Abstract
Objectives
Lead and homocysteine are both linked to cardiovascular disease. With this in mind, the authors evaluated the relation between blood lead and homocysteine in people aged 19–66 years in two Asian populations.
Methods
This cross‐sectional study comprised 183 workers from a lead stabiliser factory in Singapore and 323 workers from a battery factory in Vietnam. Workers were occupationally exposed to lead. Blood lead was analysed using atomic absorption spectrophotometry while plasma homocysteine was measured using high performance liquid chromatography.
Results
Chinese subjects had the lowest blood lead levels while the Indians had the highest. Controlling for age, sex and race, an increase of 1 μg/dl in blood lead was associated with an increase of 0.04 μmol/l of homocysteine on the log scale. Gender and ethnicity seemed to be strongly associated with the relation between lead and homocysteine. The positive relation between lead and homocysteine among the Vietnamese subjects was significant (Pearson's r = 0.254, p<0.01). When blood lead levels were divided by quartiles, the correlation coefficient between blood lead levels in the 4th quartile and homocysteine among the Vietnamese was higher (r = 0.405, p<0.01). Overall, an increase of 1 μg/dl in blood lead in all the Vietnamese subjects was associated with an increase of 0.05 μmol/l increase in homocysteine on the log scale. However, in the 4th quartile, the same increase was associated with an increase of 0.41 μmol/l of homocysteine on the log scale.
Conclusions
Blood lead was found to be associated with homocysteine levels in this Asian sample. Although we cannot determine causality from cross‐sectional data, it is sensible to consider the probability that this relation could explain one of the mechanisms of the impact of lead on the cardiovascular system. More studies would be needed to confirm this inference.
Lead is a well‐documented hazardous substance and its toxicity is a major concern as millions of people are estimated to be exposed to excessive levels of lead through environmental and occupational settings. Lead is not eliminated from the body easily and can reside in the system for years. Low blood lead levels have been associated with increased blood pressure, elevated risk of hypertension, increased circulatory and cardiovascular mortality, kidney damage, anaemia, toxicity to the reproductive system and progressive decline in cognitive function over time, even after cessation of exposure.1 Despite the vast amount of research done on lead toxicity, the mechanisms for these effects remain unclear.
Homocysteine has also been linked with cardiovascular disease.2 Elevated homocysteine levels increase the risk of heart disease, stroke, peripheral vascular disease and cognitive impairment.3 Homocysteine may cause vascular damage by impairing vascular endothelial smooth muscle cell function.4 This impairment may be brought about by proliferation of vascular smooth muscle cells, altered elasticity of the vascular wall, inhibition of nitric oxide synthesis and increased oxidative stress.
Although the similarities in these health effects are apparent, there have been few studies associating homocysteine and lead to date. To our knowledge, there has only been one study by Schafer et al5 to report an association between blood lead and homocysteine among an elderly population (50–70 years of age) with no known exposure to inorganic lead. In this study, we report the relation between blood lead and homocysteine in a study of people aged 19–66 years in Singapore and Vietnam. Participants, who were occupationally exposed to lead, were selected from a factory that deals with lead additives (Singapore) and a factory that manufactures car batteries (Vietnam). The main objectives were (a) to evaluate relations between blood lead levels with homocysteine, controlling for age, race/ethnicity, gender and other potential confounding factors; and (b) to evaluate whether these relations were modified by age, gender or race/ethnicity.
Materials and methods
The design of the study was cross‐sectional in nature. The study was approved by the National University Hospital Ethics Committee. Informed consent was obtained from each worker before the start of the study.
Study population
The study population consisted of 183 workers from a PVC lead stabiliser factory in Singapore and 323 workers from a battery factory in Hai Phong City, Vietnam. All workers from the production line as well as in the division of management and quality control were recruited into this study. Of the 323 Vietnamese workers, 246 were occupationally exposed to lead, and the remaining 77 were not directly exposed to lead. Although these 77 workers were not directly exposed to lead, many had a previous history of exposure. Some were still exposed, albeit less than in the group of 246. Therefore, these workers were also included in the study. Twenty of the exposed lead workers were not present during the study period, and 17 did not want to participate. Of the 77 workers who were not directly exposed to lead, 10 were unwilling to give their blood for analysis. Because the study was strictly on a voluntary basis, the workers' decision to not provide blood was respected. Hence, 47 workers were excluded in the study, giving a response rate of 85.4% (276 of 323). The 183 Singaporean workers comprised the three major ethnic groups in Singapore, namely Chinese, Indian and Malay. The main exposure in this group was lead oxide dust. The workers also filled out a standard questionnaire with the help of a trained technician. For the Vietnamese group, the interview was conducted by a Vietnamese interviewer using a translated questionnaire. After completing the questionnaire, 10 ml of blood was collected from each worker during a medical examination by an occupational health practitioner. Signed consent was obtained from each worker before these samples were taken for subsequent analysis.
Questionnaire
All data were collected by trained research assistants. A structured questionnaire included information such as age, years of education, detailed occupational history, and current and previous smoking habits. Alcohol intake was also documented. Actual amount of alcohol consumption per day (that is, types of drink consumed and amount estimated by number of bottles consumed) and number of years of drinking were noted.
Blood lead measurement
We obtained blood samples by venipuncture with lead‐free disposable syringes and stored the samples in heparinised lead‐free polypropylene tubes. The blood samples were centrifuged within two hours of the blood collection and plasma separated into different vials. Plasma samples were stored at −70°C until analysis. Blood lead was analysed using atomic absorption spectrophotometry (Varian Spectra AA‐30; SiberHegner Pte Ltd, Victoria, Australia) with a graphite furnace. Strict external quality control was carried out under the External Quality Assessment Scheme (NEQAS) in the UK and the Inter‐laboratory Comparison Programme in Canada. We calculated the absolute difference between the reference laboratory and our laboratory result. The mean percentage (%) difference between NEQAS values and our results was <5% for the last 10 years. We analysed all samples within one month of arrival in Singapore at our laboratory.
Homocysteine measurement
The plasma total homocysteine was measured using high performance liquid chromatography (HPLC) with electrochemical detection using a method modified from Malinow et al6 and Wu et al.7 Briefly, 50 μl of plasma and 50 μl of water were mixed thoroughly with 150 μl of 9 M urea (pH 9.0). A volume of 25 μl of n‐amylalcohol was then added as an antifoaming agent and 25 μl of 10% (wt/vol) sodium borohydride which was prepared in 0.1 N NaOH was incubated with samples at 45°C for 30 min. After the reaction was stopped by adding 200 μl of 20% (wt/vol) trichloroacetic acid, the sample was centrifuged at 15 000 g for 2 min. The supernatant was further cleaned up using Costar Spin‐X micro‐filtration tube and centrifuged for another 2 min. A 10 μl aliquot of the filtrate was injected onto a PartiSphere 5 C18 column (5 μm, 110×4.7 (id) mm, Whatman, Clifton, NJ, USA) for HPLC analysis. The HPLC system comprised a Waters Alliance 2695 Separation Module (MA, USA) and an Agilent Model 1049A electrochemical detector (Palo Alto, CA, USA) equipped with a solid state silver/silver chloride reference electrode over a gold‐working electrode (potential +150 mV, full instrument scale at 50 nA). Isocratic elution was carried out with a flow rate of 1 ml/min of mobile phase (pH 2.25) containing 0.1 M sodium dihydrogen phosphate, 4% (vol/vol) acetonitrile, 1 mM octanesulfonic acid and 20 mM phosphoric acid. Data acquisition was performed with Waters Empower software and homocysteine of a pure standard (50 μmol/ml) and an unknown sample (23.6 μmol/ml) were detected at 3.8 min. For quality control, two control samples with 5.9 and 18.8 μmol/ml of homocysteine were analysed in between every 15 unknown samples. The coefficients of variations of these control samples was <8% for within‐day assay (n = 3) and <15% for between‐day (n = 5) analysis.
Statistical analysis
Of the 459 people enrolled, 33 were missing homocysteine values, 10 had no blood lead values, 2 were missing information on alcohol consumption and 1 was missing information on tobacco use. Subjects with missing homocysteine data were not statistically different regarding blood lead, age, gender or race/ethnicity. The distributions of the variables were checked individually with histograms and standardised normal probability (P‐P) plots. In order to achieve normal distribution, necessary transformation was done on important variables.
Pearson's correlation test was used to study the correlation between blood lead and homocysteine levels. We used multiple linear regression to assess predictors of homocysteine levels, controlling for covariates. Models were constructed including known covariates (for example, age, gender and race/ethnicity) and other potential confounding variables such as smoking status, alcohol consumption and exposure duration. Variables were retained in the final models if they were significantly associated with homocysteine levels (p<0.05). The resulting model included age, race/ethnicity and gender. As elaborated below, we did the regression using the dataset without the outlier.
When we examined the data, there appeared to be an outlying subject whose homocysteine level was 41.08 μmol/l and blood lead level was 44.8 μg/dl. Univariate analysis such as box plot had also indicated that this subject might be an outlier, and so in consideration that this outlier might be the result of a data entry error, we performed regression analysis with the exclusion of this subject. Similar results were obtained before dropping this observation.
Non‐linearity was checked by fitting a fractional polynomial of at most degree 2. This was performed for both continuous covariates, namely blood lead level and age. The best‐fitting powers for blood lead level and age were 3 (p = 0.037) and 1 (p<0.001) respectively, both of degree 1. The resulting fractional polynomial plot, which included the fitted regression line and its 95% confidence band, is shown in the next section.
We evaluated effect modification by including cross‐product terms in the model. However, no statistically significant effect modification was found, using a p value cut‐off of 0.05. To assess the validity of the final model, regression diagnostics were performed. All statistical analyses were performed using Stata version 8.0 (Stata Corporation, College Station, TX, USA).
Results
A total of 459 subjects participated in the study. Study subjects were 84% male and had a mean age of 38.7 (19–66) years and mean exposure duration of 12.9 (0.1–41) years. Of the Singapore group, 21.6% were Chinese, 9.6% Malay and 8.7% Indian. The other 60.1% made up the Vietnamese group. The mean homocysteine level was significantly higher (p<0.0001) for males (8.0 μmol/l, n = 384) compared with females (4.6 μmol/l, n = 75). The mean homocyteine level of the Vietnamese (6.2 μmol/l, n = 276) was significantly lower (p<0.0001; for all) compared to the Chinese, Malays and Indians who had mean homocysteine levels of 9.3 μmol/l, 10.4 μmol/l and 9.3 μmol/l; respectively. This finding was still true even after the means were adjusted for possible confounders such as age and gender (table 1).
Table 1 Characteristics of study populations by blood lead and homocysteine concentrations.
| n (%) | Geometric mean (SD) | p Value | Geometric mean (SD) | p Value | Adjusted geometric mean* | |
|---|---|---|---|---|---|---|
| Blood lead | Homocysteine | Homocysteine | ||||
| Nation | ||||||
| Vietnam | 276 (60.1) | 20.6 (1.9) | <0.001 | 6.2 (1.4) | <0.001 | |
| Singapore | 183 (39.9) | 16.8 (1.9) | 9.6 (1.4) | |||
| Gender | ||||||
| Male | 384 (83.7) | 20.2 (1.9) | <0.001 | 8.0 (1.4) | <0.001 | |
| Female | 75 (16.3) | 13.5 (2.0) | 4.6 (1.3) | |||
| Ethnic group | ||||||
| Vietnamese | 276 (60.1) | 20.6 (1.9) | <0.001† | 6.2 (1.4) | <0.001† | 5.5 |
| Chinese | 99 (21.6) | 11.9 (2.0) | 9.3 (1.3) | 8.3 | ||
| Malays | 44 (9.6) | 25.0 (1.4) | 10.4 (1.2) | 10.1 | ||
| Indians | 40 (8.7) | 25.5 (1.5) | 9.3 (1.2) | 9.6 | ||
| Smoke | ||||||
| Smoker | 227 (49.3) | 18.2 (1.9) | 0.187 | 6.6 (1.5) | <0.001† | |
| Non‐smoker | 230 (50.3) | 20.0 (1.9) | 8.1 (1.3) | |||
| Drink | ||||||
| No | 105 (22.9) | 15.9 (1.9) | 0.002† | 5.5 (1.4) | <0.001† | |
| Occasional | 205 (44.9) | 19.8 (1.8) | 7.7 (1.4) | |||
| Regular | 147 (32.2) | 20.0 (2.0) | 8.3 (1.3) |
*Adjusted for age and gender (drinking and smoking habits were taken out as they did not contribute significantly to the model).
†ANOVA results.
Mean blood lead: significant differences between Chinese and Malays, Chinese and Indians, Chinese and Vietnamese (p all <0.001).
Mean homocysteine: significant differences between Vietnamese and Chinese, Vietnamese and Malays, Vietnamese and Indians (p all <0.001). Similar results for adjusted homocysteine means.
Mean blood lead: significant differences between non‐drinkers and occasional drinkers (p = 0.015), non‐drinkers and regular drinkers (p = 0.019).
Mean homocysteine: significant differences between non‐drinkers and occasional drinkers (p = 0.015), non‐drinkers and regular drinkers (p all <0.001).
Table 2 shows the demographic characteristics of the study population by lead quartile subsets. The mean homocysteine level in the 4th blood lead quartile (8.5 μmol/l) was significantly higher (p = 0.022) than the mean homocysteine level in the 1st blood lead quartile (7.2 μmol/l). In the Singapore group, the Chinese subjects had the lowest blood lead levels compared to the Malay and Indian subjects (table 2). Comparing data across the two countries however showed that the Vietnamese subjects had higher blood lead levels (data not shown). Subjects in different blood lead quartiles did not differ greatly in terms of smoking and alcohol habits.
Table 2 Demographic characteristics of the study population by lead quartile subsets.
| Total (n = 449) | Blood lead quartile | p Value | ||||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 112) | Quartile 2 (n = 113) | Quartile 3 (n = 113) | Quartile 4 (n = 111) | |||
| Blood lead level, μg/dl (mean (range)) | 22.7 (2.0–66.9) | 8.7 (2.0–13.2) | 16.9 (13.3–20.4) | 24.8 (20.6–29.4) | 40.4 (29.7–66.9) | |
| Homocysteine, μmol/l (mean (SD)) | 7.8 (3.3) | 7.2 (2.8)* | 8.1 (3.0) | 7.5 (2.7) | 8.5 (4.3)* | 0.022 |
| Age, years (mean (SD)) | 38.7 (10.7) | 39.9 (9.9) | 39.7 (11.0) | 37.5 (11.7) | 37.5 (9.7) | 0.153 |
| Exposure duration, years (mean (SD)) | 12.9 (9.8) | 14.1 (9.8) | 13.1 (9.9) | 11.9 (9.9) | 12.3 (9.1) | 0.375 |
| Race/ethnicity (%) | ||||||
| Vietnamese | 21.6 | 23.3 | 22.9 | 25.6 | 28.2 | |
| Chinese | 9.6 | 46.5 | 32.3 | 18.2 | 3.0 | |
| Malay | 8.7 | 6.8 | 20.5 | 34.1 | 38.6 | |
| Indian | 60.1 | 2.5 | 27.5 | 30.0 | 40.0 | |
| Current cigarette use (%) | ||||||
| Smoker | 49.8 | 54.5 | 47.8 | 49.6 | 46.4 | |
| Non‐smoker | 50.2 | 45.5 | 52.2 | 50.4 | 53.6 | |
| Alcoholic beverage use (%) | ||||||
| None | 23.0 | 33.3 | 16.8 | 28.3 | 11.8 | |
| Social drinker (<1 per week) | 44.9 | 37.8 | 51.3 | 41.6 | 48.2 | |
| Regular drinker (at least once a day) | 32.2 | 28.8 | 31.9 | 30.1 | 40.0 | |
*Significant difference (p = 0.03) one‐way ANOVA.
We next used multiple linear regression to evaluate predictors of homocysteine levels, controlling for covariates (table 3). Controlling for age, gender, and race/ethnicity, the results showed that an increase of 1 μg/dl in log(blood lead) was associated with an increase of 0.04 μmol/l of log(homocysteine). There appeared to be a strong negative association between lead and homocysteine in the comparison of females to males, as well as Vietnamese to Chinese (table 3).
Table 3 Predictors of homocysteine levels in subjects with complete data (n = 422), adjusted for variables in table.
| Total (n = 422) | ||
|---|---|---|
| β (95% CI) | p Value | |
| Blood lead level (μg/dl) | 0.040 (−0.001 to 0.082) | 0.058 |
| Age (years) | 0.005 (0.003 to 0.008) | <0.001 |
| Female | −0.415 (−0.486 to −0.344) | <0.001 |
| Race* | ||
| Malay | 0.029 (−0.074 to 0.132) | 0.577 |
| Indian | −0.022 (−0.126 to 0.082) | 0.672 |
| Vietnamese | −0.363 (−0.429 to 0.296) | <0.001 |
R2 = 0.537; adjusted R2 = 0.530.
*Reference group = Chinese subjects.
There was a significant relation (r = 0.255, p<0.01) between blood lead and homocysteine levels among the Vietnamese subjects. When the blood lead levels were divided by quartiles, the correlation coefficient (r) between blood lead levels in the 4th quartile and homocysteine was higher (r = 0.405, p<0.01) for Vietnamese while the other ethnic groups showed tendencies in the same direction, but not with statistical significance (table 4). We were not able to divide the group further by gender as the numbers in each cell became too small for some of the cells. Multiple linear regression equation models were constructed to assess predictors of homocysteine levels by blood lead controlling for covariates of age, drinking, smoking and gender for the all the blood lead levels and those in the 4th quartile. Covariates that were found to have significant contribution (p<0.05) are shown in table 5. Considering only the Vietnamese subjects, an increase of 1 μg/dl in log(blood lead) was associated with an increase of 0.05 μmol/l of log(homocysteine). However, at blood lead levels in the 4th quartile, an increase of 1 μg/dl in log(blood lead) was associated with an increase of 0.41 μmol/l of log(homocysteine) (table 5).
Table 4 Correlation between blood lead and homocysteine levels by race/ethnic groups and blood lead quartiles.
| Correlation coefficient for blood lead and homocysteine levels | |||||
|---|---|---|---|---|---|
| Total (n = 422) | Quartile 1 (n = 103) | Quartile 2 (n = 108) | Quartile 3 (n = 108) | Quartile 4 (n = 103) | |
| Race/ethnicity | |||||
| Vietnamese | 0.255* (n = 263) | 0.232 (n = 61) | −0.041 (n = 61) | −0.058 (n = 68) | 0.405* (n = 73) |
| Chinese | 0.117 (n = 92) | −0.045 (n = 42) | −0.002 (n = 30) | 0.006 (n = 17) | −0.904 (n = 3) |
| Malay | 0.021 (n = 34) | −† | −0.061 (n = 7) | −0.214 (n = 14) | 0.413 (n = 13) |
| Indians | 0.286 (n = 33) | −† | −0.168 (n = 10) | −0.571 (n = 9) | 0.031 (n = 14) |
*p<0.01.
†Insufficient observations.
Table 5 Predictors of homocysteine levels in Vietnamese subjects for all blood lead levels and those in the 4th quartile, adjusted for significant independent variables (p<0.05).
| All Vietnamese subjects (n = 263) | Vietnamese subjects with blood lead levels in the 4th quartile for (n = 73) | |||
|---|---|---|---|---|
| (R2 = 0.359) | (R2 = 0.342) | |||
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Blood lead level (μg/dl) | 0.051 (−0.003 to 0.106) | 0.066 | 0.406 (0.056 to 0.756) | 0.024 |
| Female | −0.435 (−0.515 to −0.355) | <0.001 | −0.425 (−0.624 to −0.227) | <0.001 |
| Age (years) | 0.007 (0.004 to 0.010) | <0.001 | 0.004 (−0.002 to 0.011) | 0.198 |
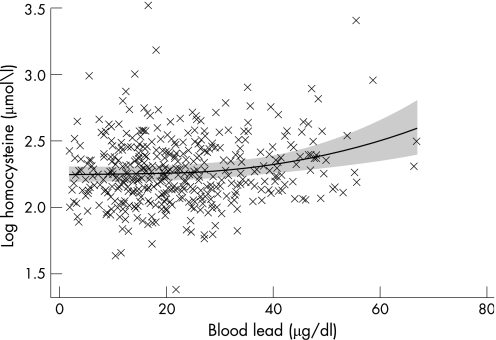
A fractional polynomial plot was used to give a composite picture of the relation between blood lead and homocysteine levels adjusted for the possible confounders: age, gender and race (including the Vietnamese). Figure 1 shows the fractional polynomial plot of log(homocysteine) and blood lead after adjusting for age, gender and race. At blood lead levels below 20 μg/dl, the relation between log(homocysteine) and blood lead is not so apparent. But at blood lead levels above 20 μg/dl, there is a corresponding increase in log(homocysteine); and blood lead increases with the slope of the curve increasing even more at blood lead levels above 40 μg/dl.
Figure 1 Fractional polynomial plot of log‐transformed homocysteine and blood lead adjusted for age, gender and race.
Discussion
To our knowledge, this is the first study to investigate associations between blood lead and homocysteine levels in Asian populations. We detected a significant association between blood lead and homocysteine among factory workers exposed to lead in a Vietnamese population, after controlling for age and gender. As previously observed, gender and age were predictors of homocysteine levels.8,9 This relation between homocysteine and blood lead was also evident in a recent investigation by Schafer et al.5 Exposure duration, alcohol intake and cigarette smoking did not have apparent effects on the relationship between blood lead and homocysteine levels in our sample. More than 70% of the workers had blood lead level below 30 μg/dl. The mean blood lead level in the 4th quartile was 40 μg/dl (range 29.7–66.9 μg/dl; 75th percentile <46 μg/dl). Thus, this population was generally not exposed to high levels of lead. The study therefore indicates an association at blood lead levels that among occupationally exposed workers can be regarded as moderate.
Homocysteine levels are on average higher among males compared to females in this study. This observation confirms the results of a previous study by Saw et al.8 There were no significant differences in the mean homocysteine levels among the Chinese, Malays and Indians. In an earlier study involving Chinese, Malays and Indians in Singapore, Hughes and Ong also reported no significant differences in the plasma homocysteine levels among these three ethnic groups.10 The Vietnamese subjects had significantly lower homocysteine levels compared to the three ethnic groups (Chinese, Malays and Indians) in Singapore; even after adjusting for age and gender. We are not aware of any published report of adult Vietnamese plasma homocysteine levels. It is not surprising that the two nationalities produced varying results because lifestyle, dietary and genetic factors could vary widely between Singaporeans and Vietnamese.
Several vitamins function as cofactors and substrates in the metabolism of homocysteine. A number of studies have shown inverse relationships of blood homocysteine concentrations with plasma/serum levels of folic acid, vitamin B6, and vitamin B12.11 Several randomised controlled trials have evaluated the effect of multivitamin supplementation on fasting homocysteine levels. All treatment regimens, including a combination of folic acid (1–5 mg), vitamin B6 (5–50 mg) and vitamin B12 (0.02–1 mg) have reported lowered fasting plasma homocysteine levels by 20% to 50%.12 Folic acid is present in most foods, particularly organ meats (especially liver), fresh green vegetables, and some fresh fruits. Vitamin B12 is mainly present in animal protein particularly organ meats (especially liver) and bivalves (clams and oysters), and to a lesser extent in seafood, milk and milk products.10 We did not have information on the use of vitamin supplements among our subjects. However, we are aware that Vietnamese are known to consume large quantities of fresh vegetables daily as these are freely available in Vietnam (Mai Anh. Personal communication, 2007) while Singapore has to import all its vegetable needs. Genetic variation at the methylenetetrahydrofolate reductase (MTHFR) locus have also been reported to affect plasma homocysteine levels.8 Hence, these factors could contribute to the lower homocysteine levels found in our Vietnamese subjects.
Race/ethnicity was an important determinant of the association, with the Vietnamese showing a stronger and significant relation (p<0.01) between blood lead and homocysteine, while the other ethnic groups showed tendencies in the same direction, but not with statistical significance (table 4). With the Vietnamese subjects only, an increase of 1 μg/dl in log(blood lead) was associated with an increase of 0.05 μmol/l of log(homocysteine). At blood lead levels in the 4th quartile, an increase of 1 μg/dl in log(blood lead) was associated with an increase of 0.41 μmol/l of log(homocysteine) (table 5, fig 1). At higher blood lead levels, the association between blood lead and homocysteine was much stronger. Schafer et al have elegantly summarised their theory on the interaction of lead and homocysteine with sulfhydryl group to explain this association: “… furthermore, homocysteine itself contains a sulfhydryl group, so if lead has an affinity for this sulfhydryl group, the metabolism of homocysteine could be directly inhibited, leading to an accumulation of homocysteine.”5
Lead and homocysteine are both related to an increased risk of cardiovascular disease. An occupational cohort of 496 men with previous lead exposure was found to have an average increased systolic blood pressure of 0.64 mmHg (SE 0.25) for every SD increase in blood lead at baseline.13 A recent meta‐analysis of 30 prospective and retrospective studies showed that a 25% lower homocysteine level was related to an 11% (OR 0.89, 95% CI 0.83 to 0.95) lower ischaemic heart disease risk and a 19% (OR 0.81; 95% CI 0.69 to 0.95) lower stroke risk.3 In one large epidemiological study it was reported that each 5 μmol/l increase in plasma homocysteine was associated with an increase in systolic and diastolic blood pressure of 0.7/0.5 mmHg in men and 1.2/0.7 mmHg in women, which was independent of renal function and vitamin B status.14 Mechanisms that have been postulated to explain the relation between homocysteine and blood pressure include “homocysteine‐induced arteriolar constriction, renal dysfunction and increased sodium reabsorption, and increased arterial stiffness … the hypothesis that homocysteine increases blood pressure must be considered unproven.”15 There is only circumstantial evidence that these mechanisms are operative in humans. In addition, confounding by subtle renal dysfunction or by unmeasured dietary and lifestyle factors cannot be excluded as an explanation for the association between homocysteine and blood pressure.
Schafer et al (2005), in a population of older (50–70 years) non‐exposed adults (n = 1140), reported that an increase of 1 μg/dl in blood lead was associated with an increase of 0.35 μmol/l of homocysteine. The mean blood lead was much lower at 3.5 μg/dl (range 0.1–27.3 μg/dl), with the 4th quartile having mean lead level of 6.5 μg/dl (range 4.5–27.5 μg/dl). Our increase of 0.04 μmol/l of log(homocysteine) is lower but our population is younger and of different racial groups compared to Schafer's subjects. However what we found was that subjects in a higher blood lead category had a higher incremental rise in the plasma homocysteine level for every increase in unit of blood lead. At blood lead level in the 4th quartile, an increase of 1 μg/dl in log(blood lead) was associated with an increase of 0.41 μmol/l of log(homocysteine) (table 5). Our findings, together with Schafer et al's report,5 suggest that lead in blood and plasma homocysteine levels may be associated with one another and could have a role to play in the causation of elevated blood pressure.
There are several limitations to this study. Tibia lead levels were not measured. In a population‐based study of older adults, tibia lead was found to be modestly correlated with blood lead (Pearson's r = 0.12, p<0.01).5 However, we used exposure duration as a surrogate, and this factor was not found to be significant in our analysis. The sample size for the Malays and Indians was not large and these ethnicities were not represented fairly by both genders. Although blood lead, and not exposure duration, was associated with homocysteine levels, we cannot determine from this cross‐sectional study whether recent lead exposure, cumulative lead exposure, or both, is the likely cause of lead that describes the relationship.
In conclusion, blood lead was found to be associated with homocysteine levels at higher blood lead levels in the Vietnamese subjects. Although we cannot determine causality from cross‐sectional data, it is sensible to consider the probability that this relation between blood lead and homocysteine could explain one of the mechanisms of the impact of lead on the cardiovascular system. We cannot conclude based on the study's finding whether lead elevates homocysteine or vice versa. But it is clear that the relation exists especially at moderate blood lead levels in the Vietnamese subjects. Future studies should involve a larger cohort of Chinese, Malay and Indian subjects so as to ensure sufficient power to investigate if there are any significant associations between blood lead and homocysteine.
Main messages
A significant positive association was detected between blood lead and homocysteine among workers exposed to lead in a Vietnamese population, after controlling for age and gender. This association was only seen at higher blood lead levels.
Homocysteine levels are on average higher among males compared to females in this study. There were no significant differences in the mean homocysteine levels among the Chinese, Malays and Indians.
The Vietnamese subjects had significantly lower homocysteine levels compared to the three ethnic groups (Chinese, Malays and Indians) in Singapore, even after adjusting for age and gender.
Acknowledgements
This project was supported by the Office of Life Sciences, National University of Singapore. We also wish to thank all the factory workers who took part in this study.
Abbreviations
HPLC - high performance liquid chromatography
Footnotes
Funding: This project was made possible through the funding of Office of Life Sciences, National University of Singapore grant no: WBS R329‐000‐008‐712.
Competing interests: None.
The study was approved by the National University Singapore Intuitional Review Board. Informed consent was obtained from each worker before the commencement of the study.
References
- 1.Papanikolaou N C, Hatzidaki E G, Belivanis S.et al Lead toxicity update: a brief review. Med Sci Monit 200511RA329–RA336. [PubMed] [Google Scholar]
- 2.Dufouil C, Alperovitch A, Ducros V.et al Homocysteine, white matter hyperintensities, and cognition in healthy elder people. Ann Neurol 200353214–221. [DOI] [PubMed] [Google Scholar]
- 3.Homocysteine Collaboration Homocysteine and risk of ischemic heart disease and stroke: A meta‐analysis. JAMA 20022882015–2022. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo R, Passalacqua W, Araya J.et al Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J Cardiovasc Pharmacol 200342453–461. [DOI] [PubMed] [Google Scholar]
- 5.Schafer J H, Glass T A, Bressler J.et al Blood lead is a predictor of homocysteine levels in a population‐based study of older adults. Environ Health Perspect 200511331–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malinow M R, Kang S S, Taylor L M.et al Prevalence of hyperhomocysteinemia in patients with peripheral arterial occlusive disease. Circulation 1989791180–1188. [DOI] [PubMed] [Google Scholar]
- 7.Wu L L, Wu J, Hunt S C.et al Plasma homocyst(e)ine as a risk factor for early familial coronary artery disease. Clin Chem 199440552–561. [PubMed] [Google Scholar]
- 8.Saw S M, Yuan J M, Ong C N.et al Genetic, dietary, and other lifestyle determinants of plasma homocysteine concentrations in middle‐aged and older Chinese men and women in Singapore. Am J Clin Nutr 200173232–239. [DOI] [PubMed] [Google Scholar]
- 9.Jacques P F, Bostom A G, Wilson P W.et al Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr 200173613–621. [DOI] [PubMed] [Google Scholar]
- 10.Hughes K, Ong C N. Homocysteine, folate, vitamin B12, and cardiovascular risk in Indians, Malays, and Chinese in Singapore. J Epidemiol Community Health 20005431–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malinow M R, Bostom A G, Krauss R M. Homocysteine, Diet, and Cardiovascular Diseases: A Statement for Healthcare Professionals From the Nutrition Committee, American Heart Association. Circulation 199999178–182. [DOI] [PubMed] [Google Scholar]
- 12.Booth G L, Wang E L, with the Canadian Task Force on Preventive Health Care Preventive health care, 2000 update: screening and management of hypohomocysteinemia for the prevention of coronary artery disease events. CMAJ 200016321–29. [PMC free article] [PubMed] [Google Scholar]
- 13.Glenn B S, Stewart W F, Links J M.et al The longitudinal association of lead with blood pressure. Epidemiology 20031445–60. [DOI] [PubMed] [Google Scholar]
- 14.Lim U, Cassano P A. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 20021561105–1113. [DOI] [PubMed] [Google Scholar]
- 15.Stehouwer C D, van Guldener C. Does homocysteine cause hypertension? Clin Chem Lab Med 2003411408–1411. [DOI] [PubMed] [Google Scholar]