Abstract
For almost half a century it has been thought that the heart rhythm originates on the surface membrane of the cardiac pacemaker cells and is driven by voltage-gated ion channels (membrane clocks). Data from several recent studies, however, conclusively show that the rhythm is initiated, sustained, and regulated by oscillatory Ca2+ releases (Ca2+ clock) from the sarcoplasmic reticulum, a major Ca2+ store within sinoatrial node cells, the primary heart’s pacemakers. Activation of the local oscillatory Ca2+ releases is independent of membrane depolarization and driven by a high level of basal state phosphorylation of Ca2+ cycling proteins. The releases produce Ca2+ wavelets under the cell surface membrane during the later phase of diastolic depolarization and activate the forward mode of Na+/Ca2+ exchanger resulting in inward membrane current, which ignites an action potential. Phosphorylation-dependent gradation of speed at which Ca2+ clock cycles is the essential regulatory mechanism of normal pacemaker rate and rhythm. The robust regulation of pacemaker function is insured by tight integration of Ca2+ and membrane clocks: the action potential shape and ion fluxes are tuned by membrane clocks to sustain operation of the Ca2+ clock which produces timely and powerful ignition of the membrane clocks to effect action potentials.
MeSH keywords: Heart Rate, Sinoatrial Node, Calcium, Sarcoplasmic Reticulum, Ryanodine Receptor Calcium Release Channel, Ion Channels
1. Introduction
The current dogma of the initiation and regulation of rhythmic heart beats has been evolved from the intertwining of theory and experimentation for almost 200 years [1, 2]. Shortly after the development of quantitative membrane excitation theory by Hodgkin and Huxley in 1952 [3], the mechanism of the cardiac pacemaker initiation has been thought largely to result from interplay of voltage-gated ion membrane currents [4]. Since that time pacemaker cell research has largely focused on these essentially membrane-delimited pacemaker mechanisms. Although the ensemble of voltage and time-dependent rhythms of surface membrane ion channels (“Membrane clocks”, Fig. 1A), represents the immediate cause for excitation and indeed execution of action potentials (AP) in sinoatrial nodal cells (SANC), it does not necessarily follow that this ion channel ensemble is the formal, i.e., initiating, cause of spontaneous, rhythmic APs.
Figure 1.
A: Schematic representation of the idea that Ca2+ clock ignites ion channel membrane clocks to entrain normal automaticity in cardiac pacemaker cells (see text for details). B: A schematic illustration of the fine structure of the diastolic depolarization (DD) following of spontaneous action potential in rabbit SANC shown together with inward ion currents and related Ca2+ signals. The nonlinear, exponentially rising, late DD part is shown as a gray area between membrane potential curve and an extrapolation of the initial, linear DD part (modified from [37]). C,D: Confocal line scan images of Ca2+ signals (Fluo-3) measured in rabbit SANC with different orientation of the scanning line. Trace in panel C is simultaneous action potential recordings in perforated patch clamp configuration. Trace in panel D is the time course of the average Ca2+ signal along the scan line. Local Ca2+ Releases (shown by white arrows) occur under cell surface membrane in the later part of diastolic depolarization. Modified from [14].
Recent, robust experimental evidence, based on confocal cell imaging, and supported by numerical modeling, puts forth a novel concept to explain normal, firing rhythm in SANC: intracellular Ca2+ cycling (“Ca2+ clock”, Fig. 1A) ignites membrane clocks, effecting rhythmic APs. The SANC’s Ca2+ clock is manifested by spontaneous, but precisely timed, rhythmic, submembrane, local Ca2+ releases from sarcoplasmic reticulum (SR) that appear shortly before firing of the next AP (Fig. 1B–D). These Ca2+ releases rhythmically activate Na+/Ca2+ exchanger (NCX) inward current, prompting the membrane clocks to generate those rhythmic APs (Fig. 1B). The occurrence and rhythmicity of local Ca2+ releases are tightly controlled by a high degree of basal and reserve protein kinase A (PKA)-dependent protein phosphorylation [5]. Phosphorylation-dependent gradation of the Ca2+ clock, by regulation of the speed of Ca2+ cycling, is the essential regulatory mechanism of normal pacemaker rate and rhythm. Although membrane clocks do not initiate pacemaker AP, they tune the AP shape and ion fluxes to sustain function of the Ca2+ clock. Thus, the intracellular Ca2+ clock does not merely fine tune SANC AP firing, but is, in fact, the formal cause of both the basal and reserve pacemaker rate rhythms, insuring pacemaker robustness, and responsiveness by rhythmically integrating multiple Ca2+-dependent functions, including those of membrane clocks and ion transporters. We believe that the new concept of the heartbeat origin replaces the current dogma of cardiac pacemaker mechanism, and thus releases the field from an “intellectual phase lock” of the membrane-delimited approach that has been perpetuated since 1960. Perhaps, the most significant implication of the new findings is that two separate field of cardiac research of how fast and strong the heart beats, are now merged within a novel, general, unifying theory [2, 6]: intracellular Ca2+ cycling initiates the heartbeat in cardiac pacemaker cells, and, in ventricular myocytes, executes the heartbeat.
2. Spontaneous local Ca2+ releases in SANC, the primary heart’s pacemaker
In rabbit SANC, fluorescence imaging over the last decade has documented the occurrence of an AP-induced Ca2+ transient, and, importantly, in the absence of Ca2+ overload, confocal imaging has permitted detection of the occurrence of localized, Ca2+ releases (LCRs) during normal cell function [7, 8]. During spontaneous beating, LCRs occur during late DD in the form of multiple locally propagating wavelets beneath the cell surface membrane (Fig. 1C,D) [7], larger than the spontaneous Ca2+ sparks in ventricular cells, but markedly less than well organized Ca2+ waves that can propagate the length of ventricular cells[9]. While LCRs occur during the spontaneous diastolic depolarization (DD) in spontaneously firing rabbit SANC, they do not require triggering by the depolarization: spontaneous LCRs in SANC occur in the absence of membrane potential changes, i.e., during voltage clamp (“Control” in Fig. 2C and Fig. 3C), or following “chemical skinning” of the surface membrane with the detergent saponin (“Control” in Fig. 2Aa and Fig. 3A) [5, 10]. Of note, however, in cat, latent atrial pacemaker cells, T-type Ca2+ current, activated during DD, is thought to trigger LCRs, as these are blocked by 50 μM Ni2+ in concentrations that inhibit T-type Ca2+ channels [8]; in contrast, in primary rabbit SANC, Ni2+ at these concentrations does not inhibit LCRs (Fig. 4)[11].
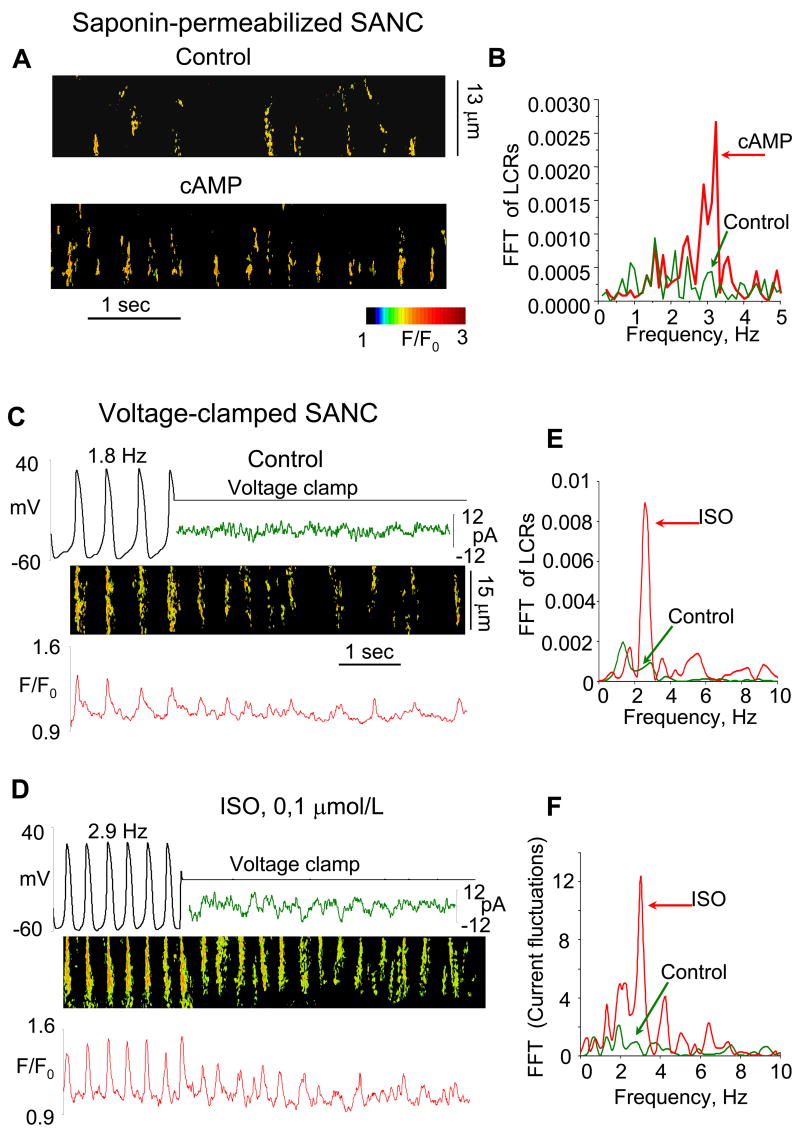
Figure 2.
Suppression of cAMP-mediated, PKA-dependent signaling in SANC decreases frequency and size of LCRs in permeabilized and intact SANC. A: Confocal linescan images of a representative saponin-permeabilized SANC bathed in 100 nmol/L free [Ca2+] before (top), and after (bottom) superfusion with 15 μM PKI, a specific peptide inhibitor of PKA catalytic submit. B: The average frequency (normalized per 1 s and 100 μm and size of LCR in skinned SANC in control conditions (72 LCR, n = 4) and 15 μM PKI (20 LCR).*P < 0.05. Error bars indicate standard error of mean. C and D: Simultaneous recordings of membrane potential or current (top), confocal linescan image (middle), and normalized fluo-3 fluorescence (bottom) averaged over the linescan image, in a representative spontaneously beating SANC with intact sarcolemma before and during voltage clamp to −10 mV in control (C) and following PKA inhibition (8 μM PKI) (D). Fast Fourier transform (FFT) of Ca2+ (E) and membrane current (F) fluctuations during voltage clamp in control and after PKI. Because current fluctuations during voltage clamp were imposed on the total membrane current, each data set was fit with a nonlinear regression line that was subtracted to give a difference signal to minimize frequency interference. From [5].
Figure 3.
Stimulation of cAMP-mediated, PKA-dependent signaling in SANC increases internal Ca2+ oscillation frequency. A: Confocal linescan images of a representative saponin-skinned SANC bathed in 100 nM [Ca2+], before (top), and after (bottom) superfusion with 10 μM cAMP. B: Fast Fourier transform (FFT) of Ca2+ fluctuations of the cell in panel A in control conditions and during superfusion with cAMP. C and D: Simultaneous recordings of membrane potential or current (top), linescan image (middle), and normalized fluo-3 fluorescence (bottom) averaged over the linescan image, in a representative spontaneously beating cell with intact sarcolemma before and during voltage clamp to −10 mV in control (C) and following exposure to a β-AR agonist (0.1 μM isoproterenol, ISO) (D). E and F: FFT of Ca2+ (E) and membrane current fluctuations (F) during voltage clamp in control and after ISO. From [5].
Figure 4.
Local Ca2+ releases persist during spontaneous beating and under voltage clamp to -70 mV, when T-type Ca2+ current is blocked by Ni2+ in rabbit SANC. A: recordings of APs (top), line-scan image (middle) and normalized subsarcolemmal fluorescence averaged spatially over the image width in a representative SANC during superfusion with 50 μM Ni2+. B: control recordings of line-scan image and normalized subsarcolemmal fluorescence in the same cell before exposure to Ni2+. From [10].
3. Spontaneous LCRs are rhythmic: The SR is a “Ca2+ clock”
In the absence of regularly occurring APs, LCRs occurrences are roughly periodic and rhythmic in SANC (see Fourier spectrum of LCR activity in voltage-clamped cells in Fig. 2E “Control”). Thus, the SR can be considered as a Ca2+ clock. During spontaneous SANC firing, i.e. in the presence of rhythmically occurring APs, the LCR period or the speed at which Ca2+ Clock ticks, is reflected in the delay between the prior AP-induced, L-type Ca2+ channel-triggered, relatively synchronized cytosolic Ca2+ transient and LCR emergence later in diastole (Fig. 1D, “LCR period"). The Ca2+ clock’s period is, thus, slightly shorter than the cycle length (Fig. 1B); it approximately coincides with the LCR period measured during the first “would-be” cycle period after switch from spontaneous beating to voltage clamp or the period of Ca2+ oscillations under voltage clamp [10]. A key point to note is that the LCR period is not fixed, but rather depends upon the speed at which SR Ca2+ cycling mechanisms have restituted following the Ca2+ release and SR Ca2+ depletion, and RyR inactivation effected by the prior AP, similar to what has been observed in ventricular cells [12]. Neither the SR Ca2+ load, nor Ca2+ cycling restitution status is fixed, but each changes in response to variations in 1) rate or pattern of APs, which determine net cell Ca2+ balance, 2) the velocity and extent of SR Ca2+ pumping into the SR, and 3) activation status of SR release mechanisms. Recent studies have shown that the speed at which the SR Ca2+ Clock “ticks” is under tight regulation by the degree of PKA-dependent phosphorylation [5]. In skinned or voltage clamped rabbit SANC, inhibition of PKA-dependent phosphorylation by PKI, a specific peptide PKA inhibitor, slows the Ca2+ clock speed (LCRs in Fig. 2). This indicates a crucial role of intracellular PKA-dependent phosphorylation of SR Ca2+ cycling proteins in the control of the Ca2+ clock speed, thus permitting the generation of LCRs in SANC in the basal state. Direct elevation of cAMP, or stimulation of βARs, increases the Ca2+ clock speed (i.e. reduces the LCR period)(Fig. 3).
4. High levels of basal cAMP and PKA-dependent phosphorylation drive the “high speed” SANC Ca2+ clock in the basal state
While, basal, diastolic Ca2+ levels in rabbit SANC and ventricular cells do not differ, [10, 13, 14] the SR Ca2+ clock within spontaneously firing SANC, in the basal state, restitutes and generates LCRs with an LCR period (<0.5 s), which is substantially less than in ventricular myocytes under basal conditions (~10 s [15]) thus allowing for LCRs to emerge sooner (following prior AP) in SANC than in ventricular cells. A recent discovery provides the clue as to why the basal SANC SR Ca2+ Clock speed is so rapid: high basal levels of cAMP and cAMP-dependent phosphorylation are present within SANC, i.e. even in the absence of β-AR stimulation (Fig. 5).
Figure 5.
High basal level of cAMP and cAMP-mediated, PKA-dependent phospholamban (PLB) phosphorylation in SANC. A: Average content of cAMP in suspensions of SANC, atrial or ventricular myocytes, and changes in cAMP level in SANC following suppression of adenylyl cyclase activity with 400 μM MDL (an adenylyl cyclase inhibitor) or β-AR stimulation (1 μM isoproterenol, ISO). B: Left, Western blots of the basal level of phosphorylated at serine16 (P-PLB) and total PLB in SANC and ventricular myocytes; right, average values of phosphorylated PLB normalized to total PLB. C: Phosphorylated PLB and total PLB at base line and in response to graded increases inAC inhibition by MDL (note that the concentration of MDL increases from right to left). D: Typical Western blots of the basal level of PLB phosphorylation and that following PKA inhibition (15 μM PKI, a specific peptide inhibitor of PKA catalytic subunit), or β-AR stimulation (1 μM ISO). E: Average changes in phosphorylated PLB induced by maneuvers in (C and D) (MDL concentration was 400 μM). *P < 0.05. Error bars indicate standard error of means. From [5].
The high levels of basal cAMP are due to high constitutive adenylyl cyclase activity that is not driven by constitutive β-AR activation [5]. Preliminary evidence indicates that two adenylyl cyclase isoforms, Type 2 and Type 8, a Ca2+-activated isoform highly expressed in the brain, are expressed in SANC, but not in other cardiomyocytes [16]. Interestingly, basal phosphodiesterase activity also appears to be elevated in SANC [17], likely as a mechanism to keep basal cAMP levels in check. Among proteins highly phosphorylated in the basal state are phospholamban (Fig. 5) and RyRs [5]; and indirect evidence suggests that L-type Ca2+ channels are likely to be as well [18]. PKA dependent phosphorylation of these proteins presents the SR with an increased Ca2+ to be pumped (Ca2+ influx via ICaL), speeds up the SR Ca2+ filling rate (phospholamban), and likely alters the Ca2+ release threshold (ryanodine receptors). The net effects of this phosphorylation create conditions that are required for LCR spontaneous activation during the DD in the basal state.
5. The rhythmic intracellular Ca2+ clock entrains normal automaticity in SANC via activation of inward NCX current
Recent experimental data, supported by numerical modeling in spontaneously firing, rabbit SANC [5, 7, 10, 11, 14, 19, 20] conclusively show that, LCRs emerging during the later part of DD, ignite NCX inward current (Fig. 6 and schematically illustrated in Fig. 1A–B). This imparts an exponential increase to the later part of DD [7, 20](“Nonlinear DD” in Figs 1B and 6A), prompting activation of surface membrane, voltage-gated, L-type Ca2+ channels to initiate an AP. An analysis of the detailed fine structure of the exponentially rising DD in SANC (Fig. 7) reveals noticeable beat-to-beat membrane potential fluctuations (Fig. 7C–D) that coincide with the occurrence of LCRs (Fig. 7E). These fluctuations reflect activation of local NCX currents by individual LCRs resulting from a stochastic nature of RyR openings [20, 21]. Blocking LCRs with ryanodine (Fig. 6A–B) or blocking NCX by brief, rapid Na+-free superfusion (Fig. 6C–D) in rabbit SANC reduces the LCR-initiated inward current and markedly slows or abolishes spontaneous SANC AP firing. In voltage-clamped SANC, persistent, rhythmic LCRs generate rhythmic current fluctuations with the same periodicity (Fig. 2 and 3); both periodic LCRs and current fluctuations are abolished by ryanodine [22]. Both the DD variance and the amplitude of the late, nonlinear DD component are closely correlated with the changes in beating rate produced by a variety of chronotropic interventions known to influence period and/or amplitude of LCRs (e.g. ryanodine, BAPTA, nifedipine or isoproterenol)(Fig. 7F).
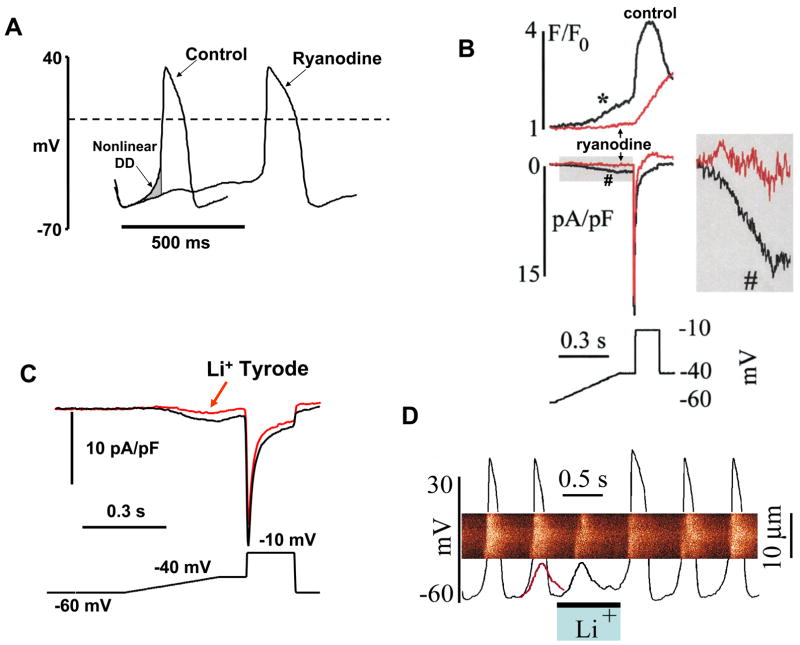
Figure 6.
Local Ca2+ releases (LCRs) ignite rhythmic APs via activation of NCX current imparting an exponential increase to the later part of the diastolic depolarization (“nonlinear DD”) in rabbit SANC. A: Ryanodine abolishes the LCR-mediated AP ignition early in the cycle by inhibition of the nonlinear DD (gray area shown by arrow). Modified from [22]. B: Ryanodine (3 μM, 4 min) inhibits submembrane Ca2+ increase (*) and inward current (#) during a simulated diastolic depolarization (bottom) without affecting peak ICaL. Inset shows the indicated part of the current record at greater magnification. C: An inhibitory effect of a 10s Na+-free spritz (arrow) on inward current during the voltage ramp protocol (bottom). D: Linescan image of Ca2+ release with superimposed AP records during rapid and brief superfusion with a solution in which Na+ was replaced by Li+. Note that the maneuver blocked the subsequent AP firing. The line superimposed on the last AP preceding spritz of Na+-free solution is a copy of the residual membrane potential oscillation observed during the Li+ solution spritz. From [7].
Figure 7.
Membrane potential fluctuations resulting from LCRs in rabbit SANC impart an exponential phase to the late DD that controls their chronotropic state. A: Schematic illustration of the assessment of the non-linear DD component (NDDC). B: Eight successive APs with seven 90-ms recordings of DD marked. C: Superimposition of 7 individual DDs marked in B and their mean (bold). D: Residual voltage after subtracting the mean of a group records from 7 successive DDs. Note the larger beat-to-beat deviations as the DD approaches the AP upstroke. E: Time course of DD variance (curve) and of the relative LCR occurrences (columns) observed at different times before AP upstroke (166 measurements in 24 cells under control conditions). F: Changes in DD fluctuations and NDDC amplitude versus changes in beating rate with respect to chronotropic interventions. From [20].
Entrainment of automaticity in SANC by rhythmic LCR/INCX signals originating from an internal Ca2+ clock is not really surprising, since it has been known for long time from both experimental and theoretical studies that generation of APs in SANC can be easily entrained by rhythmic electric current pulses, if these pulses are applied in the “right” phase of the pacemaker cycle (so called “1:1 entrainment zone” in the phase response curves) [23, 24]. A 2:1 entrainment pattern and other complex Wenckebach-like patterns (e.g., 5: 4, 4:3, and 3:2) were also observed when pulses were applied out of the 1:1 zone. Although depolarization pulses from LCR/INCX signals are generated from within the cell by the SR Ca2+ clock, their electrogenic influence on the excitable SANC membrane is obviously identical to that applied by an experimenter in a current-clamp configuration. Interestingly, in rabbit SANC LCRs predominantly occur within a time window of ~20% of the cycle length before an excitation [10, 14], i.e., exactly where “1:1 entrainment zone” was originally discovered [23]. Also, arrhythmic beating showing various alternation patterns can be observed in rabbit SANC when the SR clock is slowed by chelation of intracellular Ca2+ [25], by disabling Ca2+ release by ryanodine [7] or by inhibition of PKA signaling [5, 26].
6. Phosphorylation-dependent gradation of Ca2+ clock speed is the essential regulatory mechanism of normal pacemaker rate and rhythm (Fig. 8)
Figure 8.
A: Schematic illustration of functional integration and regulation of membrane and submembrane Ca2+ cycling to control pacemaker function via NCX-mediated ignition of rhythmic APs. The thick line indicates spontaneous SR Ca2+ cycling (see text for details). Modified from [5]. B: Spontaneous beating of rabbit SANC critically depends upon Ca2+-related mechanisms and protein phosphorylation. Bars show a decrease in the beating rate (% control) induced by different drugs that affect Ca2+ cycling [ryanodine (Ry), BAPTA-AM], NCX (Li+ substitution for Na+), protein phosphorylation (PKI, H-89, MDL), or ion channels: If (Cs+) or T-type Ca2+ current (Ni2+). PKI and H-89 are protein kinase A inhibitors, and MDL is an adenylyl cyclase inhibitor. From [22]. C: Disabling RyR markedly reduces the stimulatory effects of CPT-cAMP on the spontaneous beating rate of rabbit SANC. Left panel: typical examples of the change in the spontaneous beating rate of rabbit SANC in response to CTP-cAMP, before and after exposure to ryanodine; right panel: the average effects; error bars indicate standard error of mean, * P<0.05. From [5].
6.1. Slowing of the Ca2+ clock by PKA-inhibition
Graded reduction of PKA-dependent phosphorylation prolongs the LCR period or abolishes LCRs in permeabilized, voltage-clamped, or spontaneously beating SANC (Fig. 2, Fig. 9A–C). In spontaneously beating cells this produces parallel effects on the spontaneous AP cycle length of SANC (Fig. 8B, “Phosphorylation”). The dose response of the suppression of the spontaneous beating rate, due to the inhibition of PKA phosphorylation, closely parallels that of the suppression of phospholamban phosphorylation at serine 16 (Fig. 10B). This effect of de-phosphorylation also likely involves graded effects on L-type Ca2+ channels [18] to reduce Ca2+ influx and cytosolic Ca2+, and on SR Ca2+ cycling proteins to decelerate Ca2+ cycling. In slowing the basal SR Ca2+ Clock and prolonging the LCR period (Fig. 9A–C), submaximal basal PKA inhibition concomitantly slows the spontaneous AP firing rate (Fig. 10). The extent to which the LCR period in individual, isolated SANC becomes increased by PKA inhibition is highly correlated with the concomitant prolongation of the spontaneous AP cycle length (Fig. 10). The time-dependent effects on beating rate, of submaximal, basal PKA inhibition and its reversal in individual SANC, are also highly correlated with the concomitant time-dependent changes effected in LCR period [5, 26]. After a prolonged time of submaximal PKA inhibition or following higher levels of PKA inhibition, LCRs are no longer detectable, and the rate of spontaneous AP’s markedly slows, becomes highly irregular and often ceases [5].
Figure 9.
Suppression (PKI, a specific inhibitor of PKA, 14–22 amide) or stimulation (isoproterenol, ISO) of PKA-dependent phosphorylation have opposite effects on frequency and spatio-temporal properties of LCRs in intact, spontaneously firing rabbit SANC. A: Confocal linescan images of a representative nodal cells depicting AP-induced Ca2+ transients and LCRs during spontaneous beating in control and when PKA phosphorylation was inhibited by PKI or stimulated by ISO. B and C: Histograms of LCR period and size (full width at half maximum) in control (n = 4 cells, 58 LCRs) and after superfusion with 5 μM PKI (n=4 cells, 25 LCRs). D and E: Histograms of LCR period and size in control (8 cells, 42 LCRs) and after superfusion with 0.1 μM ISO (8 cells, 89 LCRs). From [5]
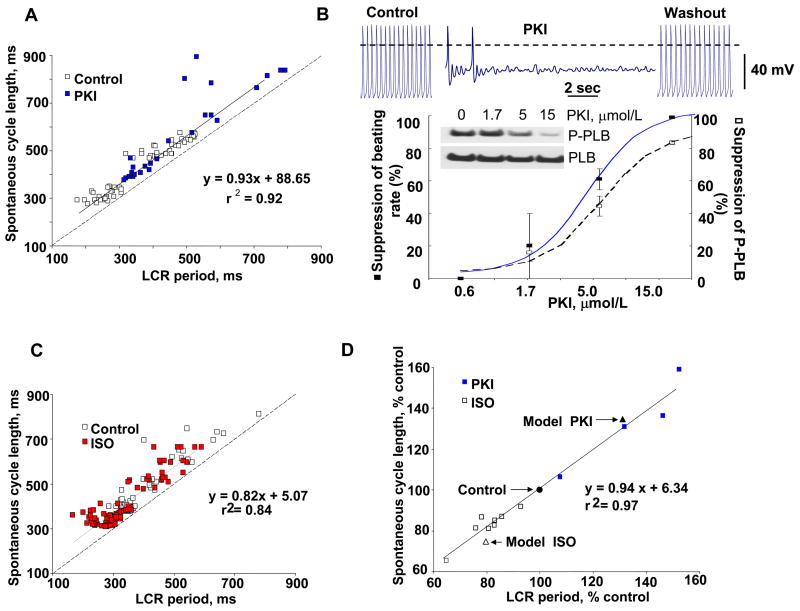
Figure 10.
A: The PKI effect to increase the spontaneous cycle length is linked to its effect on the LCR period. Note that this relationship lies above the line of identity (dashed line), indicating that the period of LCRs is shorter than the spontaneous cycle length. B: Typical example of APs recorded in a rabbit SANC before, during superfusion with 15 μM PKI, and during washout of the drug. The relationship of PKI-induced suppression of SANC beating rate (solid line) and PLB phosphorylation of SANC suspensions (dashed line). Inset shows Western blots of phosphorylated PLB and total PLB in response to increasing PKI concentrations. Error bars indicate standard error of mean. C: Relationship between LCR period and spontaneous cycle length is shifted to shorter periods by β-AR stimulation with 0.1 μM ISO. Dashed line is the line of identity. D: The relative PKI and ISO effects to alter spontaneous cycle length over a wide range are linked to their effects on the LCR period within the same cells. Note that this relationship conforms to the line of identity. Square symbols and solid line depict the experimentally obtained data; triangular symbols depict the average data simulated by numerical model using experimentally measured changes in LCR characteristics and phase. Modified from [5].
6.2. Acceleration of the Ca2+ clock by PKA-activation
As noted in above, increasing PKA-dependent phosphorylation reduces the LCR period (i.e. speeds up the Ca2+ clock) in permeabilized, voltage-clamped, or spontaneously beating SANC (Fig. 3; Fig. 9A, D–E). In spontaneously beating cells, the extent to which this occurs is highly correlated with the concomitant increase in the spontaneous beating rate (Fig. 10C,D). The crucial role of RyR-initiated LCRs in mediating the chronotropic response to cAMP-dependent signaling by β-AR stimulation has been demonstrated by employing different chronotropic perturbations in the presence and absence of ryanodine. In vitro, in single SANC, the effects of the cell-permeable cAMP [16], or β-AR stimulation [11], and in the intact organism, in vivo [16], to increase the spontaneous AP firing rate or heart rate are markedly blunted in the presence of ryanodine.
6.3. The set point of physiological regulation of pacemaker rate and rhythm is determined by PKA-dependent regulation of the Ca2+ clock
The relative change in AP firing rate among cells and the relative LCR period prolongation by PKI, or relative LCR period reduction by β-AR stimulation are extremely highly correlated, (r2 = 0.97 and this relationship lies on the line of identity, Fig. 10D). Since the Ca2+ clock controls the chronotropic state of the SANC via NCX (previous section), this phosphorylation-dependent gradation of the speed at which the Ca2+ clock ticks is the essence of both basal rate regulation and regulation of chronotropic reserve of SANC by neuromodulators [16]. This data (Fig. 10) correlating the relative beating rate increase or decrease and relative changes in PKA activation or inhibition, respectively, reveals that the basal SANC pacemaker clock speed is set to result in basal beating rate that is close to mid-point of the rate regulation range of spontaneously beating rates observed in isolated rabbit SANC (from approximately −30% to +20%, i.e. it is slightly shifted towards higher rates). Thus, the speed at which Ca2+ clock ticks and generates LCRs is variable, matching the chronotropic demand for a given condition, and is governed by the SR Ca2+ loading and Ca2+ release characteristics, which in turn, are governed by the degree of phosphorylation of its aforementioned Ca2+ cycling proteins.
7. Membrane ion channels are necessary but not sufficient to sustain normal pacemaker function and regulation
7.1. Membrane ion channels and transporters regulate the intracellular Ca2+ clock
Robust regulation of SANC pacemaker function requires tight integration of membrane and intracellular Ca2+ cycling. The Ca2+ cycling proteins of the SR, and sarcolemmal ion channels, mutually entrain and thus tightly control each other during spontaneous basal and β-AR-stimulated SANC firing. Multiple control points are mediated by the functional dependencies of the involved proteins on Ca2+, cAMP, phosphorylation, and membrane potential (Fig. 8A). The key integration elements include the L-type Ca2+ channels and NCX as their functions are both voltage and Ca2+ dependent (Fig. 1A). ICaL–related Ca2+ influx triggers global Ca2+ transient that causes a global SR Ca2+ depletion. This resets local SR Ca2+ clocks within the cell (Fig. 1B, see “Ca2+ clock phases: reset”) and thus synchronizes the appearance of their LCRs later in diastole. ICaL is finely tuned by the Ca2+ transient that it triggers (via its Ca2+ dependent inactivation and CaMKII dependence [25]), and NCX operation is tuned by the membrane voltage during the DD. Ca2+ influx via ICaL (and, possibly, via reverse NCX function) also “refuels” the Ca2+ clock by supplying Ca2+ to be pumped into the SR. The basal, sustained SANC Ca2+ clock function is supported not only by enhanced function of SR Ca2+ cycling proteins but also results from an enhanced L-type Ca2+ channel activity, as these channels seem to be highly phosphorylated in the basal state by PKA [18]. In general, modulatory changes of ion channel characteristics produced by Ca2+, cAMP, and cAMP/PKA-dependent phosphorylation (see review [2] for details) can be viewed as supporting the enhanced Ca2+ clock operation (dotted lines in Fig. 8A), as they tune and optimize LCRs: 1) a larger Ca2+ influx via Ca2+ current enhances the SERCA Ca2+ pumping rate; 2) a larger IKr effects a more rapid repolarization and shorter AP duration, providing sufficient time for removal of inactivation of Ca2+ channels within a shorter cycle length; 3) shift in If activation by high basal cAMP level and increase in the sustained current (Ist) limit the extent of hyperpolarization by the increased IKr, ensuring that a) LCR-activated INCX operates within the voltage range close to the ICaL activation threshold and b) cell Ca2+ is preserved from lost that would occur via enhanced NCX function at low voltages.
7.2. While ion channels define membrane excitability, they do not initiate AP in SANC: Pacemaker cycle begins from the Ca2+ clock-induced acceleration of membrane depolarization during the late DD
It is important to note that while ion channels tune and support long term operation of the Ca2+ clock, the Ca2+ clock operates independently of ion channels. Under voltage clamp at the maximum diastolic potential the Ca2+ clock can generate LCRs for at least several cycles (Fig. 11A) until SR Ca2+ depletion occurs (Fig. 11B). A failure to generate the critical LCR/INCX signals and exponentially rising DD phase leads to pacemaker failure, when Ca2+ influx via ICaL is normal (under ryanodine or NCX blockade [7]) or even enhanced, e.g. in ischemia-like conditions [27]. Robust pacemaker function requires the activation of LCRs and INCX during the late DD [7, 14, 20], i.e. that crucial time when voltage-gated K+ conductance has been stabilized and If has faded (due to its relatively low reversal potential), but ICaL is not yet activated (Fig. 1B). Thus, the initiation of the subsequent cycle begins with LCR ignition of NCX current and DD acceleration. This deviates categorically from the present dogma about pacemaker automaticity, which envisions that pacemaker ignition begins from maximum diastolic potential. Our hypothesis relegates the events that determine the maximum diastolic potential and early DD as “after effects” of the previous AP, known as an afterpotential (i.e. after hyperpolatrization in this case) that is a common feature of many cardiac cell types [1]. Earlier studies have shared our vision of initiation of true pacemaker activity that the pacemaker cycle begins with the DD acceleration. A detailed analysis of AP shapes in various regions of rabbit sinoatrial node revealed that the late acceleration of depolarization is observed as an earliest event within the pacemaker region [28]. Thus, intracellular Ca2+ cycling provides primary (i.e. depolarization-independent) rhythmic signals, which entrain sinoatrial node rhythmic excitations.
Figure 11.
A: LCR occurrences in the absence of changes in membrane potential in SANC with intact sarcolemma. Recordings of membrane potential (i), linescan image (ii), and normalized subsarcolemmal fluorescence (iii) averaged spatially over the band indicated by double headed arrow in (ii). (iv) Average total signal mass of LCRs during spontaneous beating and during voltage clamp (n = 14). B: Voltage clamp (VC) at the maximum diastolic potential decreases SR Ca2+ content and intracellular [Ca2+] in rabbit SANC. Spontaneous Ca2+ transients and caffeine-induced Ca2+ releases, indexed by F/F0, in a representative cell during spontaneous beating (i) and in a representative cell after several seconds of voltage clamp (iii). (ii) Average response to caffeine normalized to Ca2+ transient in cells during spontaneous beating and cells subjected to several seconds of voltage clamp. (iv) Averages of submembrane diastolic [Ca2+] before, during, and after voltage clamp in nine cells. *P<0.05. Error bars indicate standard error of mean. From [10].
β-AR stimulation enhances ICaL in the presence of ryanodine, to the same extent as in the absence of ryanodine, but the chronotropic effect of this stimulation is markedly blunted (both in vitro and in vivo) [5, 11]. A minor role of If in cardiac pacemaker mechanism has been also previously suggested based on a variety of other considerations, including its activation kinetics, voltage-dependency, and a minor change of the basal beating rate after its blockade [10, 11, 19, 29]. The impact of a greatly increased If found under ischemic-like conditions [27] fails to drive membrane potential towards the lower take-off potential (defined by the increased ICaL), similarly questions a major role of this so called “the pacemaker current” [30] in the pacemaker function of SANC.
Further, graded inhibition of PKA activity with PKI reveals a wide range of reserved/basal regulation of SANC spontaneous beating rate via PKA (discussed in previous section, Figs. 8B and 10); but this does not involve If, because this current is cAMP-(but not PKA-) dependent [31, 32]. As discussed above, β-AR stimulation of SANC beating rate is markedly blunted in the presence of ryanodine, indicating a minor role of the membrane clock (including If) in the rate regulation following βAR stimulation [5]. Although, Bucchi et al [33] found almost no difference in the effect of membrane-permeable cAMP (CPT-cAMP) on the SANC beating rate in the presence or absence of ryanodine, this result has been reinterpreted [5], as the increase in beating rate produced by CPT-cAMP in control conditions (Fig. 8C) was two-fold greater than reported by Bucchi et al. Further, a blockade of If by Cs+ resulted in minor changes of β-AR stimulation effect [11]. Thus, in our view the SANC rate regulation by a βAR stimulation or exogenously applied cAMP is mediated mainly via the ryanodine-sensitive, cAMP-PKA-modulated Ca2+ release mechanism, rather than Cs-sensitive, cAMP- modulated If. Finally, while knocking out either cardiac RyR (type 2) or NCX results in embryonic lethality (review [2]), hearts of mouse embryos lacking HCN4 (i.e. If channel) contract rhythmically, albeit at a reduced (approx. 50%) rate, that is, “a basal heart rate can be sustained without If” [34]. Thus, the portrayal of If as “the pacemaker current” (i.e. almost entirely mediating the cardiac pacemaker function) is no longer valid.
8. Testing control points of the Ca2+ clock: evidence for Ca2+-driven pacemaker mechanism in SANC
Multiple lines of evidence support the novel concept that normal pacemaker function is based on functional integration of membrane and submembrane Ca2+ cycling (Figs. 1A,B, and 8A). Any factors or interventions that either directly or indirectly influence LCRs (i.e. their period and/or signal mass) and/or global Ca2+ transient (resetting LCRs) have major consequences for spontaneous beating:
Graded suppression or complete blockade of Ca2+ cycling by buffering intracellular Ca2+ (Fig. 8B, “Cytosolic Ca2+“) slows and blocks beating. In addition to abolishing Ca2+ release following excitation and abolishing LCRs, chelation of Ca2+ by BAPTA may inhibit Ca2+-calmodulin dependent facilitation of ICaL[25] and affect other Ca2+ -dependent components of membrane clocks.
Ryanodine, a specific inhibitor of RyR function, produces graded suppression or blockade of LCRs, and, in a concentration dependent manner (IC50=3 μM), reduces the spontaneous SANC beating rate; at higher concentrations, ryanodine can abolish beating [7] (Fig. 8B, “Ryanodine receptors”).
In contrast to ryanodine, which specifically blocks the Ca2+ clock, a nifedipine blockade of L-type Ca2+ channels directly blocks the membrane clock and ICaL-mediated Ca2+ influx. This prevents resetting and refueling (see section 7.1) the Ca2+ clock leading to SR Ca2+ depletion and LCR abolition, culminating in pacemaker failure.
Cyclopiazonic acid, a selective Ca2+ ATP-ase antogonist, interferes with SR Ca2+ pumping function and slows SR Ca2+ clock. Application of this drug significantly increases LCR period and decreases spontaneous beating rate (~ by half) of both guinea pig SANC [35] and rabbit SANC [36].
Under conditions simulating ischemia in rabbit SANC, pacemaker function fails when ICaL and If increase, IKr is unchanged, and ICaT and INCX decrease [27, 37]. The failure of normal automaticity in this case is associated with the elimination of the late exponential phase of the DD. While ICaT is activated during the late DD, this current has a minor contribution into SAN pacemaker function in relatively large mammals, such as rabbit [38]. LCRs, in contrast, have major effects on the rabbit SANC beating rate via activation of NCX and accelerating the late DD (section 5) and are reduced by myocardial ischemia [39, 40].
Variations in the basal LCR period or the first “would-be” cycle during voltage clamp (both parameters assess the Ca2+ clock period) among different cells are tightly linked to variations in their spontaneous cycle length [10]. The LCRs emergence closely precedes the spontaneous APs, and thus can indeed prompt activation of the APs.
Cessation of spontaneous AP’s by voltage clamp at the maximum diastolic potential leads to SR Ca2+ depletion and LCR abolition (Fig. 11). Removal of the voltage clamp is followed by the gradual recovery of the control spontaneous cycling rate, and this gradual recovery is highly correlated with the return of LCRs and the recovery of the control LCR period [10].
Physiological regulation of Ca2+ clock by neuromediators: There is tight coupling among the degree of PKA-dependent protein phosphorylation, the speed at which the SR Clock generates LCRs, and the concomitant rate at which spontaneous AP’s are generated (Figs. 2,3,8B,9,10; see section 6 for details).
9. Novel numerical pacemaker models to test Ca2+ clock-driven pacemaker mechanism
Initial tests of the ability of LCRs during the DD to command the AP firing rate utilized a primary rabbit SANC model developed by Zhang et al. [41] When experimentally measured, submembrane, LCR waveforms were introduced into this model, spontaneous AP firing rate was easily entrained at a rate determined by the LCR period [19].
Subsequently, Kurata et al., model [42] described Ca2+ concentration in sub-membrane space and predicted strong negative chronotropic effect of Ca2+ chelation in this critical location. A novel pacemaker model [14] included LCR in the sub-membrane space and reproduced, for the first time, the negative chronotropic effect of ryanodine, and predicted a new powerful mechanism of rate regulation by varying Ca2+ release rate and phase: the larger release resulted in the larger INCX that, in turn, allowed wider rate regulation range by the phase of the release covering basically the entire physiological range. In terms of the fine DD structure, the model showed that the LCR-activated NCX current imparts exponentially rising part to the late DD that culminates in an AP upstroke [14].
A subsequent upgrade of the local Ca2+ release model reproduced individual stochastic LCRs (a multicompartment SR model) [5, 20]. Varying frequency, size, and phase of the LCRs according to experimental data, this model predicted the wide range of chronotropic effects that were experimentally observed with graded PKA activity, that is, basal and reserve rate regulation (Fig. 10D, “Model PKI” and “Model ISO”) via a stochastic ensemble of local NCX currents induced by the LCRs [5]. The model also provided further details of fine DD structure including its fluctuations and exponentially rising late DD component observed experimentally shortly before the excitation (Fig. 7, described in section 5), as well as modest beat-to-beat cycle variations due to the stochastic occurrence of the LCRs [20]. This model identified substantial benefits of interactions of the rhythmic LCRs and the membrane ion channel clock in terms of overall performance of the new pacemaker mechanism. Under conditions when ion channels operating alone in numerical models fail to generate rhythmic AP’s, stable and rhythmic AP firing resumes when the timely and powerful prompt to the membrane sent by SR via LCRs and NCX current is introduced into the model [26].
The numerical model approaches described above illustrated that the concept of Ca2+ release- ignited excitation is indeed operational in primary SANC, based on the existing knowledge on LCRs and SANC-specific ion channels. However, the Ca2+ release function in these model simulations was, either substituted by the experimental waveform, or approximated by a phenomenological mathematical function (in fact, a sine function). The most recent approaches to quantitatively describe Ca2+ integrated SANC function has included spontaneous Ca2+ release mechanisms [43] and local Ca2+ dynamics [44]. More specifically, this approach has developed a new model [43] of primary rabbit SANC, in which an integrated Ca2+ release mechanism is controlled by the SR Ca2+ load (modified from Shannon et al. 2004 [45]). This more mechanistic model now predicts SR Ca2+ depletion-refilling dynamics measured by Fluo-5N [36, 43] in spontaneously beating SANC and exhibits spontaneous Ca2+ oscillations under voltage clamp. Varying the Ca2+ clock model parameters results in a graded control of the timing of spontaneous Ca2+ release and the pacemaker rate. This result was corroborated by the experimental finding that cyclopiazonic acid, an inhibitor of SR Ca2+ ATPase, effects an ~50% reduction of the spontaneous beating rate of rabbit SANC [36]. These recent numerical model studies thus show that: 1) periodic SR Ca2+ refilling and release indeed define (quantitatively rather than speculatively or phenomenologically) the Ca2+ clock within SANC; and 2) beat-to-beat interaction of this Ca2+ clock with the membrane clocks results in a robust pacemaker function with an effectively controlled beating rate.
The great acceleration of computational power of modern computers permits development of an extension of the presently available models to simulate local Ca2+ dynamics in the submembrane space of SANC, based on approximation of Ca2+ diffusion and stochastic activation of RyR clusters (Ca2+ release units) [44]. These simulations predict the occurrence of local stochastic Ca2+ wavelets with spatiotemporal properties similar to those found for LCRs in SANC. In perspective, when the above two modeling approaches converge, a rather sophisticated, powerful SANC model integrating function of membrane channels, SR Ca2+ release mechanism, and local Ca2+ dynamics will likely emerge.
SUMMARY
The true cardiac pacemaker mechanism is complex and includes a balanced integration of both intracellular and membrane delimited processes. While the SANC ion channel membrane clocks (Fig. 1A) are necessary to effect an AP, they are not sufficient in our opinion to ignite an AP, or to generate rhythmic APs at normal rates. Rather, both experimental data and numerical simulations reviewed here demonstrate that an intracellular Ca2+ clock (Fig. 1A) and the rhythmic LCRs it generates do not merely “fine tune” the normal automaticity of SANC, but by igniting excitations from within the cell (see “Ignition” in Fig. 1A), represent the formal cause of APs. Thus, the rate at which the Ca2+ clock ticks, controls rhythmic, spontaneous AP firing across the broad range of physiologic firing rates. In short, variations of internal Ca2+ cycling within SANC result in corresponding variations in LCRs that are tightly linked to gradations in their normal automaticity. The spontaneous, but tightly controlled, rhythmic, SR Ca2+ clock of SANC insures the stability of the basal rhythm and sets the basal firing rate by integrating multiple Ca2+-dependent functions and rhythmically interacting with one, igniting the ensemble of surface membrane clocks to effect APs. Variable degrees of intrinsic PKA-dependent protein phosphorylation, and its attendant variations in intracellular Ca2+ regulate the time that the SR Ca2+ clock keeps, thus regulating the rate at which LCRs occurs, and modulate the voltage- and time-dependent kinetics of the membrane clocks, thus insuring that they produce APs with characteristics commensurate with given rhythmic ignition rates commanded by LCRs. Interfering with SR internal Ca2+ cycling interferes with normal pacemaker functions, just as does interfering with membrane ion channels that generate the AP upstroke and repolarization (Fig. 8B). Thus, the true initiation of cardiac pacemaker automaticity begins during the late part of the DD when the Ca2+ clock ignites the surface membrane excitation. Earlier events such as the maximum diastolic potential and early, slow DD ensure recovery of the membrane clocks that are activated during the prior AP and need to be reset before next AP.
In the intact organism, integrated autonomic nervous system signals confer another layer of regulation of the intrinsic cAMP/PKA/Ca2+ signaling and of the SANC, SR Ca2+ Clock and membrane clocks of SANC: vagal stimulation of cholinergic receptors predominates in the basal state, keeping the SR Ca2+ clock in check; in response to a demand for higher heart rates, stimulation of cholinergic receptors wanes, and sympathetic stimulation of β-adrenergic waxes; at the maximum chronotropic rate, β-AR activation of PKA-dependent phosphorylation is maximal, and the SR Ca2+ clock runs at full speed, activating NCX and igniting the membrane clock at a maximum rate in order to achieve the maximum heart rate.
The evidence for the crucial role of Ca2+ and rhythmic spontaneous Ca2+ releases in the initiation and regulation of normal cardiac automaticity provides the key that reunites pacemaker and ventricular cell research [2, 6]. The separate achievements of reductionist approaches to elucidate how strength of contraction of ventricular myocytes and the spontaneous beating rate of pacemaker cells are regulated now converge: Ca2+ cycling into and from the SR by proteins common to both ventricular myocytes and pacemaker cells can be portrayed as Ca2+ clock. The mutual entrainment of the Ca2+ clock and surface membrane ion channel clocks regulate the duty cycle of both cell types. Thus, a general theory of cardiac inotropy and chronotropy emerges.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cranefield PF. Action potentials, after potentials, and arrhythmias. Circ Res. 1977;41:415–23. doi: 10.1161/01.res.41.4.415. [DOI] [PubMed] [Google Scholar]
- 2.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–69. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- 3.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–44. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature. 1960;188:495–7. doi: 10.1038/188495b0. [DOI] [PubMed] [Google Scholar]
- 5.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–14. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG. Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium. 2004;35:629–42. doi: 10.1016/j.ceca.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–8. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 8.Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524(Pt 2):415–22. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- 10.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004;94:802–9. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- 11.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–9. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 12.Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–89. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997;272:H657–68. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- 14.Maltsev VA, Vinogradova TM, Bogdanov KY, Lakatta EG, Stern MD. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: numerical modeling of the coupling process. Biophys J. 2004;86:2596–605. doi: 10.1016/S0006-3495(04)74314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capogrossi MC, Suarez-Isla BA, Lakatta EG. The interaction of electrically stimulated twitches and spontaneous contractile waves in single cardiac myocytes. J Gen Physiol. 1986;88:615–33. doi: 10.1085/jgp.88.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinogradova TM, Ruknudin AM, Zhu W, Lyashkov AE, Volkova M, Boheler KR, Xiao RP, Spurgeon H, Lakatta EG, et al. High basal cAMP content markedly elevates PKA-dependent protein phosphorylation and sustains spontaneous beating in rabbit sinoatrial nodal pacemaker cells (SANC) Biophys J. 2006;90:155a. Abstract. [Google Scholar]
- 17.Vinogradova TM, Lyashkov AE, Zhu W, Spurgeon H, Maltsev VA, Lakatta EG. Constitutive phosphodiesterase activity confers negative feedback on intrinsic cAMP-PKA regulation of local rhythmic subsarcolemmal calcium releases and spontaneous beating in rabbit sinoatrial nodal. Biophysical Journal. 2005;88:303a. Abstract. [Google Scholar]
- 18.Petit-Jacques J, Bois P, Bescond J, Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993;423:21–7. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- 19.Lakatta EG, Maltsev VA, Bogdanov KY, Stern MD, Vinogradova TM. Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res. 2003;92:e45–50. doi: 10.1161/01.res.0000055920.64384.fb. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006;99:979–87. doi: 10.1161/01.RES.0000247933.66532.0b. [DOI] [PubMed] [Google Scholar]
- 21.Bers DM. The beat goes on: diastolic noise that just won’t quit. Circ Res. 2006;99:921–3. doi: 10.1161/01.RES.0000249859.10103.a9. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Vinogradova T, Lyashkov A, Sirenko S, Zhu W, Ruknudin A, Maltsev VA. The Integration of Spontaneous Intracellular Ca2+ Cycling and Surface Membrane Ion Channel Activation Entrains Normal Automaticity in Cells of the Heart’s Pacemaker. Ann N Y Acad Sci. 2006;1080:178–206. doi: 10.1196/annals.1380.016. [DOI] [PubMed] [Google Scholar]
- 23.Anumonwo JM, Delmar M, Vinet A, Michaels DC, Jalife J. Phase resetting and entrainment of pacemaker activity in single sinus nodal cells. Circ Res. 1991;68:1138–53. doi: 10.1161/01.res.68.4.1138. [DOI] [PubMed] [Google Scholar]
- 24.Abramovich-Sivan S, Akselrod S. A single pacemaker cell model based on the phase response curve. Biol Cybern. 1998;79:67–76. doi: 10.1007/s004220050459. [DOI] [PubMed] [Google Scholar]
- 25.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res. 2000;87:760–7. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 26.Maltsev VA, Vinogradova TM, Stern MD, Lakatta EG. Local subsarcolemmal Ca2+ releases within rabbit sinoatrial nodal cells not only regulate beating rate but also ensure normal rhythm during protein kinase A inhibition. Biophys J. 2005;88:89a–90a. Abstract. [Google Scholar]
- 27.Du YM, Nathan RD. Ionic basis of ischemia-induced bradycardia in the rabbit sinoatrial node. J Mol Cell Cardiol. 2006 doi: 10.1016/j.yjmcc.2006.10.004. in print. [DOI] [PubMed] [Google Scholar]
- 28.Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980;46:11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Vassalle M. The pacemaker current (I(f)) does not play an important role in regulating SA node pacemaker activity. Cardiovasc Res. 1995;30:309–10. [PubMed] [Google Scholar]
- 30.DiFrancesco D. The contribution of the ‘pacemaker’ current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiFrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiFrancesco D, Mangoni M. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J Physiol. 1994;474:473–82. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. I(f)-dependent modulation of pacemaker rate mediated by cAMP in the presence of ryanodine in rabbit sino-atrial node cells. J Mol Cell Cardiol. 2003;35:905–13. doi: 10.1016/s0022-2828(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 34.Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A. 2003;100:15235–40. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 2006;571:639–49. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinogradova TM, Brochet DX, Sirenko SG, Lyashkov AE, Maltsev VA, Yang D, Cheng H, Lakatta EG. Sarcoplasmic reticulum (SR) Ca2+ refilling kinetics controls the period of local subsarcolemmal Ca2+ releases (LCR) and the spontaneous beating rate of sinoatrial node cells (SANC) Biophys J. 2007:31a. [Google Scholar]
- 37.Maltsev VA, Lakatta EG. Cardiac pacemaker cell failure with preserved I(f), I(CaL), and I(Kr): A lesson about pacemaker function learned from ischemia-induced bradycardia. J Mol Cell Cardiol. 2007;42:289–294. doi: 10.1016/j.yjmcc.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vassort G, Talavera K, Alvarez JL. Role of T-type Ca2+ channels in the heart. Cell Calcium. 2006;40:205–20. doi: 10.1016/j.ceca.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Stern MD, Weisman HF, Renlund DG, Gerstenblith G, Hano O, Blank PS, Lakatta EG. Laser backscatter studies of intracellular Ca2+ oscillations in isolated hearts. Am J Physiol. 1989;257:H665–73. doi: 10.1152/ajpheart.1989.257.2.H665. [DOI] [PubMed] [Google Scholar]
- 40.Weiss RG, Gerstenblith G, Lakatta EG. Calcium oscillations index the extent of calcium loading and predict functional recovery during reperfusion in rat myocardium. J Clin Invest. 1990;85:757–65. doi: 10.1172/JCI114501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Holden AV, Kodama I, Honjo H, Lei M, Varghese T, Boyett MR. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol. 2000;279:H397–421. doi: 10.1152/ajpheart.2000.279.1.H397. [DOI] [PubMed] [Google Scholar]
- 42.Kurata Y, Hisatome I, Imanishi S, Shibamoto T. Dynamical description of sinoatrial node pacemaking: improved mathematical model for primary pacemaker cell. Am J Physiol Heart Circ Physiol. 2002;283:H2074–101. doi: 10.1152/ajpheart.00900.2001. [DOI] [PubMed] [Google Scholar]
- 43.Maltsev VA, Brochet DX, Parsons SP, Vinogradova TM, Sirenko SG, Cheng H, Stern MD, Lakatta EG. A numerical model of a Ca2+ clock within sinoatrial node cells: Interactive membrane and submembrane Ca2+ cycling provides a novel mechanism of normal cardiac pacemaker function. Biophys J. 2007:77a. [Google Scholar]
- 44.Maltsev AV, Maltsev VA, Sirenko SG, Maltseva LA, Parsons SP, Lakatta EG, Stern MD. A simple stochastic mechanism of a roughly periodic Ca2+ clock within cardiac cells. Biophys J. 2007:344a. doi: 10.1016/j.bpj.2010.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–71. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]