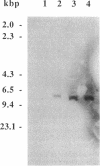
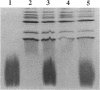
Abstract
Protease III, the product of the ptr gene, is a 110-kDa periplasmic protease with specificity towards insulin and other low-molecular-weight substrates (less than 7,000 molecular weight) in vitro (Y.-S.E. Cheng and D. Zipser, J. Biol. Chem. 254:4698-4706, 1979). Escherichia coli strains deficient in protease III were constructed by insertional inactivation of the ptr gene. This mutation did not appear to affect the function of the adjoining recB and recC genes. Expression of protein A-beta-lactamase, a protease-sensitive secreted polypeptide, was increased approximately twofold in ptr cells. A comparable increase in the half-life of protein A-beta-lactamase was observed by pulse-chase experiments, suggesting that protease III is involved in the catabolism of high-molecular-weight substrates in vivo, ptr mutants exhibited no detectable phenotypic alterations except for a slight reduction in growth rate. When the ptr mutation was transferred to a strain deficient in the secreted protease DegP, a further decrease in growth rate, as well as an additive increase in the expression of the fusion protein, was observed. A ptr degP ompT mutant strain resulted in a further increase in expression in minimal medium but not in rich medium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anba J., Bernadac A., Lazdunski C., Pagès J. M. Improving the stability of a foreign protein in the periplasmic space of Escherichia coli. Biochimie. 1988 Jun;70(6):727–733. doi: 10.1016/0300-9084(88)90101-0. [DOI] [PubMed] [Google Scholar]
- Baneyx F., Georgiou G. In vivo degradation of secreted fusion proteins by the Escherichia coli outer membrane protease OmpT. J Bacteriol. 1990 Jan;172(1):491–494. doi: 10.1128/jb.172.1.491-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A. M., Smith G. R. Escherichia coli recBC deletion mutants. J Bacteriol. 1984 Nov;160(2):788–791. doi: 10.1128/jb.160.2.788-791.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D. Purification and characterization of protease III from Escherichia coli. J Biol Chem. 1979 Jun 10;254(11):4698–4706. [PubMed] [Google Scholar]
- Claverie-Martin F., Diaz-Torres M. R., Kushner S. R. Analysis of the regulatory region of the protease III (ptr) gene of Escherichia coli K-12. Gene. 1987;54(2-3):185–195. doi: 10.1016/0378-1119(87)90486-0. [DOI] [PubMed] [Google Scholar]
- Cook R. A. Periplasmic proteases of Escherichia coli. Crit Rev Biotechnol. 1988;8(3):159–175. doi: 10.3109/07388558809147554. [DOI] [PubMed] [Google Scholar]
- Dykstra C. C., Kushner S. R. Physical characterization of the cloned protease III gene from Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):1055–1059. doi: 10.1128/jb.163.3.1055-1059.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra C. C., Prasher D., Kushner S. R. Physical and biochemical analysis of the cloned recB and recC genes of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):21–27. doi: 10.1128/jb.157.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Wilson R. E., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of the Escherichia coli ptr gene encoding protease III. Nucleic Acids Res. 1986 Oct 10;14(19):7695–7703. doi: 10.1093/nar/14.19.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Kuys Y., Zwieb C., Taatjes D., Taatjes H., Bannwarth W., Stueber D., Ibrahimi I. Association of degradation and secretion of three chimeric polypeptides in Escherichia coli. J Bacteriol. 1988 May;170(5):2212–2220. doi: 10.1128/jb.170.5.2212-2220.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou G., Baneyx F. Expression, purification, and immobilization of a protein A-beta-lactamase hybrid protein. Ann N Y Acad Sci. 1990;589:139–147. doi: 10.1111/j.1749-6632.1990.tb24240.x. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Genetics of proteolysis in Escherichia coli*. Annu Rev Genet. 1989;23:163–198. doi: 10.1146/annurev.ge.23.120189.001115. [DOI] [PubMed] [Google Scholar]
- Greenwood J. M., Gilkes N. R., Kilburn D. G., Miller R. C., Jr, Warren R. A. Fusion to an endoglucanase allows alkaline phosphatase to bind to cellulose. FEBS Lett. 1989 Feb 13;244(1):127–131. doi: 10.1016/0014-5793(89)81177-9. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Maintenance of the bacteriocinogenic plasmid Clo DF13 in Escherichia coli cells. II. Specific recombination functions involved in plasmid maintenance. Mol Gen Genet. 1982;188(2):338–344. doi: 10.1007/BF00332698. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Beppu N., Mizushima S. Protease IV, a cytoplasmic membrane protein of Escherichia coli, has signal peptide peptidase activity. J Biol Chem. 1984 Aug 10;259(15):9853–9857. [PubMed] [Google Scholar]
- Ichihara S., Suzuki T., Suzuki M., Mizushima S. Molecular cloning and sequencing of the sppA gene and characterization of the encoded protease IV, a signal peptide peptidase, of Escherichia coli. J Biol Chem. 1986 Jul 15;261(20):9405–9411. [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski A. M. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. FEMS Microbiol Rev. 1989 Sep;5(3):265–276. doi: 10.1016/0168-6445(89)90035-1. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Park S. C., Goldberg A. L., Chung C. H. Protease So from Escherichia coli preferentially degrades oxidatively damaged glutamine synthetase. J Biol Chem. 1988 May 15;263(14):6643–6646. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Oliver D. B., Goldberg E. B. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol. 1977 Nov;116(4):877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. Protease IV and protease V. J Biol Chem. 1982 Apr 25;257(8):4333–4339. [PubMed] [Google Scholar]
- Palmer S. M., St John A. C. Characterization of a membrane-associated serine protease in Escherichia coli. J Bacteriol. 1987 Apr;169(4):1474–1479. doi: 10.1128/jb.169.4.1474-1479.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman J. E., Levine R. L. Purification of a protease from Escherichia coli with specificity for oxidized glutamine synthetase. J Biol Chem. 1987 Feb 15;262(5):2101–2110. [PubMed] [Google Scholar]
- Schultz D. W., Taylor A. F., Smith G. R. Escherichia coli RecBC pseudorevertants lacking chi recombinational hotspot activity. J Bacteriol. 1983 Aug;155(2):664–680. doi: 10.1128/jb.155.2.664-680.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Halpern Y. S., Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971 May 25;246(10):3320–3329. [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Nishihara T. Purification, characterization, and primary structure of Escherichia coli protease VII with specificity for paired basic residues: identity of protease VII and OmpT. J Bacteriol. 1988 Dec;170(12):5625–5632. doi: 10.1128/jb.170.12.5625-5632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Itoh A., Ichihara S., Mizushima S. Characterization of the sppA gene coding for protease IV, a signal peptide peptidase of Escherichia coli. J Bacteriol. 1987 Jun;169(6):2523–2528. doi: 10.1128/jb.169.6.2523-2528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenhan D., Wackernagel W. Functional analyses of Proteus mirabilis wild-type and mutant RecBCD enzymes in Escherichia coli reveal a new mutant phenotype. Mol Microbiol. 1989 Dec;3(12):1777–1784. doi: 10.1111/j.1365-2958.1989.tb00163.x. [DOI] [PubMed] [Google Scholar]