Abstract
Epidemiological evidence of an association between Alzheimer's disease (AD) and the most frequently studied occupational exposures—pesticides, solvents, electromagnetic fields (EMF), lead and aluminium—is inconsistent. Epidemiological studies published up to June of 2003 were systematically searched through PubMed and Toxline. Twenty‐four studies (21 case–control and 3 cohort studies) were included. Median GQI was 36.6% (range 19.5–62.9%). Most of the case–control studies had a GQI of <50%. The study with the highest score was a cohort study. Likelihood of exposure misclassification bias affected 18 of the 24 studies. Opportunity for bias arising from the use of surrogate informants affected 17 studies, followed by disease misclassification (11 studies) and selection bias (10 studies). Eleven studies explored the relationship of AD with solvents, seven with EMF, six with pesticides, six with lead and three with aluminium. For pesticides, studies of greater quality and prospective design found increased and statistically significant associations. For the remaining occupational agents, the evidence of association is less consistent (for solvents and EMF) or absent (for lead and aluminium).
Keywords: Alzheimer's disease, occupational exposure, pesticides, solvents, electromagnetic fields
Alzheimer's disease (AD) is the most common cause of dementia in the elderly, accounting for 60–70% of the cases of progressive cognitive impairment. The prevalence of AD is up to 40% in those aged 85 years and older. The population of patients with AD will nearly quadruple in the next 50 years if the current trend continues.1 The diagnosis of this disease is considered probable when other alternative causes of dementia have been excluded, but only necropsy allows a definitive diagnosis of AD.2,3
Several risk factors for AD have been identified in epidemiological studies in addition to age and female sex. The strongest and most consistent risk factor is the apolipoprotein E genotype epsilon 4 allele (APOE4). Other risk factors evaluated include head injury, low serum levels of folate and vitamin B12, raised plasma and total homocysteine levels, a family history of AD or dementia, fewer years of formal education, lower income and lower occupational status.1 The evidence for increased risk of AD for occupational exposures is generally not consistent.4,5 The most widely studied occupational agents have been pesticides, solvents, electromagnetic fields (EMF), lead and aluminium.
The lack of evidence between AD and occupational exposures might be explained by the problem of validity, given the presence of characteristic biases in epidemiological studies on AD such as the bias derived from use of surrogate informants in retrospective studies, as the cognitive state of the patients makes it necessary to gather relevant information from the family or close friends, and diagnosis misclassification bias due to the difficulties of differential diagnosis for AD.6,7,8
Epidemiological research of occupational risks for AD has also frequent precision problems, because occupational exposures of interest are relatively uncommon and large studies would be required to show even relatively moderate risks. In 1991, under the assumption of an insufficient sample size, 11 case–control studies were reanalysed to assess with increased statistical power potential risk factors, including environmental factors. However the heterogeneity of the studies prevented the pooling of data.9,10
Different reanalysis and meta‐analysis of observational studies have been carried out, but as far as we know, except for the reanalysis mentioned above, none into occupational risk factors and AD. This study aimed at assessing, with a standardised and systematic approach, the strength of the associations between AD and pesticides, solvents, EMF, lead and aluminium in the workplace, evaluating the quality of published studies.
Methods
Data collection
Epidemiological studies on the association between AD and occupational exposure were located through electronic searches on PubMed and Toxline and further searching the references of relevant articles found.
The search was carried out in June 2003. For the search in PubMed a combination of the MeSH terms “Occupational exposure” and “Alzheimer disease” was used, with a further search with “Alzheimer*” and “occupatio*” in free text. In Toxline “Alzheimer*” and “occupatio*” in free text were used as searching terms. In both databases no limit was applied to the search strategy. Two hundred and forty‐one references were obtained in PubMed and 199 in Toxline. A first selection of relevant articles was made, including all epidemiological studies with individualised data, written in English, Spanish, French or Italian, in which it was possible to calculate measurements of relative risk for AD between those exposed at least once and those never exposed. Therefore ecological studies or studies focusing exclusively on aetiopathogenic mechanisms were excluded. Studies assessing environmental exposures which did not occur in the workplace were also excluded. Only original articles assessing specific exposures to those most widely studied agents (pesticides, solvents, EMF, aluminium and lead) were considered. Papers in which exposure was related to the starting age of AD or the evolution of the disease, but not to its aetiology, were also excluded. Also, only original papers, in which the effect analysed was a specific diagnosis of AD, were included. The clinical diagnosis of AD was based, therefore, on the application of the criteria of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA), the Diagnostic and Statistical Manual for mental disorders, revised 3rd and 4th editions (DSM III‐R, DSM IV), and the International Classification of Diseases, 9th and 10th revisions (ICD‐9, ICD‐10) or equivalent criteria. Hence, studies not applying diagnostic criteria of AD or studies limited to the assessment of cognitive impairment or presenile dementia were also excluded.
Inclusion and exclusion criteria were applied to the retrieved references, by reading the abstracts or, when necessary, full paper. We found two studies which had carried out reanalysis of previous data. The first one10 was conducted with data from four previously published studies.11,12,13,14 This reanalysis was excluded, as the original articles complying with our inclusion criteria were already included in our selection. The second reanalysis15 was based on three independent studies, unpublished at the time. The quality of these studies was therefore analysed separately in our review.
If there was more than one publication of the same study, we included the most recent, provided that it included the information of the previous studies. If these related papers presented different aspects of the study they were all selected but a note was made in the description explaining that all came from the same research.
Quality evaluation of the studies
A specially designed questionnaire was applied to each of the selected articles in order to assess the quality of each study and determine the presence of the main types of biases6,7,8 which might affect the results. On the basis of the design of the studies (cases and controls or cohorts) appropriate specific questionnaires were drawn up. The questionnaires were designed on the basis of protocols and questionnaires used previously with similar aims.16,17,18,19,20,21,22
Data collection followed the recommendations of Chalmers23 and Delgado‐Rodriguez and Sillero‐Arenas16 in order to minimise observer bias: each article was allocated an identification number and the details of the journal, authors and affiliations were removed. Every article was evaluated independently by two expert epidemiologists (FB and AMG). In cases of disagreement, the final evaluation was obtained by a consensus meeting between them.
The questionnaire for evaluation of the case–control studies contained 39 items measuring the quality of studies, with a maximum score of 111 points. These items were distributed in seven sections: (1) selection of cases and controls; (2) inclusion and exclusion criteria; (3) occupational exposure measurement; (4) control of confounding variables; (5) precision of the study; (6) internal and external validity of the study; and (7) general assessment of the presence or absence of biases (table 1 and Appendix I, available at http://ard.bmjjournals.com/supplemental). Thirty‐seven of these 39 items were distributed in five dimensions. Each dimension assessed a specific type of bias. Some of the items contributed to more than one dimension. The potential of the study for the presence of selection bias (17 items, maximum score of 44 points), disease misclassification (8 items, 23 points), exposure misclassification (11 items, 29 points), bias arising from the use of surrogate informants (7 items, 21 points) and misclassification bias of the confounding variables (5 items, 25 points) was analysed. Lower scores mean more potential for bias, while higher scores point to a smaller potential for bias in the study (table 2 and Appendix I, available at http://ard.bmjjournals.com/supplemental).
Table 1 Maximum scores and items for each section in the questionnaires assessing quality of the research in case–control and cohort studies.
| Questionnaire sections | Design | ||
|---|---|---|---|
| Case–control | Prospective cohort | Retrospective cohort | |
| Definition and follow‐up of the cohort | |||
| Maximum score | NA | 11 | 18 |
| Items | NA | 1–7 | 1–8 |
| Losses to follow‐up | |||
| Maximum score | NA | 13 | 11 |
| Items | NA | 8–12 | 9–12 |
| Measurement of disease incidence | |||
| Maximum score | NA | 6 | 5 |
| Items | NA | 13–16 | 13–15 |
| Selection of the cases and controls | |||
| Maximum score | 25 | NA | NA |
| Items | 1–11 | NA | NA |
| Inclusion and exclusion criteria for cases and controls | |||
| Maximum score | 9 | NA | NA |
| Items | 12–16 | NA | NA |
| Occupational exposure measurement | |||
| Maximum score | 18 | 9 | 8 |
| Items | 17–28 | 17–20 | 16–18 |
| Control of confounding variables | |||
| Maximum score | 9 | 9 | 9 |
| Items | 29, 30 | 21, 22 | 21, 22 |
| Precision | |||
| Maximum score | 2 | 2 | 2 |
| Items | 31, 32 | 23, 24 | 23, 24 |
| Internal and external validity | |||
| Maximum score | 8 | 8 | 8 |
| Items | 33, 34 | 25, 26 | 25, 26 |
| Likelihood for biases | |||
| Maximum score | 40 | 32 | 32 |
| Items | 35–39 | 27–30 | 27–30 |
| Global Quality Index* | |||
| Maximum score | 111 | 90 | 93 |
NA, not applicable.
*In the text the Global Quality Index (GQI) for each study is presented as a percentage of these maximum scores.
Table 2 Maximum scores for dimensions and items in the questionnaires assessing the likelihood for biases in case–control and cohort studies.
| Questionnaire dimensions* | Design | ||
|---|---|---|---|
| Case–control | Prospective cohort | Retrospective cohort | |
| Selection bias | |||
| Maximum score† | 44 | 40 | 43 |
| Items‡ | 1–12, 15, 16, 33–35 | 1,2, 4–6, 8–13, 25–27 | 1,2,4, 6–12, 25–27 |
| Disease misclassification bias | |||
| Maximum score | 23 | 24 | 31 |
| Items | 12–16, 33, 34, 36 | 7, 13–16, 25, 26, 28 | 5–7, 13–15, 25, 26, 28 |
| Exposure misclassification bias | |||
| Maximum score | 29 | 25 | 32 |
| Items | 17–24, 33, 34, 37 | 17–20, 25, 26, 29 | 6, 7, 16–18, 25, 26, 29 |
| Bias arising from use of surrogate informants | |||
| Maximum score | 21 | NA | NA |
| Items | 25–28, 33, 34, 38 | NA | NA |
| Bias arising from confounding | |||
| Maximum score | 25 | 25 | 33 |
| Items | 29, 30, 33, 34, 39 | 21, 22, 25, 26, 30 | 6, 7, 21, 22, 25, 26, 30 |
NA, not applicable
*Categories of biases assessed through the different items in the questionnaires.
†Maximum score obtained from the sum of scores for items included in each dimension.
‡Items of the questionnaire (Appendices I and II, available at http://ard.bmjjournals.com/supplemental) included in each dimension.
The questionnaire for cohort studies contained some common items with the questionnaire for case–control studies and specific items for prospective and retrospective cohorts, distributed in eight sections: (1) definition and follow‐up of the cohort; (2) follow‐up losses; (3) measurement of disease incidence; (4) occupational exposure measurement; (5) control of confounding variables; (6) precision of the study; (7) internal and external validity; (8) general assessment of the presence or absence of biases. Thirty items measure the quality of the prospective cohort studies with a maximum score of 90 points, and 28 items measure the quality in retrospective cohorts with a maximum score of 93 points (table 1 and Appendix II, available at http://ard.bmjjournals.com/supplemental). A total of 27 items (25 in retrospective cohorts) were distributed in four dimensions. The potential of the study for the presence of selection bias (14 items for prospective cohorts and 13 items for retrospective cohorts, with maximum scores of 40 and 43 points respectively), disease misclassification (8 items, 24 points, for prospective cohorts and 9 items, 31 points, for retrospective cohorts), exposure misclassification (7 items, 25 points, for prospective cohorts and 8 items, 32 points, for retrospective cohorts), and misclassification of the confounding variables (5 items, 25 points, for prospective cohorts and 7 items, 33 points, for retrospective cohorts) was analysed (table 2, Appendix II, available at http://ard.bmjjournals.com/supplemental).
For each study, a Global Quality Index (GQI) was calculated according to the total sum of the points for each item mentioned above. As a result of the different number of items with different maximum scores, this index is presented as a percentage of the maximum possible value (100%) that each study can achieve (GQI = (score obtained/corresponding maximum score) ×100).
To determine the presence or absence of bias in each study, we calculated in the same way the percentage of the possible maximum score in each dimension which assessed each specific type of bias ((score obtained in the dimension/maximum score for that dimension) × 100). Then, these percentages were grouped in five categories for each particular dimension (referred to each particular source of bias): highly probable (when the percentage of the maximum score for that dimension was <20%), probable (20–40%), possible (>40–60%), improbable (>60–80%) and highly improbable (>80%).
Lastly, in the last section of the questionnaires the experts reviewing the studies should establish, for each bias identified as probable or highly probable, the pattern for the bias (non‐differential or differential for classification biases) and the effect or direction of the bias on the associations observed in the study (Appendices I and II, available at http://ard.bmjjournals.com/supplemental).
For the data management we used the statistical packet SPSS, version 11.0 and the Excel spreadsheet.
Results
According to selection criteria, 22 original articles11,12,13,14,15,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 (19 case–control studies and three cohort studies) were included. One of the articles, as mentioned above, is a reanalysis of three independent series of case–control studies on AD and EMF.15 Therefore, the questionnaires were finally applied to 24 original studies.
Table 3 presents a summary of the main methodological aspects of the studies analysed, including population studied, data collection period, assessment of exposure and control of confounding bias.
Table 3 Summary of main methodological aspects of the epidemiological studies on the relationship between Alzheimer's disease and selected occupational exposures.
| Authors/Design/Year/Place* | Gender/Ascertainment period/ Data collection methods† | Study population‡ | Diagnostic criteria§ | Exposure assessment¶ | Control of confounding variables |
|---|---|---|---|---|---|
| Savitz et al. Retrospective cohort. 1998. USA33 | Men. 1950–86. Based on death certificates | Information for 20 068 workers who worked for at least 6 months in electric companies. In 56 deaths, AD was indicated | ICD‐9, code 331.0 | Exposure to EMF. Duration of work in exposed jobs was assessed and cumulative exposure at the time of death in five intervals was evaluated | Adjusted for age, calendar year, social class, work status, polychlorinated biphenyl exposure and solvent exposure |
| Kukull et al. Case–control. 1995. USA‐Seattle27 | Both sexes. 1987–92. Personal interview to proxy respondents for cases and controls through structured questionnaire | 193 Probable AD cases and 243 control subjects free of dementia and neurological disease, randomly selected from the study base | DSM III‐R and NINCDS‐ADRDA probable | Exposure to solvents through specially designed questions that incorporate duration of exposure. Different groups of solvents are assessed separately | Frequency matching for age and sex. Adjusted for age, sex, education, proxy relationship, alcohol consumption |
| Tyas et al. Prospective cohort. 2001. Manitoba, Canada38 | Both sexes. 1991/2–1996/7. Subjects free of dementia received the questionnaire to complete and return by mail | 694 Subjects who screened as cognitively intact were follow up for 5 years. 36 developed AD | Screening with 3MS. NINCDS/ADRDA possible or probable | Methods to measure exposure to specific agents (pesticides, solvents) through questionnaire are not defined. Different groups of solvents are assessed separately | Adjusted for age, sex, education |
| Baldi et al. Prospective cohort. 2003. Paquid Study‐France40 | Both sexes. 1992–8. Personal interview through structured questionnaire to the cohort | 1507 Subjects older than 65 years were followed up for 5 years. 96 AD cases were identified | DSM III‐R and NINCDS‐ADRDA. Cases were definitively classified by considering the results of jointly available complementary examinations | Exposure to pesticides: insecticides, herbicides, and fungicides was evaluated with a JEM made for 4 experts through detailed occupational histories. Cumulative exposure was calculated and quartiles were considered | Adjusted for age, tobacco consumption and education |
| Salib and Hillier. Case–control. 1996. UK28 | Both sexes. 1991–3. Proxy respondents of both case and control groups in a direct interview using a structured questionnaire | 198 Cases were compared with 164 controls with other dementias and 176 controls free of dementia | NINCDS–ADRDA possible or probable | Exposure to aluminium through occupational history from questionnaire. Subjects were labelled under “Aluminium occupation” category without additional information on criteria applied | Adjusted for age, sex, age of onset, duration of work, duration of condition and family history of dementia |
| Savitz et al. Case–control. 1998. USA‐25 different states35 | Men. 1985–1991. Information was obtained from death certificates, available from the National Center for Health Statistics for selected states | 256 Male cases who died of AD in 25 states, were compared with controls (ration 1:3) who died from causes other than leukaemia and brain cancer | ICD‐9, code 331.0 | EMF: occupations reported on the death certificate were classified as electrical and non‐electrical occupation according to a previous study. Electrical occupations were additionally classified in 10 different groups | Frequency matching by year of death and age at death. Adjusted for age, calendar year, social class and race |
| Graves et al. Case–control. 1999. USA‐Seattle36 | Both sexes. 1987–92. Direct interview only to the cases and control spouses | From the same study population base as in ref 27. Only people who have spouses as informants who agreed to collaborate, were considered eligible (89 population cases). 89 Population controls free of dementia. | NINCDS‐ADRDA probable | After data collection, IHs scored each job from detailed occupational history for potential exposure to EMF. Exposures were also classified according to duration and intensity | Matching by age, sex and source of information. Adjusted for age and education |
| Chandra et al. Case–control. 1987. USA‐Denver13 | Both sexes. 1975–85. Structured interview through standardised questionnaire applied to the next of kin of both patients and controls | 64 Hospital cases and 64 non‐demented hospital controls | NINCDS‐ADRDA probable | Question on “ever exposure” to some metals was included. Specific exposure to lead was collected in the questionnaire | Matching by age, sex, race and type of proxy |
| Gauthier et al. Case–control. 2001. Canada‐Quebec37 | Both sexes. Ascertainment period. not specified Interview structured through standardised questionnaire to the next of kin of both patients and controls | 68 Population cases were matched with 68 non‐demented population controls | Screening with 3MS, DSM IV, ICD‐10 and NINCDS‐ADRDA possible or probable | IH assess exposition to pesticides from detailed occupational history. Cumulative exposures were calculated | Matching by age and sex. Adjusted for education level, presence of family cases of AD, and presence of at least one ApoE epsilon4 allele |
| CSHA** et al. Case–control. 1994. 10 Canadian provinces26 | Both sexes. 1991–2. Questionnaire completed by the proxies themselves (usually a close relative) both cases and controls. In seven centres an interviewer administered it | 258 Cases with onset of symptoms within 3 years of diagnosis, and 535 population controls confirmed to be cognitively normal | Screening with 3MS. DSM III‐R and NINCDS‐ADRDA probable | Methods to measure exposure to specific agents (pesticides, solvents) through questionnaire are not defined | Frequency matching by study centre residence in community or institution, and age group. Adjusted for age, sex, residence in community or institution, and education |
| O'Flynn et al. Case–control. 1987. England and Wales24 | Men. 1970–9. From death certificates | 557 Cases who died of “presenile dementia” were randomised and compared with the same number of controls | Cases were further selected in order to exclude dementias other than AD | The person's most recent full time paid employment as reported to the Registrar at the time the death was registered. Occupations were graded by one of the investigators and an IH into one of three categories according to probable exposure to organic solvents and to lead” | Matching by age and sex |
| Graves et al. Case–control. 1998. USA‐Seattle32 | Both sexes. 1987–92. Direct interview only with the cases and control spouses | From the same study population base as in ref 27. Only people who had spouse informants who agreed to collaborate, were considered eligible (89 population cases). 89 Non‐demented population controls | NINCDS‐ADRDA probable | An IH scored each job from detailed occupational history for potential exposure to aluminium and 5 types of solvent. Exposures were also classified according to duration and intensity | Matching by age, sex and source of information. Adjusted for age and education |
| Feychting et al. Case–control. 1998. Sweden34 | Both sexes. 1989–91. Direct interview of cases' relatives (most often spouse or adult offspring) and of controls through structured questionnaire | From a cohort of twins taken by a register‐based sample of twins, 55 cases were identified (only a case when more than one twin demented). Cases were compared with non‐demented twins controls in two groups of 228 and 238 people | Screening with MMSE. DSM‐III‐R and NINCDS/ADRDA possible and probable | Occupations were linked to a JEM for exposure to EMF. Investigators had account of each subject's primary occupation, the last occupation in the person's work life, and the occupation with the highest magnetic field exposure. Exposures were categorised in three levels | Adjusted for age, sex and education |
| Noonan et al. Case–control. 2002. USA‐Colorado39 | Men. 1987–96. Death certificate data were collected from the Vital Statistics Unit of the Colorado Department of Public Health | 1556 Cases older than 60 years, were identified and compared with the same number of controls who died of other causes: leukaemia, brain cancer, or breast cancer | ICD‐9, code 331.0 | Exposure to EMF was assessed from primary occupation with three different methods: Electrical/no electrical occupation, according to 4 levels with a different probability of exposure, and according to different exposure levels given by a JEM | Frequency matched by 5‐year age intervals and year of death. Adjusted for age, race and occupational grouping |
| Sobel et al. 1996. Case–control. USA‐California29 | Both sexes. Ascertainment period not specified. Data were collected from the ADDTC at RLAMC | A clinical series of 326 cases who were at least age 65 at the time of their first examination at RLAMC were compared with 152 controls who were cognitively impaired or presented dementia other than vascular dementia | NINCDS‐ADRDA at least probable | Primary occupation was obtained from hospital records. The same method as previous investigation15 was used to measure exposure to EMF in high/low risk | Adjusted in men for sex and age at onset of symptoms. In women adjusted for education too |
| Shalat et al. 1988. Case–control. USA‐Bedford14 | Hombres. 1975–85. Questionnaire completed by the next of kin themselves for both cases and controls | 98 Cases obtained from hospitals were compared with 162 population controls | DSM III, NINCDS‐ADRDA (complete description of the diagnostic procedure reported previously) | Exposure to organic solvents and lead was assessed through specific items from the questionnaire and a detailed occupational history. Three IHs assigned likelihood and a semiquantitative level of exposure | Matched for sex, year of birth and town of residence. Adjusted for years of education |
| Li et al. Case–control. 1992. China25 | Both sexes. 1988–9. Direct interview to surrogate informants of both cases and controls through structured questionnaire | 70 Cases (54 hospital and 16 population cases) were compared with 140 non‐demented controls (neighbours) | Screening with MMSE. ICD‐10, NINCDS‐ADRDA possible and probable | Method to assess exposure to painting/other organic solvents through questionnaire is not defined | Matched by age and sex. Priority was given to those living closest to the matched patient. Adjusted for solvents is made, but confounding variables are not described |
| French et al. Case–control. 1985. USA‐Minneapolis12 | Men. 1979–82. Direct interview through structured questionnaire or by telephone (6% of completed interviews) to surrogates respondents (usually next of kin) of both cases and controls | 78 Hospital cases. 76 hospital controls and 48 neighbourhood controls. Controls with psychiatric disorders, CNS disorders, and alcoholism were excluded | NINCDS‐ADRDA probable. (Histologically confirmed in a subset of study subjects) | Method to assess exposure to pesticides, solvents and lead through questionnaire is not defined | Matching by age, sex and race |
| Gun et al. Case–control. 1997. Australia30 | Both sexes. 1986–9. Direct interview through structured questionnaire to proxy respondents of both cases and controls | 170 Hospital cases were compared with 170 population controls | NINCDS‐ADRDA possible and probable | Exposure to solvents, aluminium, lead and pesticides was assessed by a panel of three IHs, using occupational histories and a JEM. Cumulative exposures were calculated | Matching by age and sex. Adjusted for sex, education, family history of AD, early‐ or late‐onset AD cases, and possible versus probable AD cases |
| Heyman et al. Case–control. 1984. USA‐Durhan11 | Both sexes. Ascertainment period unspecified. Direct interview through structured questionnaire to proxy respondents of both cases and controls | 46 Hospital cases were compared with 92 population controls free of dementia | Similar to NINCDS‐ADRDA. (Histologically confirmed in a subset of study subjects) | Exposure to solvents and lead was directly assessed through an item from questionnaire which asked about “ever exposure” of at least 10 hours at week at least during 6 months in any occupation of the cases or controls | Matched for sex, race and 5‐year age intervals |
| Sobel et al. Case–control. 1995. Finland and USA‐California15 | Both sexes. Series 1: 1982–5, series 2: 1977–8, series 3: 1984–93. Direct interview through structured questionnaire to proxy respondents of cases, but direct interview to controls | Three clinical series analysed globally and independently with different types of controls: 53, 198, 136 cases were compared with 70, 299, 136 controls, respectively | NINCDS‐ADRDA, or similar to NINCDS‐ADRDA | Exposure to EMF was evaluated by an IH from primary occupations. Exposures were also classified according to intensity in high/low level | Adjusted for age at onset, age at examination, sex, education and social class |
| Palmer et al. Case–control. 1998. England and Wales31 | Men. 36–38 months. Short postal questionnaire either to the patient himself, or if he had died, to the next of kin | From CT records, 204 dementia cases (105 AD) were identified, who were compared with 225 controls with brain cancer and 441 controls with other neurological diseases like cerebrovascular disease, benign tumours, migraine or headache | From CT records of neuroradiology centres. Clinical diagnosis not specified | To assess exposure to solvents only occupations for more than 1 year recalled in the questionnaire were examined. These occupations were linked to a classification of occupations by likely exposure to organic solvents into three levels (high exposure, intermediate or uncertain exposure and low exposure) | Adjusted for age at the CT, neuroradiology centre, and distance of residence at the time diagnosis from the neuroradiology centre |
*Papers are listed in decreasing order of their Global Quality Index, see explanation in the text.
†ADDTC, Alzheimer Disease Diagnosis and Treatment Centre; RLAMC, Rancho Los Amigos Medical Centre.
‡AD, Alzheimer's disease; CNS, central nervous system; CT, computed tomography.
§ICD‐9 and ICD‐10: criteria for Alzheimer's disease from the International Classification of Diseases, 9th and 10th revisions; NINCDS‐ADRDA: criteria for Alzheimer's disease from the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; DSM III‐R and DSM‐IV: criteria for Alzheimer's disease from the Diagnostic and Statistical Manual for mental disorders, revised 3rd and 4th editions; MMSE, Mini‐Mental State Examination; 3MS, modified Mini‐Mental State Examination.
¶EMF, electromagnetic fields; IH, industrial hygienist; JEM, job exposure matrix.
**CSHA, Canadian Study of Health and Aging investigators.
In Table 4 the results of the expert evaluation of the studies are presented. For the 24 studies, the median for the GQI was 36.6%. The article with the highest score reached a GQI of 62.9%. There was great variability in the quality of the different studies, with a range of 43.4% between the papers with the highest and lowest score. All the case–control studies but one27 showed a GQI below 50%. Five case–control studies scored below 25%, and the lowest score in the total sample was for a case–control study (GQI = 19.4%). Quality in the three cohort studies was greater and more homogeneous than that seen in the case–control studies. The lowest value in the cohort studies corresponded to a prospective cohort study (GQI = 50.5%).40
Table 4 Relative risks, quality (Global Quality Index, see explanation in the text) and likelihood for the presence of biases in epidemiological studies on the relationship between Alzheimer's disease and selected occupational exposures.
| Authors/Design/Year/ Place* | Studied exposures. RR for exposure classified as ever/never exposed† | Global quality index | Selection bias‡ | Disease misclassification bias‡ | Exposure misclassification bias‡ | Bias due to surrogates informants‡ | Misclassification of confounding bias ‡ |
|---|---|---|---|---|---|---|---|
| Savitz et al. Retrospective cohort. 1998. USA33 | EMF: aRR = 2.1 (95% CI 0.6 to 6.8) | 62.9 | – – | +/− | – | 9 | – |
| Kukull et al. Case–control. 1995. USA‐Seattle27 | Solvents: Men: aRR = 6.3 (95% CI 2.2 to 18.1). Women: aRR = 0.6 (95% CI 0.2 to 1.9). Both: aRR = 1.8 (95% CI 1.1 to 3.1) | 55.6 | – | – | – | +/− | – |
| Tyas et al. Prospective cohort. 2001. Manitoba, Canada38 | Solvents: Degreasers: aRR = 0.88 (95% CI 0.31 to 2.50) Pesticides: Defoliants, fumigants: aRR = 4.35 (95% CI 1.05 to 17.90). Pesticides/fertilisers: aRR = 1.45 (95% CI 0.57 to 3.68) | 53.8 | +/− | – | + (?) | 9 | – |
| Baldi et al. Prospective cohort. 2003. Paquid study‐France40 | Pesticides: Men: aRR = 2.39 (95% CI 1.02 to 5.63). Women: aRR = 0.89 (95% CI 0.49 to 1.62) | 50.5 | +/− | – | +/− | 9 | +/− |
| Salib and Hillier. Case–control. 1996. UK28 | Aluminium: Demented controls: aRR = 0.95 (95% CI 0.5 to 1.8). Non‐demented controls: aRR = 0.95 (95% CI 0.5 to 1.9) | 48.1 | – – | +/− | + (↓) | +/− | – |
| Savitz et al. Case–control. 1998. USA‐25 different states35 | EMF: Electrical/non‐electrical occupation: aRR = 1.2 (95% CI 1.0 to 1.4) | 44.4 | – | + (↓) | ++ (↓) | 99 | +/− |
| Graves et al.Case–control. 1999. USA‐Seattle36 | EMF: Hygienist 1: aRR = 0.74 (95% CI 0.29 to 1.92); hygienist 2: aRR = 0.95 (95% CI 0.29 to 1.92) | 42.6 | +/− | – | +/− | + (↑) | +/− |
| Chandra et al. Case–control. 1987. USA‐Denver13 | Lead: aRR = 0.25 (95% CI 0.03 to 2.24)§ | 41.7 | +/− | +/− | + (↓) | + (?) | + (?) |
| Gauthier et al. Case–control. 2001. Canada‐Quebec37 | Pesticides: aRR = 0.97 (95% CI 0.38 to 2.41). Herbicides: aRR = 1.07 (95% CI 0.39 to 2.54). Insecticides: aRR = 1.62 (95% CI 0.64 to 4.11) | 41.7 | +/− | +/− | +/− | +/− | – |
| CSHA** et al. Case–control. 1994. 10 Canadian provinces26 | Solvents: aRR = 0.76 (95% CI 0.38 to 1.54) Pesticides: Pesticides/fertilisers: aRR = 1.58 (95% CI 0.81 to 3.10) | 39.8 | +/− | – | + (↓) | + (?) | +/− |
| O'Flynn et al. Case–control. 1987. England and Wales24 | Solvents: unadjusted RR = 1.11 (not significant) Lead: unadjusted RR = 0.86 (not significant) | 39.8 | – | ++ (↓) | ++ (↓) | 99 | + (?) |
| Graves et al. Case–control. 1998. USA‐Seattle32 | Solvents: aRR = 1.77 (95% CI 0.81 to 3.90) Aluminium: unadjusted RR = 1.46 (95% CI 0.63 to 3.42) | 37.0 | +/− | – | + (↓) | +/− | + (?) |
| Feychting et al. Case–control. 1998. Sweden34 | EMF: Control group 1:aRR = 2.4 (95% CI 0.8 to 6.9). Control group 2: aRR = 2.7 (95% CI 0.9 to 7.8). | 36.1 | +/− | – | +/− | + (?) | +/− |
| Noonan et al. Case–control. 2002. USA‐Colorado39 | EMF: aRR = 1.21 (95% CI 0.83 to 1.76) | 34.3 | +/− | ++ (↓) | ++ (↓) | 99 | +/− |
| Sobel et al. 1996. Case–control. USA‐California29 | EMF: Men: aRR = 4.90 (95% CI 1.3 to 7.9). Women: aRR = 3.40 (95% CI 0.8 to 16). Both: aRR = 3.93 (95% CI 1.45 to 10.56) | 34.2 | + (↓) | +/− | ++ (↓) | 99 | + (?) |
| Shalat et al. 1988. Case–control. USA‐Bedford14 | Solvents: aRR = 1.0 (95% CI 0.5 to 1.9) Lead: aRR = 0.8 (95% CI 0.3 to 2.0) | 33.3 | + (?) | +(?) | + (?) | ++ (?) | +/− |
| Li et al. Case–control. 1992. China25 | Solvents: aRR = 1.17 (95% CI 0.31 to 4.37) | 30.6 | + (?) | +/− | + (↓) | ++ (↓) | + (?) |
| French et al. Case–control. 1985. USA‐Minneapolis12 | Solvents: aRR = 1.25 (95% CI 0.55 to 2.84) Pesticides: aRR = 0.80 (95% CI 0.29 to 2.19) Lead: aRR = 1.50 (95% CI 0.25 to 8.98)** | 28.7 | + (?) | + (?) | + (↓) | + (?) | ++ (?) |
| Gun et al. Case–control. 1997. Australia30 | Solvents: unadjusted RR = 1.31 (95% CI 0.83 to 2.07 Pesticides: Organophosphates: unadjusted RR = 2.54 (95% CI 0.41 to 27.06) Lead: unadjusted RR = 1.12 (95% CI 0.63 to 2.0) Aluminium: unadjusted RR = 0.33 (95% CI 0.01 to 4.16) | 26.8 | ++ (?) | + (?) | + (↓) | ++ (↓) | +/− |
| Heyman et al. Case–control. 1984. USA‐Durhan11 | Solvents: 0 exposed¶ Lead: aRR = 0.78 (95% CI 0.14 to 4.36)¶ | 24.1 | ++ (?) | + (↓) | ++ (↓) | +/− | +/− |
| Series 2. Sobel et al15 | EMF (see series 1 data) | 24.1 | ++ (?) | + (?) | + (↓) | ++ (↓) | +/− |
| Series 3. Sobel et al15 | EMF (see series 1 data) | 21.3 | ++ (?) | + (?) | + (↓) | ++ (↓) | +/− |
| Series 1.Sobel et al. Case–control. 1995. Finland and USA‐California15 | EMF: For the three series: Men: aRR = 1.9 (95% CI 0.8 to 5.0). Women: aRR = 3.7 (95% CI 1.7 to 8.9). Both sexes: aRR = 2.9 (95% CI 1.6 to 5.4) | 20.4 | ++ (?) | + (?) | + (↓) | ++ (↓) | +/− |
| Palmer et al. Case–control. 1998. England and Wales31 | Solvents: aRR = 0.3 (95% CI 0.1 to 1.3) | 19.4 | + (↓) | ++ (↓) | ++ (↓) | ++ (↓) | + (?) |
*Papers are listed in decreasing order of their Global Quality Index, see explanation in the text.
†Highest relative risks (and odds ratios) adjusted by the maximum number of variables in each study.
‡For bias: – – denotes highly improbable, – denotes improbable, +/− denotes possible, + denotes probable, ++ denotes highly probable. For each bias identified as probable or highly probable, (↓) denotes the experts either judged that the bias would had decreased association towards the null, (↑) denotes bias would increase the association, and (?) denotes the experts failed to reach a conclusion about the effect of that particular bias. 9: Registry‐based retrospective or prospective cohorts in which this bias is not possible; 99: Registry‐based case–control studies, in which this bias is not possible.
§Information about OR for lead was obtained from a later reanalysis.10
¶Information about OR for solvents and lead was obtained from a later reanalysis.10
**CSHA, Canadian Study of Health and Aging Investigators.
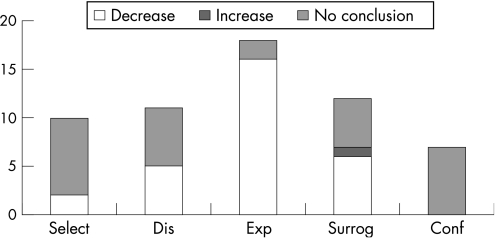
The most common potential bias is that of misclassification in the exposure, present in 18 of the 24 studies analysed (75.0%). The second in order of frequency is the potential bias arising from the use of surrogate informants, present in 12 of the 17 studies (70.6%). The third potential bias is that of misclassification of the disease, which appeared in 11 of the 24 studies (45.8%), followed by bias of selection present in 10 studies (41.7%). Confounding was considered the less frequent potential type of bias (fig 1).
Figure 1 Number of biased studies (estimated as probably or highly probably biased) and direction of bias (decreasing or increasing association, or when no conclusion was reached about direction) in selected studies on the relationship between Alzheimer's disease and occupational exposures (n = 24). Select, selection bias; dis, disease misclassification bias; exp, exposure misclassification bias; surrog, bias arising from use of surrogate informants; conf, confounding bias.
In only one case, in bias arising from the use of surrogate informants, was it judged that the effect of bias might at least probably increase the association between AD and the assessed exposure, in this case to EMF. For the remaining studies the experts either judged that the observed association was probably underestimated or they failed to reach a conclusion about the effect of the potential biases under consideration. In 16 of the 18 studies affected by potential misclassification in the exposure (88.9%), it was judged that this effect could give rise to a non‐differential misclassification which would bias the associations towards the null (fig 1 and table 4).
In the reviewed papers, for the specific occupational exposures considered, 11 studies explored the relationship of AD with solvents, seven with electromagnetic fields (EMF), six with pesticides, six with lead and three with aluminium. Solvents and pesticides are the exposures with the highest number of high quality studies (five and four studies, respectively, with a score above the median GQI), followed by EMF (three studies) and lead and aluminium (two studies for each exposure) (table 4).
For pesticides, research of greater quality and prospective design found increased and statistically significant associations with AD. Tyas et al38 reported adjusted relative risk (aRR) of 4.35 (95% CI 1.05 to 17.90) for exposure to defoliants and fumigants (a smaller and non‐significant association was found for exposure to the wider category of “pesticides, fertilisers”: aRR = 1.45, 95% CI 0.57–3.68) and Baldi et al40 found aRR for occupational exposure to pesticides in men of 2.39 (95% CI 1.02 to 5.63). The two case–control studies assessing risk associated with pesticide exposure and with GQI above the median26,37 found evidence of smaller and non‐significant associations, supporting the hypothesis that potential biases might have affected these results, decreasing the associations towards the null (table 4). Finally, one of the remaining two case–control studies assessing exposure to pesticides30 found an unadjusted RR of 2.54 (95% CI 0.41 to 27.06) for organophosphates.
For the remaining occupational agents considered in this review the evidence of an association is less consistent. For solvents, only two out of the 11 studies analysing this exposure found a significant association with AD. The two studies focused on the same population base. The first27 is a high quality case–control study (the case–control study with the highest score for GQI), an example of proper selection and diagnostic bias control, where only the aRR in exposed men was significantly increased (aRR = 6.3, 95% CI 2.2 to 18.1). The second paper32 included only cases with a spouse who was willing to collaborate in the interview, which might increase the likelihood of selection bias. With this restriction the aRR for solvent exposure during more than 18 years reached statistical significance (aRR = 2.62, 95% CI 1.07 to 7.43), although it fell to 1.77 (95% CI 0.81 to 3.90) when exposure was classified as ever/never. However, a prospective cohort study,38 also assessing exposure to solvents and with a high quality ranking (GQI) in our evaluation, did not find association with this exposure focused in degreasers (aRR = 0.88, 95% CI 0.31 to 2.50), and neither did the other two case–control studies with above the median GQI scores.24,26
Main messages
Epidemiological literature on Alzheimer's disease and occupational exposures is, in general, scarce.
Some agents have received most of the attention (pesticides, solvents, electromagnetic fields, lead and aluminium), mostly in case–control studies.
In general, results are consistent with an increased risk of Alzheimer's disease in relation to occupational exposure to pesticides.
There are three studies assessing risk for occupational exposure to EMF with high quality, well above that of the other studies,33,35,36 including the study with the highest GQI score in our ranking. However, the highest odds ratio (OR) values for this exposure correspond to the lower quality studies. Our analysis suggests that these studies are likely to be biased and that selection bias might explain these results.
For lead exposure there are no data supporting any association. All the studies are case–control studies, with a relatively low level of quality according to our classification. For aluminium, one of the three studies about this exposure is the second in the quality ranking of the case–control studies.28 Results from this study show no association (aRR = 0.95, 95% CI 0.5 to 1.9). In the other two studies associations are also non‐significant (table 4).
Policy implications
Protection and surveillance of workers exposed to pesticides should consider the potential risk of Alzheimer's disease.
Further research, and mostly follow‐up studies, can provide more conclusive evidence about this association and other risks from occupational exposures.
Discussion
The meta‐analysis protocol recommends evaluation of the quality of the primary studies included in the research16,41 through ad hoc developed questionnaires for the assessment. Recommendations have been proposed for quality assessment of observational studies.16,17,18,19,20,21,22 The recent initiative named STROBE (STrengthening the Reporting of OBservational studies in Epidemiology)42 should help to improve the quality of published epidemiological research, and some of the papers included in this review might have been improved if STROBE recommendations had been considered for their publication.
The quality of the reviewed studies was assessed with a blinded, standardised and systematic approach. Two epidemiologists independently reviewed all the studies and discrepancies were solved through consensus meetings. The percentage of global agreement between the two epidemiologists was 83.5% for all reviewed case–control studies, 93.3% for the two prospective cohort studies and 85.7% for the retrospective cohort study. Therefore, reproducibility was reasonably good.
Considering only the studies with higher quality, occupational exposure to pesticides is the risk for which, according to our analysis, there is the greatest evidence of association with AD. The quantitative synthesis of the data in a meta‐analysis, including studies with higher methodological quality, might enable a more accurate quantification of the size of this suggested potential risk. For the remaining occupational agents, the evidence of an association is less consistent. Contradictory results are found among studies assessing occupational exposure to solvents and EMF, and a lack of association in studies for lead and aluminium.
Valid assessment of exposure is always a problem in occupational epidemiology. Most of the studies evaluated did not have a good occupational exposure assessment, with too wide a range for exposure, which can cause no differential bias. Hence, it is necessary to measure occupational exposures of interest with increased specificity according to probability, intensity, and duration and time period of exposure allowing for latency periods for the disease and dose–response relationships. Also, solvents, pesticides and EMF are categories that have too wide an exposure, including exposures with highly different biological effects (e.g., benzene and toluene, organochlorines and organophosphates, radiofrequencies and extremely low‐frequency EMF). More specific definition of exposure should also be considered in research. Prospective cohort studies are a more adequate design for proper consideration of occupational exposure characteristics.
For evidence of an association for occupational exposures according to sex, 15 of the 22 articles included both men and women. In four of these 15 studies15,27,29,40 the results differed by sex. In the associations with pesticides40 and solvents,27 the strongest associations are found among men, which would be compatible with the hypothesis of an association between the exposure and AD, as it is probable that men perform activities with higher exposure to the occupational agents considered. Contrary to these results, in EMF exposures the risk in women is greater than in men in one study,15 while in another it is greater among men,29 both associations being statistically significant. These results should be interpreted with caution as the quality of these studies is relatively low. However, the possible role of sex as an interaction variable has not been fully explored.
Lastly, another well established risk factor for AD, as previously mentioned, is the allele APOE4. The genetic characteristics of individual people may modulate the expression of different environmental exposures. In our review, only one study37 introduces the APOE in the model as a confounding factor. But the interaction of this allele with the different occupational exposures was not investigated in any of the studies. This interaction should be considered for further research, but given the relatively low prevalence of this allele, even in cases of AD, this analysis will require larger samples.43
The results of our quality analysis, together with the associations observed in reviewed studies, suggest that there is evidence of an association between AD and occupational exposure to pesticides. The quantitative synthesis of the data in a meta‐analysis including studies with higher methodological quality might enable a more accurate quantification of the size of this suggested potential risk.
Supplementary Material
Acknowledgements
We thank Dr Jennifer Prieto House for her support in the translation of this paper.
Abbreviations
AD - Alzheimer's disease
APOE4 - apolipoprotein E genotype epsilon 4 allele
aRR - adjusted relative risk
EMF - electromagnetic fields
GQI - Global Quality Index
Footnotes
Competing interests: None
References
- 1.Cummings J L, Cole G. Alzheimer disease. JAMA 20022872335–2338. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of the Department of Health and Human Services. Task Force on Alzhemier's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association Diagnostic and statistical manual of mental disorders. 4th ed revised (DSM IV). Washington: APA, 1994
- 4.McDowell I. Alzheimer's disease: insights for epidemiology. Aging 200113143–162. [DOI] [PubMed] [Google Scholar]
- 5.Van Duijn C M. Epidemiology of the dementias: recent developments and new approaches. J Neurol Neurosurg Psychiatry 199660478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fratiglioni L. Epidemiology of Alzheimer's disease: issues of etiology and validity. Acta Neurol Scand 1993145(Suppl)1–70. [PubMed] [Google Scholar]
- 7.Clayton D. The Eurodem collaborative re‐analysis of case‐control studies of Alzheimer's disease: some methodological considerations. Int J Epidemiol 199120(Suppl)62–65. [DOI] [PubMed] [Google Scholar]
- 8.Kukull W A, Bowen J D. Dementia epidemiology. Med Clin North Am 200286573–590. [DOI] [PubMed] [Google Scholar]
- 9.Van Duijn C M, Stijnen T, Hofman A. Risk factors for Alzheimer's disease: overview of the EURODEM collaborative re‐analysis of case‐control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 199120(suppl)4–12. [DOI] [PubMed] [Google Scholar]
- 10.Graves A B, Van Duijn C M, Chandra V.et al Occupational exposures to solvents and lead as risk factors for Alzheimer's disease: a collaborative re‐analysis of case‐control studies. Int J Epidemiol 199120(Suppl)58–61. [DOI] [PubMed] [Google Scholar]
- 11.Heyman A, Wilkinson W E, Stafford J A.et al Alzheimer's disease: a study of the epidemiological aspects. Ann Neurol 198415335–341. [DOI] [PubMed] [Google Scholar]
- 12.French L R, Schuman L M, Mortimer J A.et al A case‐control study of dementia of the Alzheimer type. Am J Epidemiol 1985121414–421. [DOI] [PubMed] [Google Scholar]
- 13.Chandra V, Philipose V, Bell P A.et al Case‐control study of late onset `probable Alzheimer's disease'. Neurology 1987371295–1300. [DOI] [PubMed] [Google Scholar]
- 14.Shalat S L, Seltzer B, Baker E L. Occupational risk factors and Alzheimer's disease: a case‐control study. J Occup Med 198830934–936. [DOI] [PubMed] [Google Scholar]
- 15.Sobel E, Davanipour Z, Sulkava R.et al Occupations with exposure to electromagnetic fields: a possible risk factor for Alzheimer's disease. Am J Epidemiol 1995142515–524. [DOI] [PubMed] [Google Scholar]
- 16.Delgado‐Rodriguez M, Sillero‐Arenas M. Inclusion of research quality in meta‐analyses [in Spanish]. Gac Sanit 19959265–272. [DOI] [PubMed] [Google Scholar]
- 17.Realini J P, Goldzieher J W. Oral contraceptives and cardiovascular disease: a critique of the epidemiologic studies. Am J Obstet Gynecol 1985152729–798. [DOI] [PubMed] [Google Scholar]
- 18.Gomez‐Olmedo M, Fernandez Sierra M, Delgado Rodriguez M.et al Infection by the human immunodeficiency virus among the Spanish population (I). Qualitative meta‐analysis [in Spanish]. Med Clin (Barc) 199095286–291. [PubMed] [Google Scholar]
- 19.Friendenreich C M, Brandt R F, Riboli E. Influence of methodologic factors in a pooled analysis of 13 case‐control studies of colorectal cancer and dietary fiber. Epidemiology 1994566–79. [DOI] [PubMed] [Google Scholar]
- 20.Friendenreich C M. Methods for pooled analyses of epidemiologic studies. Epidemiology 19934295–302. [DOI] [PubMed] [Google Scholar]
- 21.Longnecker M P, Berlin J A, Orza M J.et al A metaanalysis of alcohol consumption in relation to risk of breast cancer. JAMA 1988260652–656. [PubMed] [Google Scholar]
- 22.Rychetnik L, Frommer M.A schema for evaluating evidence on public health interventions: version 4. Melbourne: National Public Health Partnership, 2002 [DOI] [PMC free article] [PubMed]
- 23.Chalmers T C. Problems induced by meta‐analyses. Stat Med 199110971–980. [DOI] [PubMed] [Google Scholar]
- 24.O'Flynn R R, Monkman S M, Waldron H A. Organic solvents and presenile dementia: a case referent study using death certificates. Br J Ind Med 198744259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Shen Y C, Li Y T.et al A case‐control study of Alzheimer's disease in China. Neurology 1992421481–1488. [DOI] [PubMed] [Google Scholar]
- 26.CSHA Study Group The Canadian Study of Health and Aging: risk factors for Alzheimer's disease in Canada. Neurology 1994442073–2080. [DOI] [PubMed] [Google Scholar]
- 27.Kukull W A, Larson E B, Bowen J D.et al Solvent exposure as a risk factor for Alzheimer's disease: a case‐control study. Am J Epidemiol 19951411059–1071. [DOI] [PubMed] [Google Scholar]
- 28.Salib E, Hillier V. A case‐control study of Alzheimer's disease and aluminium occupation. Br J Psychiatry 1996168244–249. [DOI] [PubMed] [Google Scholar]
- 29.Sobel E, Dunn M, Davanipour Z.et al Elevated risk of Alzheimer's disease among workers with likely electromagnetic field exposure. Neurology 1996471477–1481. [DOI] [PubMed] [Google Scholar]
- 30.Gun R T, Korten A E, Jorm A F.et al Occupational risk factors for Alzheimer disease: a case‐control study. Alzheimer Dis Assoc Disord 19971121–27. [DOI] [PubMed] [Google Scholar]
- 31.Palmer K, Inskip H, Martyn C.et al Dementia and occupational exposure to organic solvents. Occup Environ Med 199855712–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graves A B, Rosner D, Echeverria D.et al Occupational exposures to solvents and aluminium and estimated risk of Alzheimer's disease. Occup Environ Med 199855627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savitz D A, Checkoway H, Loomis D P. Magnetic field exposure and neurodegenerative disease mortality among electric utility workers. Epidemiology 19989398–404. [PubMed] [Google Scholar]
- 34.Feychting M, Pedersen N L, Svedberg P.et al Dementia and occupational exposure to magnetic fields. Scand J Work Environ Health 19982446–53. [DOI] [PubMed] [Google Scholar]
- 35.Savitz D A, Loomis D P, Tse C K. Electrical occupations and neurodegenerative disease: analysis of U.S. mortality data. Arch Environ Health 19985371–74. [DOI] [PubMed] [Google Scholar]
- 36.Graves A B, Rosner D, Echeverria D.et al Occupational exposure to electromagnetic fields and Alzheimer disease. Alzheimer Dis Assoc Disord 199913165–170. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier E, Fortier I, Courchesne F.et al Environmental pesticide exposure as a risk factor for Alzheimer's disease: a case‐control study. Environ Res 20018637–45. [DOI] [PubMed] [Google Scholar]
- 38.Tyas S L, Manfreda J, Strain L A.et al Risk factors for Alzheimer's disease: a population‐based, longitudinal study in Manitoba, Canada. Int J Epidemiol 200130590–597. [DOI] [PubMed] [Google Scholar]
- 39.Noonan C W, Reif J S, Yost M.et al Occupational exposure to magnetic fields in case‐referent studies of neurodegenerative diseases. Scand J Work Environ Health 20022842–48. [DOI] [PubMed] [Google Scholar]
- 40.Baldi I, Lebailly P, Mohammed‐Brahim B.et al Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol 2003157409–414. [DOI] [PubMed] [Google Scholar]
- 41.The MOOSE group Meta‐analysis of observational studies in epidemiology. JAMA 20002832008–2012. [DOI] [PubMed] [Google Scholar]
- 42.Von Elm M, Egger M. The scandal of poor epidemiological research. BMJ 2004329868–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García A M, Ramón N, Porta M. Occupational exposure to neurotoxic chemicals in Alzheimer disease patients and interaction with ApoE alleles [in Spanish]. Gac Sanit 200519(Suppl 3)24 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.