Abstract
The cytosine analog 5-aza-2′-deoxycytidine has been used clinically to reactivate genes silenced by DNA methylation. In particular, patients with β-thalassemia show fetal globin expression after administration of this hypomethylating drug. In addition, silencing of tumor suppressor gene expression by aberrant DNA methylation in tumor cells may potentially be reversed by a similar regimen. Consistent with its function in maintaining tumor suppressor gene expression, 5-aza-2′-deoxycytidine significantly reduces intestinal tumor multiplicity in the predisposed Min mouse strain. Despite its utility as an anti-cancer agent, the drug is highly mutagenic by an unknown mechanism. To gain insight into how 5-aza-2′-deoxycytidine induces mutations in vivo, we examined the mutational spectrum in an Escherichia coli lac I transgene in colonic DNA from 5-aza-2′-deoxycytidine-treated mice. Mutations induced by 5-aza-2′-deoxycytidine were predominantly at CpG dinucleotides, which implicates DNA methyltransferase in the mutagenic mechanism. C:G→G:C transversions were the predominant class of mutations observed. We suggest a model for how the mammalian DNA methyltransferase may be involved in facilitating these mutations. The observation that 5-aza-2′-deoxycytidine-induced mutations are mediated by the enzyme suggests that novel inhibitors of DNA methyltransferase, which can inactivate the enzyme before its interaction with DNA, are needed for chemoprevention or long term therapy.
Keywords: cancer, chemotherapy, DNA methylation, transgenic mice
The chemical synthesis of 5-aza-2′-deoxycytidine (5azaCdR) was originally aimed at generating a chemotherapeutic drug (1). Since that time, it also has been exploited by molecular biologists to manipulate genomic methylation both in tissue culture cells and in vivo to demonstrate the inverse relationship between DNA methylation and gene expression and to reactivate epigenetically silenced genes (2). The potential reversibility of epigenetic silencing of tumor suppressor genes in tumor cells has renewed interest in the use of 5azaCdR for chemotherapy (3). In fact, we recently showed that 5azaCdR significantly reduces intestinal tumor multiplicity in Min mice by reducing genomic methylation levels (4).
Mechanistically, 5azaCdR is a cytosine analogue that acts as a suicide substrate for DNA methyltransferase when incorporated into DNA at the target site for DNA methylation, CpG dinucleotides (5). Santi et al. (6) originally proposed that the normal methylation of cytosine involves a reaction intermediate in which the DNA methyltransferase is covalently bound to the 6-carbon position of cytosine. Transfer of a methyl group from the cofactor S-adenosylmethionine allows the enzyme to be released from DNA. 5azaCdR cannot accept the transfer of a methyl group because of the nitrogen in the fifth position of the cytosine ring, so the enzyme becomes trapped as an adduct on 5azaCdR-substituted DNA (7). Depletion of the DNA methyltransferase by this mechanism indirectly leads to genomic hypomethylation. In addition, the covalent binding of the DNA methyltransferase to 5azaCdR-substituted DNA has been shown to directly mediate the cytotoxic effects of 5azaCdR exploited by conventional chemotherapy (8).
Despite the protective effect of 5azaCdR against intestinal neoplasia, the drug has been shown to be highly mutagenic in prokaryotes (9–11) by a mechanism that has not been resolved. In light of the covalent interaction known between the drug and DNA methyltransferase, we examined whether the enzyme may be involved in the mechanism by which 5azaCdR induces mutations in mammalian cells. By sequencing mutations in a reporter Escherichia coli lac I transgene (12) isolated from colonic DNA from mice with and without 5azaCdR administration, we show that mutations induced by 5azaCdR are predominantly C:G → G:C transversions. Moreover, three-quarters of the mutations in 5azaCdR-treated mice occur at CpG dinucleotides. We propose a model for how the DNA methyltransferase enzyme may be involved in facilitating 5azaCdR-induced mutagenesis. The mutagenicity of this drug calls into question its utility as a chemopreventive agent for colon cancer and highlights the need to identify novel inhibitors of DNA methyltransferase for this purpose.
MATERIALS AND METHODS
Mice.
The DnmtS (13) substrain was derived from and maintained in the 129/Sv strain background. The BigBlue substrain was maintained in the C57BL/6 strain background [Stratagene (12)].
5azaCdR Administration.
5azaCdR (Sigma, catalog no. A-3656) was solubilized in sterile PBS at 2.5 mg/ml and stored in aliquots at −80°C until use. Mice were injected s.c. using a 30-gauge needle on a 0.1-ml Hamilton 700 series syringe with a Luer tip and a PB600 dispenser attachment that delivers 50 discrete units of 2 μl (4). Weekly injections were started at 7 days after birth and continued for a total of 14 weeks. Increments of 2 μl (5 μg)/5 g mouse body weight (rounded to the nearest 5 g) were delivered.
Genomic DNA Isolation.
Tissues were isolated from 100-day-old mice, frozen immediately on dry ice, and stored at −80°C until use. High molecular weight DNA was prepared by proteinase K digestion of colons pulverized in liquid nitrogen. Two extractions with phenol–chloroform and one chloroform extraction were done, and the DNA was precipitated with ethanol by spooling onto a flame-sealed pasteur pipet. Isolated DNA was resuspended in 10 mM Tris, pH 7.5/0.1 mM EDTA.
Methylation Analysis.
Genomic DNA (5 μg) was digested with EcoRI alone, EcoRI and HpaII, or EcoRI and MspI followed by agarose gel electrophoresis and Southern blotting. The membrane was hybridized with a fragment of the lacI gene (spanning from nucleotide −50 in the lac I promoter to nucleotide 601 in the coding sequence) generated by PCR amplification of the pLIZ plasmid (14) using primers BB2 (5′-CCCGACACCATCGAATGGTG-3′) and BB12 (5′-AGAACTTAATGGGCCCG-3′).
In Vitro Packaging and Mutant Isolation.
λ phages were packaged from the genomic DNAs using Transpack extracts (Stratagene) as recommended by the manufacturer. Packaged DNAs were used to infect the SCS-8 strain of E. coli (Stratagene) and were plated on NZY agar plates using top agarose containing 1.5 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside (Stratagene). Blue plaques of all intensities were isolated and replated at low density in the presence of 5-bromo-4-chloro-3-indolyl β-d-galactoside for plaque purification.
lac I Sequence Analysis.
Isolated plaques were either used for in vivo excision of the pLIZ plasmid containing the lac I gene as described (14) or used as templates for PCR amplification of the lac I gene. Plasmid DNAs or PCR fragments were cycle-sequenced using dye terminators on an automated Applied Biosystems sequencer (Whitehead Institute Sequencing Facility). Sequences were compared with the wild-type lac I gene sequence using the megalign program (DNAstar, Madison, WI) to identify mutations. All candidate mutations were resequenced on the opposite strand for verification.
RESULTS
Methylation Analysis of a lac I Mouse Transgene After 5azaCdR Administration.
To examine the spectrum of mutations induced by 5azaCdR in vivo, we used an assay that reflects the endogenous mutagenic and repair mechanisms in the intestinal cell. A mouse strain (BigBlue) that harbors a tandemly repeated transgene comprising an E. coli lac I gene in a λ bacteriophage shuttle vector provides an attractive assay for mutation screening (12). Inactivating mutations in the lac I transgene are scored as blue plaques in a background of colorless plaques after in vitro packaging of genomic DNA and plating on indicator bacteria. The lac I gene contains 94 CpG dinucleotides and a total of 299 cytosine residues in 1083 bp of coding sequence that could serve as targets for 5azaCdR mutagenesis.
The loss-of-function DnmtS allele (13) was introduced into the lac I transgenic strain to genetically alter the levels of DNA methyltransferase. The recessive embryonic lethal phenotype of the DnmtS mutation precluded analysis of homozygous mutants. Dnmt heterozygous and wild-type lac I transgenic offspring either were injected weekly with 5 μg of 5azaCdR/5 g body weight or were left untreated for 14 weeks to reproduce conditions from our previous study of polyp numbers in Min mice. As we have shown previously, this regimen results in four distinct classes of genomic methylation as measured by Southern blot hybridization with a repetitive centromeric satellite sequence (4).
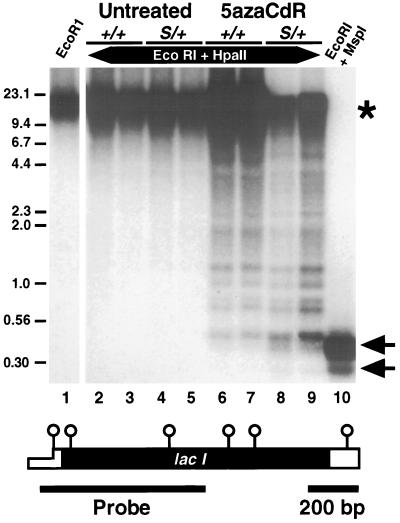
We measured the extent of demethylation caused by the DnmtS mutation and 5azaCdR administration at the lac I gene in these mice. Colonic genomic DNA was cleaved with the methylation-sensitive enzyme HpaII along with EcoRI, which cuts within the flanking λ sequences. Southern blot analysis with a lac I probe indicated that the six HpaII sites within the lac I gene and all additional HpaII sites within the λ vector were completely methylated in the Dnmt wild-type mouse colon (Fig. 1, compare lanes 2 and 3 with lane 1). Extensive methylation of the lac I transgene was also observed in DnmtS/+ colon DNAs by this assay (Fig. 1, lanes 4 and 5). 5azaCdR treatment resulted in a range of methylation states from complete methylation to near complete demethylation in both +/+ and DnmtS/+ mice (Fig. 1, lanes 6–9). The expected pattern of the unmethylated state is shown by digestion with the HpaII isoschizomer MspI that is not inhibited by CpG methylation (Fig. 1, lane 10). These results demonstrate that 5azaCdR is more effective than the DnmtS mutation at altering methylation of the lac I gene. Consequently, the mice can be categorized into two groups based on lac I methylation levels, namely untreated and 5azaCdR-treated mice.
Figure 1.
Southern blot analysis of lac I gene methylation levels. Genomic DNA (5 μg) digested with the indicated enzymes was electrophoresed on an agarose gel and analyzed by Southern blot hybridization. The lac I probe extends from the promoter region (−50, thin open box) to nucleotide 601 within the coding sequence (solid box). Position of the six HpaII/MspI sites are shown schematically as open circles over the lac I gene diagram. ∗, The position of the completely methylated lac I sequences. Arrows indicate the positions of the two complete digest products detected by this probe. Positions and sizes (in kilobases) of select marker fragments are indicated.
Spectrum of 5azaCdR-Induced Mutations.
Transitions at CpG dinucleotides account for 40% of all spontaneous mutations reported for lac I transgenic (BigBlue) mice to date; however, no sequence information has been reported for the colon (12, 15, 16). To assess the spontaneous mutation spectrum in colonic DNA from BigBlue mice and to characterize the class of mutations induced by 5azaCdR, we isolated phage-harboring lac I mutations from each of the genotype and treatment categories for sequence analysis. A total of 188 confirmed mutant isolates were plaque-purified and sequenced. Sequence analysis focused on the amino-terminal DNA binding domain of lac I (amino acids 1–60, nucleotides 29–209) due to the large number of residues in this region known to mutate to produce a phenotype. All candidate mutations were resequenced with one to three additional primers such that both strands were verified. A mutation identified multiple times from a single mouse was considered to have arisen early in development and been replicated by cell division and was therefore counted as a single, independent mutation (however, these mutations are included parenthetically in Tables 1 and 2). A total of 90 independent lac I mutations were scored across all four groups by this analysis.
Table 1.
Mutations in untreated lac I mice
| Mouse number | lac I base | Mutation, abundance | Amino acid change |
|---|---|---|---|
| Dnmt +/+ (n = 20) | |||
| 744 | 42 | C:G to T:A | T5M |
| 744 | 42 | C:G to A:T | T5K |
| 742 | 90 | C:G to T:A | S21F |
| 742 | 90-93 | C:G insertion* | R22P† |
| 742 | 92 | C:G to T:A (3X) | R22C |
| 744 | 92 | C:G to T:A | R22C |
| 742 | 93 | C:G to T:A | R22H |
| 744 | 95 | C:G to T:A | V23M |
| 744 | 117 | T:A to G:C | V30G |
| 744 | 131 | C:G to T:A | R35W |
| 742 | 167 | T:A to G:C | Y47D |
| 744 | 167 | T:A to C:G | Y47H |
| 739 | 178 | C:G to A:T | N50K |
| 741 | 180 | C:G to T:A | R51H |
| 742 | 258 | C:G to A:T | S77 Stop |
| 742 | 270 | C:G to T:A | A81V |
| 742 | 326 | C:G to A:T | E100 Stop |
| 741 | 356 | C:G to T:A | A109T |
| 744 | 437 | C:G to A:T | E137 Stop |
| 744 | 485 | C:G to T:A | Q153 Stop |
| Dnmt S/+ (n = 19) | |||
| 737 | −15 | C:G to G:C | promoter |
| 743 | 75 | C:G to A:T | S16Y§ |
| 738 | 92 | C:G to A:T | R22S |
| 743 | 92 | C:G to T:A (3X) | R22C |
| 738 | 93 | C:G to T:A | R22H |
| 743 | 95 | C:G to T:A | V23M |
| 743 | 96 | T:A to G:C | V23G |
| 743 | 117 | T:A to C:G | V30A |
| 743 | 127 | T:A to G:C (3X) | K33N |
| 743 | 137 | T:A to A:T (3X) | K37 Stop |
| 743 | 172 | T:A to G:C (2X) | I 48 M |
| 743 | 180 | C:G to T:A | R51H |
| 743 | 198 | C:G to T:A | A57V |
| 737 | 199-201 | C:G deletion‡ | G58A† |
| 743 | 200 | C:G to A:T | G58C |
| 743 | 369 | T:A to C:G | L114P |
| 743 | 381 | C:G to T:A | R118H† |
| 743 | 511 | T:A insertion | F161F† |
| 738 | 867 | C:G to G:C | S200Stop |
Sequence of independent mutations derived from untreated lac I transgenic mice. Frequency of occurance of the same mutation in one mouse is indicated in parentheses. Mutations at CpG sites are indicated by bold italics.
Mutation occurred at CCCpG, so the deleted C is not defined.
Mutation also created a frameshift.
Mutation occurred at CpGGG, so the deleted G is not defined.
Mutation was in the lac I promoter. It is not clear whether this was the inactivating mutation.
Table 2.
Mutations in 5azaCdR-treated mice
| Mouse number | lac I base | Mutation, abundance | Amino acid change |
|---|---|---|---|
| Dnmt +/+ (n = 22) | |||
| 705 | 92 | C:G to T:A | R22C |
| 705 | 93 | C:G to A:T | R22L |
| 701 | 131 | C:G to G:C | R35G |
| 705 | 131 | C:G to T:A | R35W |
| 705 | 149 | C:G deletion* | A41R† |
| 701 | 150 | C:G to A:T | A41E |
| 705 | 175 | C:G to T:A | P49P |
| 705 | 179 | C:G to T:A | R51C |
| 705 | 179 | C:G to G:C | R51G |
| 701 | 180 | C:G to G:C | R51P |
| 701 | 201 | C:G to A:T | G58V |
| 705 | 258 | C:G to G:C | S77W |
| 701 | 269 | C:G to T:A | A81T |
| 705 | 318 | C:G to G:C | S97W |
| 701 | 341 | C:G to A:T | E105 Stop |
| 701 | 383 | C:G to A:T | V119F |
| 701 | 518 | C:G to A:T | E164Stop |
| 701 | 750 | C:G to A:T (3X) | A241E |
| 705 | 792 | C:G to T:A | R255H |
| 701 | 847 | C:G to G:C | Y273Stop |
| 705 | 847 | C:G to G:C | Y273Stop |
| 701 | 917 | C:G to G:C | G297R |
| Dnmt S/+ (n = 29) | |||
| 707 | 20 | C:G to G:C | 5′UTR‡ |
| 702 | 42 | C:G to T:A | T5M |
| 707 | 49 | C:G to G:C | Y7 Stop |
| 707 | 80 | C:G to G:C | Q18E |
| 707 | 86 | C:G to G:C | V20L |
| 707 | 92 | C:G to G:C | R22G |
| 702 | 95 | C:G to T:A | V23M |
| 702 | 95 | C:G to G:C | V23L |
| 702 | 112 | C:G to G:C | S28R |
| 707 | 116 | C:G to G:C | V30L |
| 702 | 143 | C:G to G:C | E39Q |
| 702 | 150 | C:G to A:T | A41E |
| 702 | 180 | C:G to T:A | R51H |
| 702 | 180 | C:G to G:C | R51P |
| 707 | 180 | C:G to G:C | R51P |
| 707 | 186 | C:G to G:C | A53G |
| 702 | 197 | C:G to A:T | A57S |
| 702 | 198 | C:G to G:C | A57V |
| 702 | 258 | C:G to G:C | S77W |
| 707 | 270 | C:G to T:A | A81V |
| 703 | 381 | C:G to T:A | R118H |
| 707 | 381 | C:G to T:A | R118H |
| 707 | 524 | C:G to G:C | G166R |
| 702 | 744 | C:G to G:C | P239R |
| 707 | 744 | C:G to G:C | P239R |
| 702 | 794 | C:G to G:C | A256P |
| 707 | 806 | T:A to C:G | S260P |
| 702 | 928 | C:G to G:C | S300R |
| 707 | 928 | C:G to G:C | S300R |
Sequence of independent mutations derived from 5azaCdR-treated lac I transgenic mice. Frequency of occurance of the same mutation in one mouse is indicated in parentheses. Mutations at CpG sites are indicated by bold italics.
Mutation was at CpGG, so the deleted G is not defined.
Mutation also created a frameshift.
Mutation was in the 5′ untranslated region. It is not clear whether this was the inactivating mutation.
Spontaneous mutations identified in colon DNA from untreated lac I transgenic mice were consistent with the spectrum of mutations previously described for other tissues (Table 1; n = 39) (12, 15, 16). Most notably, C:G → T:A transitions at CpG sites accounted for 36% of spontaneous colonic lac I mutations (n = 14). The slight reduction of spontaneous C:G → T:A transitions at CpG dinucleotides observed in DnmtS/+ compared with +/+ mice was not statistically significant (33% and 40%, respectively; P = 0.38, χ2 test).
Mutations identified from 5azaCdR-treated mice were almost exclusively at C:G base pairs (n = 50/51), which is consistent with the fact that 5azaCdR is an analogue of cytosine (Table 2). A specific induction of C:G → G:C transversions both at CpG sites (n = 20/51) and non-CpG sites (n = 8/51) was observed. In addition, significant numbers of C:G → A:T transversions (n = 6/51) and C:G → T:A (n = 11/51) transitions each at CpG dinucleotides also were found. A comparison of spontaneous and 5azaCdR-induced mutation spectra revealed a striking increase in the percentage (29% increase; P < 0.01) of mutations at CpG dinucleotides induced by 5azaCdR (Table 3). Although the sizes of the spontaneous and 5azaCdR-induced mutation data sets are comparable, it should be noted that the frequency of mutations induced by 5azaCdR was greater than 10-fold higher than the spontaneous mutation frequency (data not shown). This indicates that at least 90% of the mutations in the induced group resulted from 5azaCdR treatment. Therefore, the identification of three–quarters of the 5azaCdR-induced mutations at CpG dinucleotides suggests that the DNA methyltransferase enzyme may be involved in the mutagenic mechanism.
Table 3.
Spontaneous and 5azaCdR-induced mutation spectra
| Mutation Class | Spontaneous, n (%)
|
5azaCdR-induced, n (%)
|
||
|---|---|---|---|---|
| CpG | non-CpG | CpG | non-CpG | |
| Transitions | ||||
| C:G → T:A | 14 (36) | 3 (8) | 11 (22) | 1 (2) |
| T:A → C:G | NA | 3 (8) | NA | 1 (2) |
| Transversions | ||||
| C:G → A:T | 3 (8) | 5 (13) | 6 (12) | 3 (6) |
| C:G → G:C | 0 | 2 (5) | 20 (39) | 8 (16) |
| T:A → G:C | NA | 5 (13) | NA | 0 |
| T:A → A:T | NA | 1 (3) | NA | 0 |
| Deletion or insertion | 0 | 3* (8) | 0 | 1* (2) |
| Total | 17 (44) | 22 (56) | 37 (73) | 14 (27) |
Mutations identified from DnmtS/+ and wild-type mice were combined in this analysis. Spontaneous, n = 39; 5azaCdR-induced, n = 51. NA, not applicable.
Some of these mutations involved multiple Cs or Gs adjacent to a CpG dinucleotide. Therefore, the exact position of the mutation cannot be determined.
DISCUSSION
We have determined the effect of DNA hypomethylation induced primarily by the drug 5azaCdR on the class of mutations scored using a lac I transgenic mouse strain. The induction of mutations predominantly at the target site for methylation, CpG dinucleotides, suggests that the mutagenicity of 5azaCdR involves the methylation reaction. These results indicate the need to develop novel inhibitors of the DNA methyltransferase that dissociate the mutagenic effect of this cytosine analogue from the chemopreventive function demonstrated previously in Min mice (4).
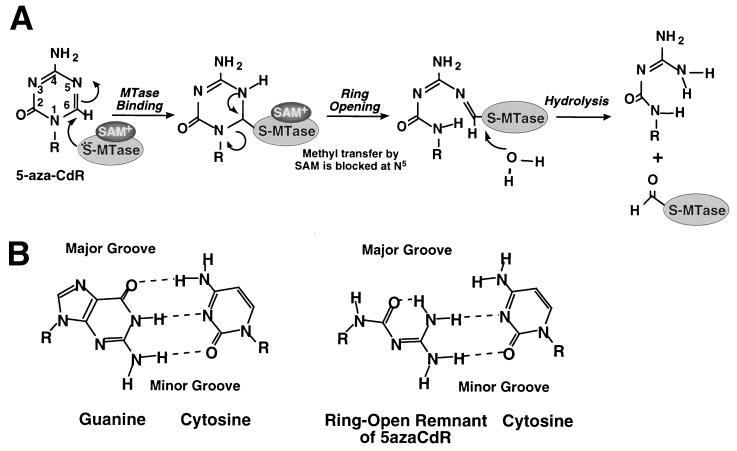
5-azaCdR is a suicide substrate of DNA methyltransferase that functions by incorporation into DNA (6, 17). Normally, during the initiation of the methylation reaction, DNA methyltransferase forms a covalent thioester linkage with the C6 position of cytosine in a CpG site. Once the methyl group is transferred from the cofactor S-adenosylmethionine to the C5 position of the cytosine residue, the DNA methyltransferase is released from its covalent linkage by β-elimination. 5azaCdR has a nitrogen in place of the C5 of cytosine. Incorporation of 5azaCdR at a CpG site allows the covalent binding of the enzyme to C6 to initiate the methylation reaction however methyl transfer is inhibited (Fig. 2A). As a result, the enzyme remains covalently attached to C6 of cytosine (7). This DNA methyltransferase–cytosine adduct is toxic to the cell if not repaired (8). Resolution of the adducts involves degradation of the enzyme, which leads indirectly to genomic hypomethylation.
Figure 2.
Model of the 5azaCdR mutagenic lesion. (A) A schematic model of the mechanism of inhibition of methylation by 5azaCdR and the subsequent chemical breakdown of the ring structure. (B) Model for a potential base pairing between cytosine and the ring open remnant of 5azaCdR compared with the normal G:C Watson–Crick base pair. Hydrogen bonds are indicated by dashed lines. SAM, S-adenosylmethionine.
5azaCdR is randomly incorporated at both CpG and non-CpG cytosine residues; however, its effects on DNA methylation level and cytotoxicity result entirely from incorporation at CpGs due to the mechanism described above. The observation that 73% of the mutations induced by 5azaCdR occur at CpG sites implicates the methylation reaction as a part of this mutagenic process. The covalent adduct of DNA methyltransferase bound to C6 of 5azaCdR in DNA may be chemically unstable because of the presence of three electronegative atoms (N1 and N5 from 5azaCdR and S from Cys 1252 in DNA methyltransferase) surrounding C6 (Fig. 2). This instability may result in ring opening by disruption of the N1–C6 bond and hydrolysis leading to the formylation and inactivation of the enzyme as has been shown (18). This ring open structure may represent the premutagenic lesion induced by 5azaCdR.
The spectrum of mutations induced by a mutagen can yield insight into the mechanism of its repair. 5azaCdR-induced mutations are predominantly C:G → G:C transversions but also include C:G → T:A transitions and C:G → A:T transversions. We speculate that C:G → G:C transversions are selected by the incorporation of a cytosine residue across from the ring open structure presented above during DNA synthesis before its repair. A tentative model of how the ring open remnant of 5azaCdR could base pair with cytosine in a manner that is nearly isosteric with the normal G:C base pair is shown in Fig. 2B. The similarity between this pair and the normal Watson–Crick base pair, particularly in the minor groove, could explain the unusual preference for C:G → G:C transversions. Appearance of C:G → T:A transitions may be most readily explained by the A-rule, in which dAMP is inserted as a single base substitution across from an abasic site. Such abasic sites are created by cleavage of the glycosylic bond between the base and deoxyribose sugar, leaving the phosphodiester backbone intact but susceptible to alkaline hydrolysis (19, 20). 5azaCdR generates alkali-labile sites within DNA (21), which suggests that at least some of the 5azaCdR-induced mutations may be due to replication across from abasic sites caused by the removal of the ring open structure by a DNA glycosylase. The mutational spectrum caused by endogenous abasic sites in E. coli is consistent with the A-rule; however the mutagenic processing of abasic sites in yeast is not (22). The mechanism by which the minor class of C:G → A:T transversions are induced by 5azaCdR is unclear at present.
Nearly one in six of the 5azaCdR-induced mutations was a C:G → G:C transversion at a non-CpG site, which suggests that the drug is also mutagenic in a nonenzyme, dependent pathway. Indeed, predominantly methyltransferase-independent C:G → G:C transversions caused by 5azaCdR have been observed in both E. coli and Salmonella mutagenicity tests (9–11). The selectivity for C:G → G:C transversions independent of the enzyme may be a consequence of the same ring opening mechanism we presented above because spontaneous hydrolysis to this ring open structure has been observed in aqueous solutions of 5-azacytidine (23). The prevalence of the enzyme-dependent mechanism in the lac I transgene within mouse tissues may be in part due to the extensive methylation of this locus.
Acknowledgments
We greatly appreciate the efficient work of Liuda Ziaugra at the Whitehead Institute Sequencing Facility. We also thank Greg Verdine for explaining the chemistry of the 5azaCdR ring opening to us and David Bartel and Thomas Tuschl for helpful discussions on the structures of base pairs and for suggesting the structure of the cytosine/5azaCdR remnant base pair we present. This work was supported by National Institutes of Health Grant R35 CA 44339 (R.J.). L.J.-G. was a recipient of fellowships from the Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation (DRG-1223) and the National Institutes of Health (AI07348–07). P.W.L. was supported by a National Research Service Award (F32 CA 09097) from the National Cancer Institute. The Undergraduate Research Opportunities Program at the Massachusetts Institute of Technology also provided funding for this work (B.J.M.).
ABBREVIATION
- 5-aza-2′-deoxycytidine
5azaCdR
References
- 1.Piskala A, Sorm F. Collect Czech Chem Commun. 1964;29:2060. [Google Scholar]
- 2.Jones P A. Cell. 1985;40:485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- 3.Jones P A. Cancer Res. 1996;56:2463–2467. [PubMed] [Google Scholar]
- 4.Laird P W, Jackson-Grusby L, Fazeli A, Dickinson S L, Jung W E, Li E, Weinberg R A, Jaenisch R. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 5.Bird A P. Nature (London) 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 6.Santi D V, Garrett C E, Barr P J. Cell. 1983;33:9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- 7.Santi D V, Norment A, Garrett C E. Proc Natl Acad Sci USA. 1984;81:6993–6997. doi: 10.1073/pnas.81.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jüttermann R, Li E, Jaenisch R. Proc Natl Acad Sci USA. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupples C G, Miller J H. Proc Natl Acad Sci USA. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin D E, Ames B N. Environ Mutagen. 1986;8:9–28. doi: 10.1002/em.2860080103. [DOI] [PubMed] [Google Scholar]
- 11.Bhagwat A S, Roberts R J. J Bacteriol. 1987;169:1537–1546. doi: 10.1128/jb.169.4.1537-1546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler S W, Provost G S, Fieck A, Kretz P L, Bullock W O, Sorge J A, Putman D L, Short J M. Proc Natl Acad Sci USA. 1991;88:7958–7962. doi: 10.1073/pnas.88.18.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei H, Oh S, Jüttermann R, Jaenisch R, Goss K A, Li E. Development (Cambridge, UK) 1996;122:3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 14.Short J M, Fernandez J M, Sorge J A, Huse W D. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler S W, Provost G S, Fieck A, Kretz P L, Bullock W O, Putman D L, Sorge J A, Short J M. Environ Mol Mutagen. 1991;18:316–321. doi: 10.1002/em.2850180421. [DOI] [PubMed] [Google Scholar]
- 16.Nishino H, Knoll A, Buettner V L, Frisk C S, Maruta Y, Haavik J, Sommer S S. Oncogene. 1995;11:263–270. [PubMed] [Google Scholar]
- 17.Jones P A, Taylor S M. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 18.Walsh C T. Annu Rev Biochem. 1984;53:493–535. doi: 10.1146/annurev.bi.53.070184.002425. [DOI] [PubMed] [Google Scholar]
- 19.Lindahl T. Nature (London) 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 20.Loeb L A. Cell. 1985;40:483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- 21.Limonta M, Colombo T, Damia G, Catapano C V, Conter V, Gervasoni M, Masera G, Liso V, Specchia G, Giudici G, D’Incalci M. Leuk Res. 1993;17:977–982. doi: 10.1016/0145-2126(93)90045-m. [DOI] [PubMed] [Google Scholar]
- 22.Kunz B A, Henson E S, Roche H, Ramotar D, Nunoshiba T, Demple B. Proc Natl Acad Sci USA. 1994;91:8165–8169. doi: 10.1073/pnas.91.17.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beisler J A. J Med Chem. 1978;21:204–208. doi: 10.1021/jm00200a012. [DOI] [PubMed] [Google Scholar]