boost. At 10 days after the boost, splenocytes were analyzed by ICS. In C–E, frequency of gag-specific IFN-γ–secreting CD8+ T cells is shown. Background values (less than 0.1%) were subtracted prior to plotting. Error bars represent SD for 5 mice.
We tested whether CD4+CD25+ cells contribute to the impairment of rAAV-induced CD8+ T cells. rAAV2/7gag-immunized mice were treated with PC61, an antibody to CD25, to functionally inactivate the CD4+CD25+ regulatory cells (31) prior to the AdC68gag boost. Control mice were treated with HRPN, a control IgG1 antibody. The frequency of gag-specific IFN-γ–producing CD8+ T cells, as determined by ICS, in mice primed with rAAV2/7gag and then treated with PC61 prior to AdC68gag boost was similar to that in untreated mice and in mice with the same vaccine regimen treated with the HRPN control antibody (Figure 7C). The proliferative capacity of rAAV-induced CD8+ T cells could thus not be rescued by inactivation of CD25+ T cells. To further confirm these results we conducted a number of adoptive transfer experiments in which CD4+CD25+ T cells from mice immunized with rAAV2/7gag were transferred into naive recipient mice, which were then immunized with AdC68gag. Adoptive transfer did not negatively affect the CD8+ T cell response to the AdC68gag vector (data not shown).
Other cell subsets may have played an inhibitory role. However, adoptive transfer of even high numbers (108 per recipient) of unsorted splenocytes or subsets of cells, including B220+ cells, CD8+ cells, CD4+ cells, and all other cells from spleens of rAAV2/7gag-immunized mice, did not impair the expansion of transgene product–specific CD8+ T cells in recipient mice upon subsequent immunization with AdC68gag (Figure 7D). In addition, serum from rAAV2/7gag-immunized mice transferred into recipient mice also did not affect the recipients’ CD8+ T cell responses to subsequent immunization with AdC68gag (Figure 7E).
Coimmunization with adjuvant does not restore proliferative capability.
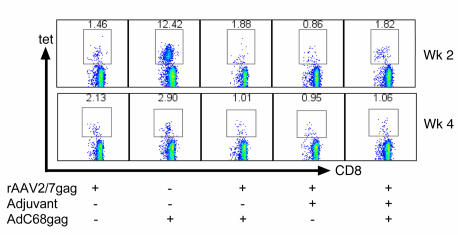
rAAV vectors do not induce a strong inflammatory response in vivo, and in vitro experiments have shown that they fail to drive maturation of immature DCs (32), which in turn are viewed as a prerequisite for induction of primary cell-mediated immune responses (33). To bypass potential lack of DC maturation upon rAAV vaccination, we immunized mice with rAAV2/7gag mixed with adjuvants that can drive DC maturation, such as incomplete Freund’s adjuvant (34) (Figure 8) or poly(I:C) (data not shown), a TLR-3 ligand. Mice were then boosted with AdC68gag 1 month later, and blood lymphocytes were analyzed 2 and 4 weeks after boost for frequencies of gag-tet+CD8+ T cells. Although mice receiving rAAV2/7gag with incomplete Freund’s adjuvant developed slightly higher frequencies than did mice that received rAAV2/7gag alone, there was no detectable increase in frequencies after the boost. Similar results were obtained with mice immunized with rAAV2/7gag mixed with poly(I:C).
Figure 8. Coimmunization with adjuvant does not restore proliferative capability.
Groups of BALB/c mice were immunized with 1011 gc rAAV2/7gag mixed in a 1:1 ratio with incomplete Freund’s adjuvant. Mice were then boosted with 1010 vp AdC68gag 1 month later, and blood was collected 2 and 4 weeks after the boost. Shown are density plots of live cells stained with an antibody to CD8 (x axis) and the gag-specific tet (y axis). Numbers within plots denote tet+CD8+ cells as a percentage of CD8+ cells.
Transgene product–specific CD8+ T cells induced by rAAV immunization express markers indicative of exhaustion.
It was shown previously in the lymphocytic choriomeningitis virus (LCMV) mouse model that continual stimulation of CD8+ T cells may lead to their functional exhaustion (35, 36). During chronic LCMV infection, CD8+ T cells cease to be lytic and lose their ability to secrete cytokines and proliferate upon antigenic stimulation (35). Although rAAV-induced CD8+ T cells did not show the same loss of functions as do exhausted T cells that develop upon infection with persisting strains of LCMV, their inability to proliferate raises the possibility of partial exhaustion.
To further explore whether rAAV-induced CD8+ T cells express markers indicative of exhaustion, gag-specific CD8+ T cells from mice immunized with rAAV2/7gag, AdC68gag, or both given sequentially were stained with a tet specific for an epitope of gag as well as antibodies to cell surface markers CD8, CD62L, CD27, CD127, CD44, program death–1 (PD-1), B and T lymphocyte attenuator (BTLA), and CD244 and to intracellular markers CLTA-4, Bcl-2, granzyme B, and Ki-67. Cells were analyzed both early after immunization, to determine the phenotype of effector cells, and later after immunization, to determine phenotypes at the memory stage. Expression of CD44, a cell surface glycoprotein involved with lymphocyte activation (37, 38), on tet+CD8+ T cells from the different mice was indistinguishable (data not shown). Expression of CD27 (T cell activation marker; ref. 39), CD127 (IL-7 receptor; refs. 40, 41), Bcl-2 (a mitochondrial membrane antiapoptotic protein), and Ki67 (proliferation marker) on or in the tet+CD8+ T cells was also not noticeably distinct between the groups (data not shown). CD62L downregulated in effector or effector memory cells (42) was high on rAAV2/7gag- and AdC68gag-induced CD8+ T cells even when tested months after immunization (data not shown). CD62L that is rapidly upregulated on CD8+ T cells stimulated by an acute infection, such as with LCMV, emerge very slowly on AdC68-induced CD8+ T cells because Ad vectors persist at low levels for prolonged times in lymphocytes, which delays progression of CD8+ T cells into central memory (43).
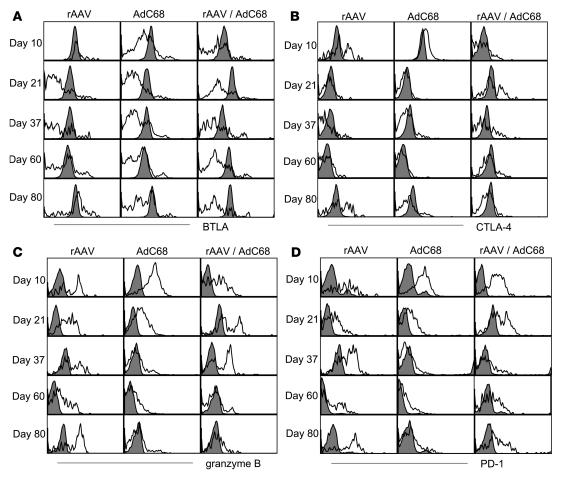
BTLA, which is constitutively expressed on CD4+ and CD8+ T cells and becomes upregulated upon activation (44–46), as well as CTL-associated protein–4 (CTLA-4), a protein that inhibits costimulation and modulates clonal expansion and differentiation (47–49), were markedly higher on all (BTLA; Figure 9A) or a portion (CTLA-4; Figure 9B) of rAAV-induced CD8+ T cells compared with AdC68gag-induced CD8+ T cells. Boosting of rAAV2/7gag-induced CD8+ T cells with the AdC68gag vector reduced expression of BTLA on some but not all gag-specific CD8+ T cells and over time caused downregulation of CTLA-4. Granzyme B, a serine protease released by cytotoxic T cells to induce apoptosis in target cells, was upregulated early in transgene product–specific CD8+ T cells induced by both the rAAV and AdC68 vaccines; AdC68gag-induced CD8+ T cells then downregulated expression of granzyme B, which remained high in rAAV2/7gag-induced CD8+ T cells (Figure 9C). Gag-specific CD8+ T cells from mice primed with AAV2/7gag and then boosted with AdC68gag initially expressed granzyme B, but this expression was significantly decreased 60 days after vaccination. Late after immunization, the rAAV vaccine-induced transgene product–specific CD8+ T cells upregulated PD-1 (Figure 9D), the hallmark indicator of T cell exhaustion (50–52). Upregulation of PD-1 was also seen on a fraction of gag-specific CD8+ T cells from mice that were primed with rAAV2/7gag and then boosted with AdC68gag. In addition, at some late time points tested, CD244 (2B4), an NK cell marker found on some T cell populations (53, 54), was upregulated on a subset of rAAV2/7-induced CD8+ T cells as well as on gag-specific CD8+ T cells from mice that had received the prime/boost regimen (data not shown). Overall, these data showed that rAAV-induced transgene product–specific CD8+ T cells resembled effector cells (CD62Llo and granzymehi) and expressed markers indicative of T cell exhaustion (PD-1 and CD244). A booster immunization only partially reverted this phenotype toward that seen on memory CD8+ T cells.
Figure 9. Transgene product–specific CD8+ T cells induced by rAAV immunization express markers indicative of exhaustion.
Groups of BALB/c mice were immunized with 1011 gc rAAV2/7gag or 1010 vp AdC68gag; a third group of mice was immunized with rAAV2/7gag and 1 month later boosted with AdC68gag. At days 10, 21, 37, 60, and 80 after the single immunization or after the booster immunization, splenocytes were stained with antibodies to BTLA (A), CTLA-4 (B), granzyme B (C), or PD-1 (D). Histograms show the expression of these markers on gag-tet+CD8+CD44+ cells (open) and gag-tet–CD8+CD44– cells (filled). Differences in negative samples were caused by differences in machine settings.
Blockage of PD-1 does not rescue the proliferative capability of rAAV-induced transgene product–specific CD8+ T cells.
It was shown in the chronic LCMV infection model that blockade of the PD-1/program death–ligand 1 (PD-1/PD-L1) pathway reinstates functionality to exhausted CD8+ T cells (36, 52). To test whether we could restore the proliferative capacity of rAAV2/7-induced CD8+ T cells, we immunized mice with rAAV2/7gag, treated them with an anti–PD-1 antibody as described previously (36, 50, 51), and then immunized them with AdC68gag. Control mice were treated with an IgG2a preparation. After 2 and 5 weeks, lymphocytes were harvested from blood and analyzed for frequencies of gag-tet+CD8+ T cells (Figure 10). Although mice immunized with the rAAV vaccine upregulated PD-1, treatment with the anti–PD-1 antibody or an antibody to PD-L1 (data not shown) did not restore the proliferative capacity of the rAAV2/7-induced CD8+ T cells.
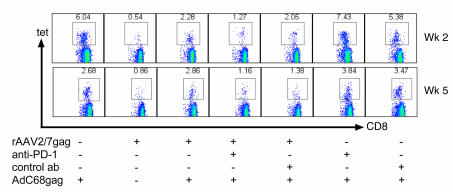
Figure 10. Blockage of PD-1 does not rescue the proliferative capability of rAAV-induced transgene product–specific CD8+ T cells.
Groups of mice were immunized with 1011 gc rAAV2/7gag and then treated with 200 μg anti-PD-1 antibody, twice prior to boost and 3 times after boost, 3 days apart, as described previously (36, 50, 51). Control mice were treated with 200 μg IgG2a. Mice were then immunized with 1010 vp AdC68gag, and 2 and 5 weeks later, lymphocytes were harvested from blood. Shown are density plots of live cells stained with an antibody to CD8 (x axis) and the tet (y axis). Numbers within plots denote tet+CD8+ cells as a percentage of CD8+ cells.
Transfer of rAAV vector-induced CD8+ T cells into antigen-free mice rescues their ability to proliferate upon reencounter of antigen.
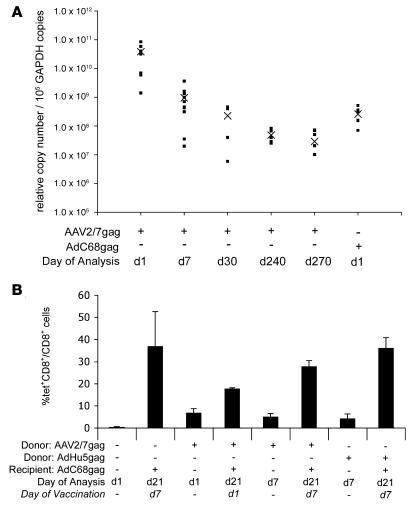
rAAV vectors used as gene therapy vehicles persist (55, 56), as demonstrated in the present study with an AAV2/7 GFP vector (Figure 4B). To test whether rAAV2/7gag vectors persist, groups of 5–10 mice were injected with 1011 gc rAAV2/7gag vector or AdC68gag as a positive control, and DNA isolated from injected leg muscles was tested for gag sequences by a nested real-time PCR at different time points over a 9-month period (Figure 11A). High levels of gag DNA were detected 24 hours after injection. By day 7, levels declined significantly (P > 0.001), presumably reflecting loss of single-stranded DNA that had not converted to double-stranded DNA. There was a further slight but statistically insignificant (P > 0.05) decrease by days 30 and 240, indicating that most of the AAV vectors persisted.
Figure 11. Persistence of antigen impairs the proliferative capability of rAAV-induced transgene product–specific CD8+ T cells.
(A) To test for vector persistence after immunization, groups of mice were administered 1011 gc rAAV2/7gag or 1010 vp AdC68gag in the lower leg muscle. DNA was isolated on days 1, 7, 30, 240, and 270 and analyzed by gag nested real-time PCR. Copy numbers normalized to GAPDH for individual mice are shown; “X” denotes the group mean. (B) Cells from rAAV2/7gag- or AdHu5gag-immunized mice were transferred into RAG–/– mice immunized on day 1 or 7 with AdC68gag. Tet analyses were performed on PBMCs before and after AdC68gag immunization. Error bars represent SD for individual mice.
To test whether persistence of the rAAV2/7gag vector, which would be expected to remain transcriptionally active as has been shown for other rAAV vectors (55, 56), affected the gag-specific CD8+ T cell response, mice were immunized with 1011 gc AAV2/7gag or 1010 vp AdHu5gag as a control. Three weeks later, splenic lymphocytes were transferred intravenously into RAG–/– mice (108 lymphocytes/mouse). The RAG–/– recipient mice were bled 1 and 7 days later to determine frequencies of gag-specific donor CD8+ T cells. AAV2/7gag-induced CD8+ T cells were detected at both time points. Frequencies were slightly lower on day 7 than on day 1, suggesting that these cells apparently failed to undergo preferential homeostatic proliferation in T cell–deficient mice. At 1 or 7 days after the adoptive transfer, groups of RAG–/– mice that had received naive lymphocytes or lymphocytes from AAV2/7gag or AdHu5gag immune-competent donors were immunized with 1010 vp AdC68gag, and frequencies of gag-specific CD8+ T cells were determined 14 days later. RAG–/– recipients that received rAAV2/7gag-immune cells developed high frequencies of gag-specific CD8+ T cells, especially if vaccination was delayed for 7 days. Frequencies were comparable to those seen in RAG–/– mice that received AdHu5gag-immune lymphocytes or lymphocytes from naive mice (Figure 11B). These data show that transfer of rAAV2/7gag immune CD8+ T cells into an antigen-free host restores their proliferative capacity.
Discussion
Vaccine-mediated protection is based on the principle that the vaccine induces an immunological memory response that mounts an accelerated and enhanced recall response after infection (57). Our present data indicate that rAAV vectors may not fulfill this very basic requirement for vaccine constructs.
An efficacious vaccine that prevents infection with HIV-1, a virus that infected 38.6 million people and killed 2.8 million people in 2005 (58), has not yet been developed. This relates to the enormous genetic diversity of the virus and the lack of immunogens suitable to induce potent cross-reactive neutralizing antibodies. Many HIV-1 vaccine candidates rely on protection through a strong cell-mediated immune response that is expected not to prevent infection, but to control viral replication and reduce viral set-point loads and CD4+ T cell loss and thus prolong survival and reduce virus shedding and transmission (57).
rAAV vectors are being used currently in human trials as vaccine carriers for HIV-1. In the initial trial, vectors based on AAV2 were shown to be well tolerated. They induced a modest HIV-1–specific T cell response in only approximately 20% of the vaccine recipients (59). Vectors based on other serotypes of AAV, most notably AAV1, are now entering trials (60).
We tested vectors from a number of different serotypes of AAV as vaccine carriers for gag of HIV-1. Several of these serotypes were derived from nonhuman primates. Humans commonly carry neutralizing antibodies to human serotypes of AAV such as AAV2 and AAV1 as a result of natural infections (61), and such antibodies have been shown in gene therapy trials to reduce gene transfer (61, 62). AAV-specific neutralizing antibodies would also be expected to reduce the potency of rAAV vaccines. Seroprevalence rates of neutralizing antibodies to the capsid of AAV serotypes isolated from nonhuman primates would be expected to be lower than those to human serotypes, which may provide the former with an advantage over the latter. In our preclinical studies in mice, we encountered a potential peril of rAAV-based vaccines that, in our opinion, requires further investigation.
rAAV vectors derived from different serotypes induced low but detectable frequencies of transgene product–specific CD8+ T cells. Responses were higher with vectors based on some serotypes (e.g., AAV1, AAV7, and AAV8) than others (e.g., AAV2, AAV5, and AAV9), which may relate to the vectors’ ability to efficiently transduce muscle cells. Unexpectedly, CD8+ T cells induced by rAAV vectors failed to efficiently proliferate upon a booster immunization with an Ad vaccine vector or other vaccine modalities carrying the same transgene. The immunomodulatory effect of priming with rAAV was seen with different AAV serotypes, was transgene product specific, and was independent of the interval between prime and boost. rAAV-induced CD8+ T cells showed an upregulation of immunoinhibitory molecules, including BTLA, PD-1, and CTLA-4. They also failed to upregulate CD62L and continued to carry intracellular granzyme B, indicating that they were unable to transition into memory cells. Although rAAV-induced gag-specific CD8+ T cells were able to kill gag peptide–pulsed lymphocytes in vivo, they did not protect as efficiently against challenge with a Vacgag vector, which is known to be controlled by CD8+ T cells. Although rAAV-induced CD8+ T cells produced cytokines such as IFN-γ in contrast to exhausted CD8+ T cells that evolve during chronic LCMV infection, levels of IFN-γ production per in vitro–stimulated cell were significantly lower than those produced by AdC68-induced CD8+ T cells (data not shown). Furthermore, rAAV-induced CD8+ T cells were unable to clear AAV-infected muscle cells. This may reflect, at least in part, the low density of MHC class I molecules on muscle cells that can be increased by IFN-γ. rAAVs, unlike other viral vectors, fail to induce a strong inflammatory response and production of proinflammatory cytokines by the innate immune system (63). Such cytokines in turn may be required to upregulate MHC class I expression on muscle cells to render them susceptible to recognition by CD8+ T cells.
Several pathways may contribute to impairing the proliferative capacity of rAAV-induced transgene product–specific CD8+ T cells. Presentation of antigen by immature DCs has been described to induce tolerance rather than activation of CD8+ T cells. DCs, which are pivotal for activation of naive T cells, exhibit an immature phenotype upon in vitro transduction with rAAV vectors: they fail to upregulate costimulatory molecules such as CD86 or MHC class II molecules and do not secrete proinflammatory cytokines or chemokines (S.-W. Lin, unpublished observations). We doubt that lack of DC maturation by the rAAV vaccines results in activation of an impaired CD8+ T cell response, as maturation of DCs through adjuvants fails to restore the rAAV vector–induced CD8+ T cells’ proliferative capacity. Furthermore, we reported previously that Ad vectors induce a potent transgene product–specific CD8+ T cell response even under conditions in which they are unable to induce DC maturation (64). We attributed this to the presence of low levels of mature DCs that can be detected in naive mice and argue that these mature DCs may also facilitate stimulation of hitherto antigen-inexperienced CD8+ T cells to an antigen presented by rAAV vectors.
We could not detect IFN-γ–producing CD4+ T cells in rAAVgag-immunized mice by ELISpot assays (data not shown), and an impaired memory response has previously been described for CD8+ T cells that are activated without T help (65, 66). This pathway also seems unlikely to explain the impaired CD8+ T cell response to rAAV vectors, because both the AAV capsid antigen (67) and the transgene product carry epitopes for CD4+ T cells (68), and we showed that rAAV vectors induced T cell–dependent antibody responses. Therefore, we assume that our failure to demonstrate a CD4+ T cell response in rAAV-immunized mice reflects insensitivity of available methods.
Regulatory cells can impair T cell activation and functionality (69–74). Although rAAV vectors caused a modest increase of CD4+CD25+GITR+ and CD4+CD25+FoxP3+ T cells, functional depletion of CD25+ cells failed to rescue the rAAV-induced CD8+ T cells’ ability to proliferate, nor did adoptive transfer of such cells or other cells or sera from rAAV-immunized mice imprint loss of proliferative capacity on T cells of recipient mice. Regulatory cells are thus an unlikely culprit to explain the impaired proliferative capacity of rAAV-induced CD8+ T cells.
CD8+ T cells that are continuously immersed in antigen become exhausted, as attested by loss of proliferation to antigen, secretion of cytokines, target cell lysis (35, 75), and upregulation of PD-1 and other negative costimulators; blockade of the PD-1/PD-L1/2 pathway through antibodies can restore functionality of exhausted CD8+ T cells (36). CD8+ T cells induced by rAAV vectors shared some of the characteristics of exhausted T cells: they upregulated PD-1 and other immunoinhibitory molecules late after activation and failed to proliferate efficiently. Nevertheless, unlike fully exhausted CD8+ T cells, they continued to lyse and secrete cytokines upon antigenic restimulation (albeit at low levels) and their proliferative capacity was not restored by blockade of the PD-1/PD-L1/2 pathway. The amount of antigen produced by rAAV vectors is most likely lower than that produced during a chronic viral infection and furthermore is restricted to the site of injection (76). One could argue that under these conditions, CD8+ T cells become partially exhausted. This partial exhaustion was reversible once T cells were removed temporarily from antigen, as AAV2/7gag vector–induced CD8+ T cells rapidly regained their proliferative capacity upon transfer into RAG–/–mice. Lack of rescue of CD8+ T cells in rAAV-vaccinated mice by antibodies to PD1 or PD-L1 may reflect distinct stages of exhaustion that differ in their responsiveness to blockade of these pathways.
The AAV vectors used in this study contained single-stranded DNA, which needs to be converted into double-stranded DNA before the transgene is transcribed and translated. In contrast, AAV vectors containing double-stranded DNA express their transgene product without delay (77). Additional studies to compare CD8+ T cell responses to AAV vectors containing single- or double-stranded DNA should elucidate whether and to what extent the delay in transgene product expression by single-stranded AAV vectors contributes to vector persistence and the resultant exhaustion of the transgene product–specific CD8+ T cell responses.
The finding that rAAV vectors induced transgene product–specific CD8+ T cells that did not efficiently proliferate upon a second encounter of the antigen has clinical implications, as human T cells might be similarly affected. The results draw attention to what we believe to be a hitherto unidentified problem for the use of rAAV vectors as a vaccine carrier in the clinic, since this vaccine may have the potential to dampen the immune response to a natural infection and thus increase the vaccine recipients’ susceptibility to progress more rapidly to AIDS. The dampening of the immune response to the transgene product may be useful for gene therapy, but it is detrimental for a vaccine carrier.
Methods
Mice.
Female 4- to 6-week-old BALB/c mice (Thy1.2 and Thy1.1) and BALB/c RAG –/– mice were purchased from The Jackson Laboratory or Ace Animals Inc. and housed at the Animal Facility of The Wistar Institute. All experiments were performed using procedures in protocols reviewed and approved by the Institutional Review Board of The Wistar Institute.
Vectors.
rAAV vectors are based on rAAV2 vectors pseudotyped with viral capsids from AAV serotypes 1, 5, 7, 8, and 9, and they carry a codon-optimized truncated gag gene (gag37; ref. 24) of HIV-1 clade B or GFP under the control of a CMV promoter. The recombinant Ad vector of human serotype 5, AdHu5, and a chimp-derived recombinant Ad vector, AdC68, also carry the truncated gag gene of HIV-1 clade B or GFP under the CMV promoter. Both sets of vectors were obtained from the University of Pennsylvania Vector Core Facility. The DNA vaccine used here was a plasmid containing the entire gag gene of HIV-1 clade B under the CMV promoter. Recombinant MVA expressing full-length HIV-1 gag was a gift from M. Feinberg (Emory University, Atlanta, Georgia, USA).
Immunization of mice.
Vaccine vectors were diluted in sterile saline to a total volume of 100 μl, and mice were immunized by injection into the lower leg muscle or orally by a feeding tube. For some experiments, incomplete Freund’s adjuvant (Sigma-Aldrich) was used at a 1:1 ratio with the rAAV vector in a total volume of 100 μl, and mice were immunized by injection into the lower leg muscle. Prime/boost regimens were generally performed with the initial rAAV immunization followed by the AdC68 booster immunization at a later time point, usually 1–2 months after the priming unless stated otherwise. For in vivo proliferation and protection assays, recipient mice were immunized i.p. with vaccinia virus vectors diluted in 100 μl sterile saline. For experiments involving the functional inactivation of CD25+ cells, 200 μg of an antibody to CD25 (PC-61; Bioexpress Inc.) or a control antibody (HRPN; Bioexpress Inc.) was administered i.p. 3 days prior to a booster immunization. Antibodies to PD-1 and PD-L1 (gifts from G. Freeman, Dana-Farber Cancer Center, Boston, Massachusetts, USA) and control isotypes (Bioexpress Inc.) were administered i.p. at a dose of 200 μg per mouse, per treatment, 2 times prior to and 3 times following booster immunization, at 3-day intervals.
ICS for IFN-γ and TNF-α.
To examine gag-specific IFN-γ– or TNF-α–producing CD8+ T cell frequencies, lymphocytes from spleens were isolated and incubated (1 × 106 cells/sample) with the AMQMLKETI peptide, which carries the immunodominant epitope of HIV-1 gag for mice of the H-2d haplotype, for 5 hours at 37°C with 5% CO2. Control cells were stimulated with an irrelevant peptide (rabies virus); background data were subtracted from sample values before plotting. Cells were surface stained with an anti-CD8 antibody conjugated to FITC, then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences — Pharmingen) for ICS with anti–IFN-γ antibody conjugated to PE and/or anti–TNF-α antibody conjugated to allophycocyanin. All antibodies, unless otherwise noted, were purchased from BD Biosciences — Pharmingen.
Tet and phenotypic marker analyses.
Tet analysis was done with allophycocyanin-conjugated gag AMQMLKETI peptide H-2Kd tets (NIAID Tetramer Facility). Antibodies for surface markers CD8, CD4, CD25, GITR, CD44, CD244, CD62L, PD-1, CTLA-4, and CD27 as well as intracellular marker Bcl-2 were purchased from BD Biosciences — Pharmingen. Antibodies for Granzyme B were purchased from Invitrogen. Antibodies for BTLA and CD127 as well as the Mouse Regulatory T cell Staining Kit, which included antibodies for FoxP3, were purchased from eBioscience. Prior to analysis, cells were fixed with 2% paraformaldehyde in PBS. Flow cytometry analysis of the cells was performed with the Beckman-Coulter XL (Beckman-Coulter) and FACSCalibur (BD) flow cytometers at The Wistar Institute Flow Cytometry Core Facility; data were analyzed with WinMDI 2.8 (Howard Scripps Institute) or FlowJo 7.1.1 (Tree Star Inc.). Statistical analysis to calculate P values was performed with Intercooled Stata 8.2 (StataCorp LP).
In vivo proliferation assay.
Lymphocytes were dissociated from spleens of donor mice (Thy1.2), and red blood cells were lysed with ACK Lysing Buffer (Invitrogen). To isolate CD8+ T cells by negative selection, cells were incubated for 30 minutes on ice with purified rat anti-mouse antibodies to MHC II, CD45R/B220, CD4, TER119, CD19, and GR1 (BD Biosciences — Pharmingen) and then for 15 minutes on ice with anti-rat IgG MACS microbeads (Miltenyi Biotech) before being passed through a MACS column in a magnetic separator (Miltenyi Biotech). Sorting with this method resulted an enrichment of 80%–85% for a given cell subset. Purified CD8+ T cells were then labeled with 5 μM CFSE using the CellTrace CFSE Cell Proliferation Kit (Invitrogen) and transferred to recipient mice (Thy1.1) in 200 μl by tail vein injection (5 × 107 cells per mouse). At 3 days after transfer, splenocytes of recipient mice were harvested; stained with antibodies to CD8, Thy1.2, and the gag-tet; and analyzed for decrease in CFSE intensity as evidence for proliferation.
In vivo killing assay.
Lymphocytes were dissociated from spleens of donor mice. Red blood cells were lysed with ACK Lysing Buffer (Invitrogen). Half the lymphocytes were pulsed with the BALB/c-specific CD8+ 9-mer AMQMLKETI peptide and then labeled with 0.5 μM CFSE using the CellTrace CFSE Cell Proliferation Kit (Invitrogen); the other half were pulsed with an irrelevant peptide derived from a sequence of the rabies virus nucleoprotein and labeled with 5 μM CFSE. Equal numbers of cells (107 cells) from the 2 populations were transferred via tail vein into immunized mice. At 24 hours after transfer, splenocytes from recipient mice were harvested for analysis by flow cytometry.
Muscle imaging.
Groups of BALB/c mice were i.m. immunized in the lower leg with 1011 gc rAAV2/7 expressing GFP or with AdC68 expressing GFP. One group of mice was immunized with both vectors, but in different legs, in a prime/boost regimen (rAAV followed by AdC68). Mice were sacrificed at the indicated time points, and the immunized and nonimmunized legs were removed and illuminated with an Illumatool Lighting System (Lightools Research). Photographs were taken with a Kodak DCS14N digital SLR camera with a 60-mm Micro Nikkor lens (Nikon). Images were captured as raw files and converted to TIFF files using Kodak DCS photodesk software.
Vaccinia virus challenge.
At 3–4 weeks after i.m. immunization with rAAV2/7gag and AdC68gag (9–10 mice per group), mice were challenged i.p. with 1 × 106 PFU Vacgag, which also expresses β-galactosidase. Nonimmunized mice served as controls. Both ovaries were harvested 5 days after infection for viral titer analysis. Ovaries were homogenized with an electric tissue grinder, plated in dilutions on confluent TK– cells, and incubated for 18 hours at 37°C and 5% CO2. Cells were overlayed with an agarose mixture containing X-gal and placed in the incubator for 24 hours. Ovary vaccinia virus titers were then determined by counting the blue plaques.
ELISA.
To measure levels of gag-specific antibodies, serum was collected from mice by retro-orbital puncture. Nunc 96-well plates (Thermo Fisher Scientific) were coated with gag protein (ImmunoDiagnostics Inc.) diluted in 0.1 M carbonate coating buffer (pH 9.6) overnight at 4°C. Plates were washed 3 times with PBS and blocked overnight at 4°C with 1% BSA-PBS. Serial dilutions of mouse serum samples were added to the wells and incubated for 1 hour at room temperature. Plates were washed 3 times with PBS, and a secondary antibody (ICN CAPPEL goat alkaline phosphatase for mouse immunoglobulin; MP Biomedicals Inc.) diluted 1:100 in 3% BSA-PBS was added for 1 hour at room temperature. After washing, the plates were developed with alkaline phosphatase for 5 minutes, and absorption was determined at OD405. Isotypes of specific antibodies were determined with the Calbiochem Hybridoma Subisotyping Kit (Calbiochem).
Cell sorting.
For some experiments, splenocytes were sorted for CD4+, CD8+, and B220+ cell populations. After incubating the cells with the appropriate antibodies (all from BD Biosciences — Pharmingen), samples were sorted or analyzed using a DakoCytomation MoFlo (DakoCytomation Inc.). Sorting resulted an enrichment of 80%–85% for a given cell subset.
Nested real-time PCR.
DNA was isolated from leg muscles using the DNeasy Tissue Kit (QIAGEN). The housekeeping gene GAPDH was quantified from each sample by real-time PCR. Samples were adjusted to equal amounts of GAPDH and amplified by PCR followed by a nested real-time PCR for gag. External 5′ and 3′ primers used for the PCR were 5′-GGAGCTAGAACGATTCGC-3′ and 5′-CTCTTGCCTTATGGCCG-3′; nested 5′ and 3′ primers for the real-time PCR were 5′-AGGGGAAGTGACATA-3′ and 5′-GCTTGCTCGTCGGCTCTTAG-3′. Standards for gag (104–108 gc) were amplified at the same time. The first PCR consisted of 25 cycles of 94°C for 40 s, 52°C for 40 s, and 72°C for 40 s. The amplicon from the first PCR product was then used as template for a second real-time PCR to quantify the gag gene. The second PCR was run for 40 cycles at 95°C for 5 s, 57°C for 4 s, 72°C for 10 s, and 84°C for 4 s. Specificity of amplicons was confirmed by analyses of melting temperature curves and gel electrophoresis of amplicons. Controls included reactions containing no DNA (water control) or samples from naive animals.
Adoptive transfer in RAG–/– mice.
Spleen cells from immune competent BALB/c mice were depleted of neutrophils using the MACS beads sorting procedure described above. All remaining cells were transferred via tail vein injection into BALB/c RAG–/– mice (108 cells per mouse).
Statistics.
Statistical analysis to calculate P values was performed with Intercooled Stata 8.2 (StataCorp LP). For analyses, a 1-tailed Student’s t test was used and P values less than 0.05 were considered significant.
Acknowledgments
This work was supported by NIH/NIAID grants PO1 A1055018-02 and PO1 HL078810-01A1. The authors wish to acknowledge Wynetta Giles-Davis for her assistance with cell culture work; Ann Cun and Lauren DiMenna for their assistance with assays; Sarah Abdulla for her assistance in purchasing antibodies; Dongming Zhou, Ang Bian, and Hua Li for their assistance with PCR assays; Jeffrey Faust, Matt Farabaugh, and Amaya Iparraguirre for their assistance with flow cytometry; and Fred Keeney for his assistance in muscle imaging. We thank Andrew Caton and E. John Wherry for helpful discussions and the Vector Core of the University of Pennsylvania for provision of vectors. We thank the NIAID Tetramer Facility for providing the gag-tet.
Footnotes
Nonstandard abbreviations used: AAV, adeno-associated virus; Ad, adenovirus, adenoviral; AdC68, adenovirus chimpanzee serotype 68; AdHu5, adenovirus human serotype 5; BTLA, B and T lymphocyte attenuator; CTLA-4, CTL-associated protein–4; gc, genome copy; GITR, glucocorticoid-induced TNF receptor; ICS, intracellular cytokine staining; i.m., intramuscular; MVA, modified vaccinia ankara; PD-1, program death–1; PD-L1, program death–ligand 1; rAAV, recombinant AAV; tet, tetramer; Vac, vaccinia; vp, viral particle.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:3958–3970 (2007). doi:10.1172/JCI33138
Scott E. Hensley’s present address is: Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Nia Tatsis’s present address is: Wyeth Pharmaceuticals, Collegeville, Pennsylvania, USA.
References
- 1.Fisher K.J., et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 2.High K. AAV-mediated gene transfer for hemophilia. Genet. Med. 2002;4:56S–61S. doi: 10.1097/00125817-200211001-00012. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z., Asokan A., Samulski R.J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y.L., et al. Therapeutic levels of factor IX expression using a muscle-specific promoter and adeno-associated virus serotype 1 vector. Hum. Gene Ther. 2004;15:783–792. doi: 10.1089/1043034041648453. [DOI] [PubMed] [Google Scholar]
- 5.Zaiss A.K., Muruve D.A. Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- 6.Wang C.H., Liu D.W., Tsao Y.P., Xiao X., Chen S.L. Can genes transduced by adeno-associated virus vectors elicit or evade an immune response? Arch. Virol. 2004;149:1–15. doi: 10.1007/s00705-003-0241-3. [DOI] [PubMed] [Google Scholar]
- 7.Bessis N., GarciaCozar F.J., Boissier M.C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl. 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 8.Xin K.Q., et al. A novel recombinant adeno-associated virus vaccine induces a long-term humoral immune response to human immunodeficiency virus. Hum. Gene Ther. 2001;12:1047–1061. doi: 10.1089/104303401750214276. [DOI] [PubMed] [Google Scholar]
- 9.Manning W.C., et al. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. . J. Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan G.J., et al. AAV vectors encoding malarial antigens stimulate antigen-specific immunity but do not protect from parasite infection. Vaccine. 2007;25:1014–1022. doi: 10.1016/j.vaccine.2006.09.072. [DOI] [PubMed] [Google Scholar]
- 11.Carter B.J. Analysis of parvovirus mRNA by sedimentation and electrophoresis in aqueous and nonaqueous solution. J. Virol. 1974;14:834–839. doi: 10.1128/jvi.14.4.834-839.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith-Arica J.R., Bartlett J.S. Gene therapy: recombinant adeno-associated virus vectors. Curr. Cardiol. Rep. 2001;3:43–49. doi: 10.1007/s11886-001-0009-x. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves M.A. Adeno-associated virus: from defective virus to effective vector. Virol. J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X., Voulgaropoulou F., Chen R., Johnson P.R., Clark K.R. Selective Rep-Cap gene amplification as a mechanism for high-titer recombinant AAV production from stable cell lines. Mol. Ther. 2000;2:394–403. doi: 10.1006/mthe.2000.0132. [DOI] [PubMed] [Google Scholar]
- 15.Gao G.P., et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol. Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- 16.Gao G., et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao G., et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao G.P., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louboutin J.P., Wang L., Wilson J.M. Gene transfer into skeletal muscle using novel AAV serotypes. J. Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar R., et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum. Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 22.Gao G.P., et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J. Virol. 2006;80:6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monahan P.E., Jooss K., Sands M.S. Safety of adeno-associated virus gene therapy vectors: a current evaluation. Expert Opin. Drug Saf. 2002;1:79–91. doi: 10.1517/14740338.1.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Qiu J.T., et al. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J. Virol. 1999;73:9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang Z., et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald J.C., et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 27.Andersson H.A., Barry M.A. Maximizing antigen targeting to the proteasome for gene-based vaccines. Mol. Ther. 2004;10:432–446. doi: 10.1016/j.ymthe.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Qiu J.T., Liu B., Tian C., Pavlakis G.N., Yu X.F. Enhancement of primary and secondary cellular immune responses against human immunodeficiency virus type 1 gag by using DNA expression vectors that target Gag antigen to the secretory pathway. J. Virol. 2000;74:5997–6005. doi: 10.1128/jvi.74.13.5997-6005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross D.A., Leboeuf M., Gjata B., Danos O., Davoust J. CD4+CD25+ regulatory T cells inhibit immune-mediated transgene rejection. Blood. 2003;102:4326–4328. doi: 10.1182/blood-2003-05-1454. [DOI] [PubMed] [Google Scholar]
- 30.Dobrzynski E., et al. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohm A.P., et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 32.Veron P., et al. Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2. J. Virol. 2007;81:5385–5394. doi: 10.1128/JVI.02516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman R.M., Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 34.Shibaki A., Katz S.I. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund’s adjuvant. Exp. Dermatol. 2002;11:126–134. doi: 10.1034/j.1600-0625.2002.110204.x. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn S.D., Wherry E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Barber D.L., et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 37.Huet S., et al. CD44 contributes to T cell activation. J. Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- 38.Shimizu Y., Van Seventer G.A., Siraganian R., Wahl L., Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J. Immunol. 1989;143:2457–2463. [PubMed] [Google Scholar]
- 39.Borst J., Hendriks J., Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Page T.H., Willcocks J.L., Taylor-Fishwick D.A., Foxwell B.M. Characterization of a novel high affinity human IL-7 receptor. Expression on T cells and association with IL-7 driven proliferation. J. Immunol. 1993;151:4753–4763. [PubMed] [Google Scholar]
- 41.Foxwell B.M., Taylor-Fishwick D.A., Simon J.L., Page T.H., Londei M. Activation induced changes in expression and structure of the IL-7 receptor on human T cells. Int. Immunol. 1992;4:277–282. doi: 10.1093/intimm/4.2.277. [DOI] [PubMed] [Google Scholar]
- 42.Mobley J.L., Rigby S.M., Dailey M.O. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J. Immunol. 1994;153:5443–5452. [PubMed] [Google Scholar]
- 43.Tatsis N., et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy K.M., Nelson C.A., Sedy J.R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez L.C., et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedy J.R., et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 47.Pandiyan P., Hegel J.K., Krueger M., Quandt D., Brunner-Weinzierl M.C. High IFN-gamma production of individual CD8 T lymphocytes is controlled by CD152 (CTLA-4). J. Immunol. 2007;178:2132–2140. doi: 10.4049/jimmunol.178.4.2132. [DOI] [PubMed] [Google Scholar]
- 48.Coenen J.J., et al. CTLA-4 engagement and regulatory CD4+CD25+ T cells independently control CD8+-mediated responses under costimulation blockade. J. Immunol. 2006;176:5240–5246. doi: 10.4049/jimmunol.176.9.5240. [DOI] [PubMed] [Google Scholar]
- 49.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman G.J., Wherry E.J., Ahmed R., Sharpe A.H. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day C.L., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 52.Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 53.Laouar A., et al. Cutting Edge: Distinct NK receptor profiles are imprinted on CD8 T cells in the mucosa and periphery during the same antigen challenge: role of tissue-specific factors. J. Immunol. 2007;178:652–656. doi: 10.4049/jimmunol.178.2.652. [DOI] [PubMed] [Google Scholar]
- 54.Rey J., et al. The co-expression of 2B4 (CD244) and CD160 delineates a subpopulation of human CD8+ T cells with a potent CD160-mediated cytolytic effector function. Eur. J. Immunol. 2006;36:2359–2366. doi: 10.1002/eji.200635935. [DOI] [PubMed] [Google Scholar]
- 55.Herzog R.W., et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conlon T.J., Flotte T.R. Recombinant adeno-associated virus vectors for gene therapy. Expert Opin. Biol. Ther. 2004;4:1093–1101. doi: 10.1517/14712598.4.7.1093. [DOI] [PubMed] [Google Scholar]
- 57.Johnston M.I., Fauci A.S. An HIV vaccine-evolving concepts. N. Engl. J. Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 58. UNAIDS. 2006. Report on the global AIDS epidemic 2006. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp. [Google Scholar]
- 59.
- 60.
- 61.Mingozzi F., et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 62.Manno C.S., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 63.McPhee S.W., et al. Immune responses to AAV in a phase I study for Canavan disease. J. Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- 64.Hensley S.E., et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther. 2007;15:393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- 65.Janssen E.M., et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 66.Shedlock D.J., Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 67.Chen J., Wu Q., Yang P., Hsu H.C., Mountz J.D. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol. Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Wu L., Kong W.P., Nabel G.J. Enhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogens. J. Virol. 2005;79:8024–8031. doi: 10.1128/JVI.79.13.8024-8031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 70.Suvas S., Kumaraguru U., Pack C.D., Lee S., Rouse B.T. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mills K.H. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 72.Belkaid Y., Rouse B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 73.McHugh R.S., Shevach E.M. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 74.Mendez S., Reckling S.K., Piccirillo C.A., Sacks D., Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. . J. Exp. Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartholdy C., Christensen J.P., Wodarz D., Thomsen A.R. Persistent virus infection despite chronic cytotoxic T-lymphocyte activation in gamma interferon-deficient mice infected with lymphocytic choriomeningitis virus. J. Virol. 2000;74:10304–10311. doi: 10.1128/jvi.74.22.10304-10311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honda H., Rostami A. Expression of major histocompatibility complex class I antigens in rat muscle cultures: the possible developmental role in myogenesis. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7007–7011. doi: 10.1073/pnas.86.18.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z., Ma H.I., Li J., Sun L., Zhang J., Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]